Development and application of a microplate method to evaluate the efficacy of essential oils against Penicillium italicum Wehmer, Penicillium digitatum Sacc. and Colletotrichum musea (Berk. & M.A. Curtis) Arx, three postharvest fungal pathogens of fruits
Univ. Liege - Gembloux Agro-Bio Tech. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: khskouassi@doct.ulg.ac.be – Cheikh Anta Diop University. Faculty of Science and Technology. Laboratory of Chemistry and Biochemistry of Natural Products. BP 5005. Dakar-Fann (Senegal).
Walloon Agricultural Research Centre (CRA-W). Life Sciences Department. Bioengineering Unit. Chaussée de Charleroi, 234. B-5030 Gembloux (Belgium).
Univ. Liege - Gembloux Agro-Bio Tech. Applied Statistics, Computer Science and Mathematics. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Pranarôm International S.A. Avenue des Artisans, 37. B-7822 Ghislenghien (Belgium).
Cheikh Anta Diop University. Faculty of Science and Technology. Laboratory of Chemistry and Biochemistry of Natural Products. BP 5005. Dakar-Fann (Senegal).
Univ. Liege - Gembloux Agro-Bio Tech. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Univ. Liege - Gembloux Agro-Bio Tech. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Received on March 5, 2010; accepted on August 24, 2010
Résumé
Développement et application d'une méthode de microplaques pour évaluer l'efficacité d’huiles essentielles contre Penicillium italicum Wehmer, Penicillium digitatum Sacc. et Colletotrichum musea (Berk. & M.A. Curtis) Arx, trois champignons pathogènes de post-récolte sur fruits. Un test microbiologique a été développé pour l'évaluation in vitro de l'activité antifongique de 30 huiles essentielles présélectionnées. Un modèle, reproductible et standardisé, basé sur 10 séries de dilutions avec huit répétitions par série reparties sur deux plaques ELISA de 96 puits chacune, a été utilisé pour identifier l'efficacité de ces huiles essentielles contre Penicillium italicum Wehmer, Penicillium digitatum Sacc. et Colletotrichum musea (Berk. & M.A. Curtis) Arx, trois champignons pathogènes de post-récolte sur fruits. La croissance mycélienne a été suivie en mesurant la densité optique à 492 nm. Cinnamomum zeylanicum, Cinnamomum verum et Eugenia caryophyllus étaient encore actives contre les trois pathogènes, même à 100 ppm. Comparé aux autres méthodes, ce test microbiologique développé s'est avéré être une méthode rapide, reproductible et efficace pour tester l'efficacité des huiles essentielles qui inhibent la germination des spores de P. italicum, P. digitatum et C. musea. Ce test nécessite relativement une faible quantité d'huile essentielle pour les essais.
Abstract
A microbioassay was developed for evaluating the in vitro antifungal activity of 30 preselected essential oils. A template based on 10 serial dilutions with eight replicates per dilution arranged on two 96-well ELISA plates was used as a reproducible and standardized design to identify the in vitro effectiveness of these essential oils against Penicillium italicum Wehmer, Penicillium digitatum Sacc. and Colletotrichum musea (Berk. & M.A. Curtis) Arx, three postharvest fungal pathogens, on fruits. Growth of mycelium was monitored by measuring optical density (492 nm). Cinnamomum zeylanicum, Cinnamomum verum and Eugenia caryophyllus were found to be still active against all the three pathogens even at 100 ppm. Compared to other methods, this microbioassay proved to be a rapid, reproducible, and efficient method for testing the efficacy of essential oils that inhibit spore germination in P. italicum, P. digitatum and C. musea. The assay requires relatively small amounts of essential oils.
1. Introduction
1The banana and citrus fruit sector is still facing serious economic losses due in part to preharvest and postharvest fungal diseases. In the postharvest context, anthracnose (caused by Colletotrichum musea [Berk. & M.A. Curtis] Arx) is the most common and important disease of banana (Muirhead et al., 2000), while green mould (caused by Penicillium digitatum Sacc.) and blue mould (caused by Penicillium italicum Wehmer) are the most commonly encountered diseases in citrus (Timmer et al., 2003).
2To cope with these diseases, fungicidal treatment has long been used. Currently, this approach to the treatment of fungal diseases is being challenged because of:
3– the presence and persistence of significant levels of fungicidal residues in fruits and vegetables (Dogheim et al., 2002; Blasco et al., 2006);
4– chemical fungicide toxicity and the consequent risks to consumer health and to the environment (Unnikrishnan et al., 2002);
5– development of resistant pathogen populations (de Lapeyre de Bellaire et al., 1997; Ghosopha et al., 2007);
6– limitations on the number of chemical fungicides used for postharvest application (Aubertot et al., 2005).
7Therefore, the fruit sector urgently needs to develop alternative postharvest treatments that are acceptable to consumers, and that have a negligible risk to human health and the environment (i.e. the so-called “natural” fungicides). Given their traditionally known antifungal activities, essential oils (liquid or vapor) have been reported to inhibit postharvest fungal growth both in vitro and in vivo (Bakkali et al., 2008). To date, no particular resistance to essential oils has been reported and the risk of development of resistant races of fungi is negligible. This may be due to the mixture of oil components with apparently different modes of action affecting several targets at the same time. Moreover, essential oils are biodegradable, non-persistent and non-toxic for human consumption at low doses (Isman, 2000). For the above-mentioned reasons, essential oils might effectively replace chemical fungicides for some specific applications.
8Despite progress in this domain, biofungicides based on essential oils are not yet well established in the market and remain largely a wish rather than a realistic alternative. Several factors explain the large disparity that persists between the accessibility of such biofungicides and the disavowal of chemical fungicides. Some of the most commonly cited disadvantages of using biofungicides are as follows:
9– difficulties involved in setting up commercial production;
10– problems with formulation;
11– doubts over their effectiveness;
12– difficulty in obtaining research funding;
13– cost of production;
14– the necessity of registering essential oil-based biofungicides.
15In order that such a biocontrol strategy for postharvest treatment becomes acceptable, it seems important to adopt an initiative to increase the chances of success in marketing biofungicides. The recommendations provided by Cos et al. (2006) for the screening of natural products effective against human diseases could be adapted to the search for antifungal essential oils for use against plant diseases. In order to be effective, such an approach would need to take into account the selection of appropriate essential oils and the use of an appropriate bioassay.
16Essential oils must be selected according to criteria that will promote their practical use. These criteria may include toxicity, cost, yield and availability of the essential oils. Regarding the appropriate bioassay, Datry et al. (1995) have critically reviewed methods used for testing the efficacy of antifungal compounds. Most of these are labor-intensive, time-consuming, expensive and often unreliable. For example, methods (Disk-diffusion, E-test) using a solid culture medium (PDA, water-agar) on Petri dishes are unsuitable for screening a large number of essential oils because they are time-consuming. Moreover, the Minimum Inhibitory Concentration (MIC) obtained by these methods is higher than that obtained using a liquid culture medium (Fernández-Torres et al., 2002). This can be explained by the fact that antifungal substances (especially essential oils) diffuse less in a solid than in a liquid medium and the surface of contact between the microorganism and active substances is greater in the liquid than in the solid medium. The poor solubility of essential oils in solid media can also explain their high MICs in these conditions. Furthermore, methods for determining the MIC of essential oils, i.e. using the counting of spore germination under an optical or binocular microscope, measuring the length of mycelial growth or colony diameter of the fungus, and estimating the visual turbidity of the solution, are more cumbersome and less accurate than those using the estimation of the fungus biomass by spectrophotometry (Banerjee et al., 1993; Arthington-Skaggs et al., 2002). For the above-mentioned reasons, the microtiter ELISA microplate method, which has proved useful for the pharmaceutical industry, is more appropriate for the rapid screening of essential oils. Using such a method, Wilson et al. (1997) and Kuhajek et al. (2003) have developed operating conditions for rapid quantitative and qualitative microscale bioassays for the discovery of novel compounds for the control of Phytophthora spp. and Botrytis cinerea, respectively. The in vitro assessment of P. italicum, P. digitatum and C. musea presents a challenge because these microorganisms may differentially be sensitive to the operating conditions defined by these authors. The development of an efficient and reproducible method for the in vitro evaluation of essential oils is thus needed for each studied fungus. The specific aims of this study were therefore:
17– to standardize the in vitro growth of P. italicum, P. digitatum and C. musea on ELISA microplates;
18– to select interesting essential oils suitable for use in biofungicides;
19– to evaluate their antifungal in vitro activity against the three postharvest phytopathogens.
2. Materials and methods
2.1. Fungal strains and production of conidia
20Penicillium italicum (strain PIRBM1), P. digitatum (strain PDRBM1) and C. musea (strain CO-CMR-65) were obtained from the Plant Pathology Unit collection (Gembloux Agro-Bio Tech, Belgium). The Penicillium strains were isolated from citrus in Morocco (ENA Meknes) and the C. musea strain from bananas in Cameroon (CARBAP). All fungal species were maintained in 25% glycerol at -80 °C. For conidia production, small agar blocks containing mycelium were cut from the fungus culture and transferred to a fresh Potato Dextrose Agar (PDA) medium for Penicillium and to a modified Mathur's medium (MM) for C. musea. Replicate plates were incubated in darkness at 23 °C to allow sporangium production.
2.2. Inoculum preparation
21The inoculum was prepared under aseptic conditions. Ten ml of 0.05% Tween 20 in sterile distilled water (SDW) were added to a Petri dish on a 14-day-old fungal colony. Conidia were dislodged by rubbing the surface area with a glass rod. The washings were then filtered through a double sterilized layer of fine cloth to remove mycelial fragments. The number of conidia was determined with a hemocytometer (Bürker) and adjusted to the desired conidia concentration.
2.3. Culture media preparation
22Concentrated orange (Penicillium) or banana (C. musea) juices, previously filtered through a membrane filter to remove particles that could interfere with photometric evaluation of the fungus growth, were autoclaved at 110 °C for 1 h and then diluted with SDW to obtain the desired juice concentration.
2.4. ELISA microplates, sealant and microplate photometer
23ELISA microplates (96 well) with flat-bottom/standard (Nunc MicroWell [untreated]; Greiner Bio-One) were used to estimate the in vitro growth of fungal strains. These microplates are well suited for their microscopic applications as well as for the precision of their optical measurements (flat bottom of the wells, 100% transmittance at ≥ 490 nm). The microplates are manufactured in a polystyrene that is characterized by its high clarity and resistance to many standard laboratory chemicals.
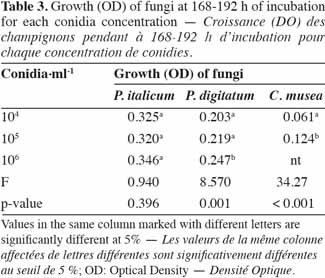
24A sealant (EASYsealTM2) coated with an acrylate adhesive was chosen to seal the microplates. It is non-cytotoxic, easy to use and characterized by its capacity to protect against evaporation and contamination. Above 330 nm, the percentage of light absorbed by the sealant is almost negligible and thus appears suitable for optical measurements.
25The 96-well microplate reader (Multiskan RC, Labystems; Thermo Scientific) was used as a photometer, which is specially used for ELISA. This device is controlled by PC software and can shake the plate to homogenate the solution before reading the whole plate in only 5 seconds.
26The microplates, the sealant and the photometer were carefully selected in order to minimize the effect of the material on fungal growth.
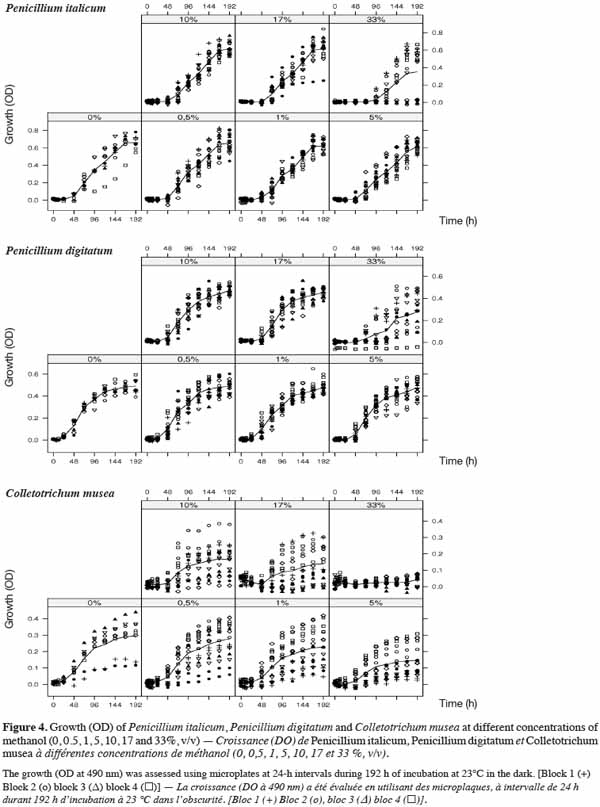
2.5. Essential oils
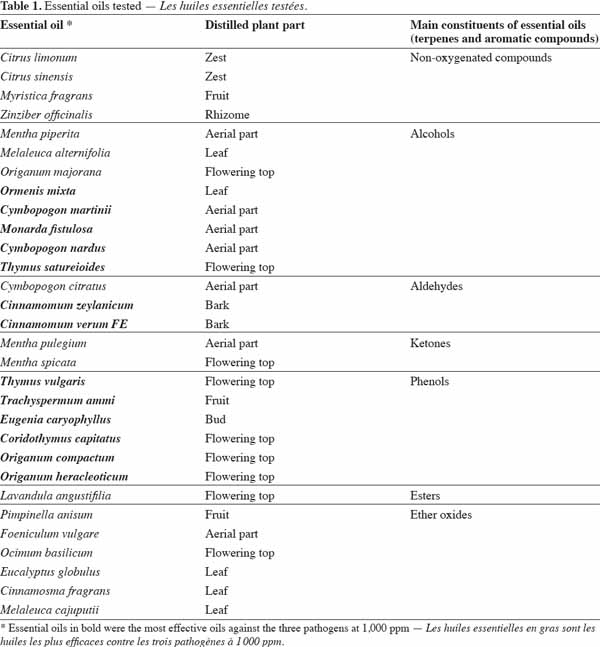
27Thirty essential oils were selected according to intrinsic (yield, toxicity) and extrinsic (availability, cost) criteria (Pranarôm International S.A, unpublished data); their classification according to their main components is shown in table 1. These essential oils were provided by Pranarôm S.A. (essential oils production company). The oils were previously diluted in methanol before testing their antifungal activity.
2.6. Experiment 1: in vitro growth of fungal strains
28This experiment was carried out in order to identify appropriate conditions for optimal fungal growth on ELISA microplates. To this end, the in vitro growth of each fungal strain was monitored using three conidia concentrations (104, 105 and 106 conidia·ml-1) and three culture media, corresponding to different dilutions of orange or banana juice (3 x 10-2, 3 x 10-3 and 3 x 10-4 v/v). The effect of each combination on the growth of each pathogen was assessed using different numbers (3 to 16) of replicates in a volume of 200 µl using sealed 96-well ELISA microplates. Given the number of replicates, all replicates of the same combination were pipetted into wells of three 96-well ELISA microplates, following a modification of the procedure reported by Cos et al. (2006). The control for each group of replicates was a well containing non-infected medium. Mycelium growth was monitored photometrically at 492 nm by measuring the optical density (OD) of each well for 196 h (at 2-h intervals during the first 12 h and then at 24-h intervals) of incubation at 23 °C in the dark. Before each measurement, microplates were shaken for 10 s. OD due to pathogen growth was obtained by subtracting the OD values of inoculated wells from those of uninoculated wells from the same group of repetitions.
2.7. Experiment 2: evaluation of the effect of methanol on the in vitro growth of fungal strains
29This second experiment was conducted in order to identify the highest methanol concentration that could be applied without a significant effect on the growth of the three studied fungi. Seven different methanol concentrations (0, 0.5, 1, 5, 10, 17 and 33%, v/v) were tested in a volume of 200 µl of diluted fruit juice (3 x 10-2, v/v) containing the fungal inoculum (104 conidia·ml-1) and using eight replicates per methanol concentration and per fungus. Growth (OD at 490 nm) was assessed using sealed microplates at 24-h intervals during 192 h of incubation at 23 °C. In order to control the heterogeneity within and between the plates observed in Experiment 1, a blocked randomized design was used (Figure 1).
2.8. Experiment 3: screening of essential oils
30The present experiment was carried out to select interesting essential oils that could be used as a biological control for postharvest diseases (anthracnose, green and blue moulds) in banana and citrus fruits. To this end, the antifungal activity of selected essential oils was evaluated using ELISA microplates with a blocked randomized design, as described previously. The growth of each pathogen was monitored in a volume of 200 µl containing diluted fruit juice (3 x 10-2, v/v), the inoculum (104 conidia·ml-1), methanol (0.5%, v/v) and the essential oil (1,000; 500 and 100 ppm). Incubation and OD measurements were carried out as for the second experiment.
3. Statistical analysis
31Statistical analyses were conducted with the R statistical software 2.7.1. (R Development Core Team, 2008).
3.1. Experiment 1: in vitro growth of fungal strains
32The optimal number of replicates was fixed using the standard error of the mean as a selection criterion. In perfect spatial homogeneity state, the standard error of the mean is represented in formula (1):

33where σ means standard deviation and n is the number of observations.
34The average standard error of the mean was then calculated for each conidia concentration and fruit juice dilution combination and for each replicate size. The selected number of replicates was the minimum number of replicates for which an increase gave no further significant improvement to the standard error of the mean.
35The observed values of the standard error for each replicate size were also compared to the theoretical standard error, calculated on all the replicates for a combination of conidia concentration and juice dilution with formula (1). Dissimilarity of the two values can give an insight into the heterogeneity of the experimental space (ELISA plates).
36The effects of conidia concentration and juice dilution on fungal growth were analyzed using a two-way ANOVA for each fungus, using the average observed OD attributed to the fungus at 168-192 h as a response, with the number of replicates selected in the first step. A multiple comparison of the means was conducted using the Tukey test.
3.2. Experiment 2: evaluation of the effect of methanol on the in vitro growth of fungal strains
37The effect of methanol concentration on fungal growth was analyzed using a two-way mixed model for each fungus (methanol concentrations and blocks), using the average observed OD attributed to the fungus at 72-96 h (active growth) and 168-192 h (plateau) as a response. A multiple comparison of the means was conducted by a contrast test, taking 0% methanol as a reference (Dunnett's test).
3.3. Experiment 3: screening of essential oils
38The effect of essential oils on fungal growth was adjusted by a two-way (oils and blocks) mixed model for each fungus and each oil concentration, using the average observed OD attributed to the fungus at 168-192 h (plateau) as a response. The percentage of inhibition of essential oils with respect to the control was calculated with the following formula (2):
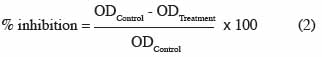
39where ODControl = [OD (X’) - OD (T’)], ODTreatment = [OD (Xn) - OD (Tn)] (Figure 1).
40Standard errors of these parameters were calculated from the mixed model. Data presented were pooled from two independent experiments.
4. Results and discussion
4.1. Experiment 1: in vitro growth of fungal strains
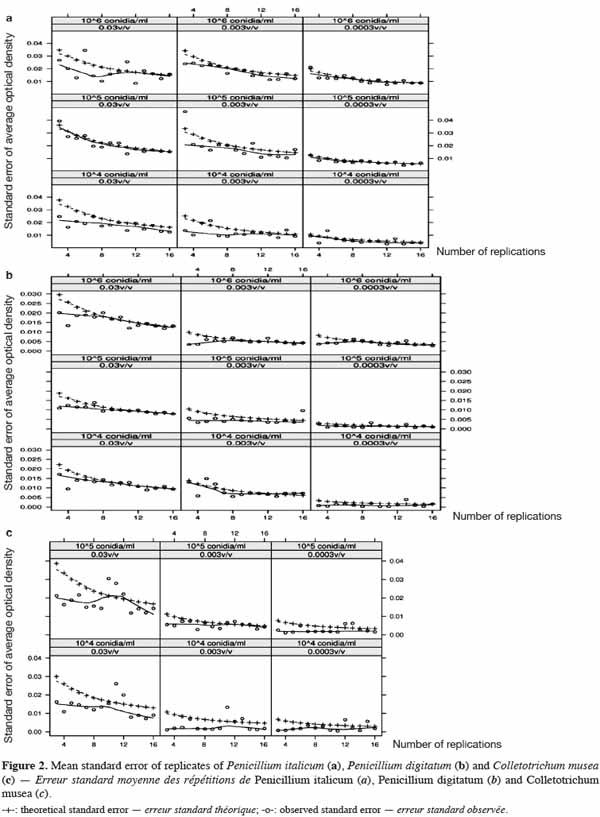
41Observed and theoretical standard errors of the means of each conidia concentration, juice dilution and replicate size combinations are shown in figure 2a (P. italicum), figure 2b (P. digitatum) and figure 2c (C. musea). As theoretical standard errors are generally higher than those observed, we expected heterogeneity in the response between and/or within ELISA plates, which would need to be taken into account in the next experimental designs. As expected, the standard errors decreased with the increase in the number of replicates. However, the decrease in the standard errors became practically negligible beyond eight replicates. Consequently, the number of replicates for further experiments was fixed to eight, following a design that controls inter- and intra-plate heterogeneity (2 plates x 2 blocks x 2 wells = 8 replicates) (Figure 1). To avoid any possibility of contamination, uninoculated wells (without pathogen) were separated from the inoculated wells (with pathogen). Unlike in the experiments of Cos et al. (2006), the wells situated at the corners of the plate were used here, as we observed no problem of evaporation from these wells. The design reported here allows more serial testing of essential oils than the design proposed by Cos et al. (2006) but with less repetition per treatment. This design saves time by reducing the number of repetitions and by increasing the number of essential oils that can be tested.
42In order to identify the appropriate juice dilution and conidia concentration for the assay, growth of each pathogen was evaluated for three different conidia concentrations and three juice dilutions (Figure 3). Growth was not photometrically quantifiable before 24 h of incubation, and the amount of growth differed depending on the medium. For the three fungi, non-significant interaction between conidia concentration and juice dilution was detected (Fmax = F4,63 = 2.09, p-value = 0.093) and juice dilutions 3 x 10-3 and 3 x 10-4 v/v showed a significantly lower growth compared to 3 x 10-2 v/v (Fmin = F2,42 = 55.19, p-value < 0.001) (Table 2). At this dilution (3 x 10-2 v/v), growth started to increase at 24 h and reached a plateau at between 168 and 192 h, whatever the conidia concentration and the pathogen tested. By 168-192 h, growth leveled off for all conidia concentrations and C. musea showed lower growth (OD = 0.174) than P. italicum (OD = 0.601) and P. digitatum (OD = 0.577). For C. musea, only 104 and 105 conidia·ml-1 were tested because that pathogen did not produce enough spores for the preparation of a concentration of 106 conidia·ml-1. Furthermore, a low in vitro growth of C. musea was observed on the ELISA plate.
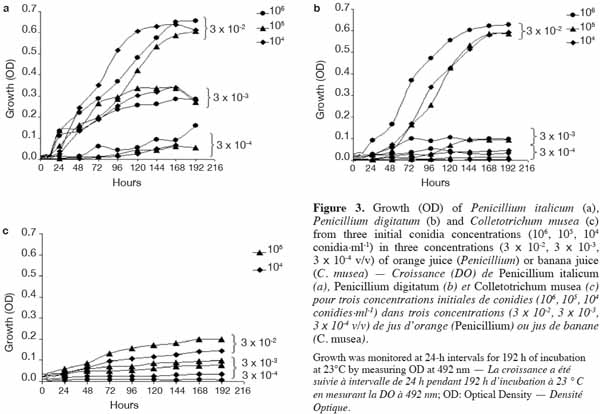
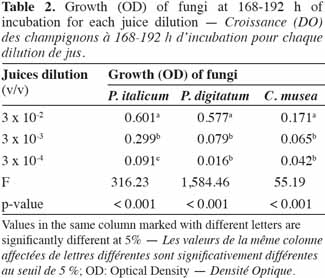
43For the 168-196 h period, conidia concentrations (104 and 105 conidia·ml-1) had no significant effect on P. italicum and P. digitatum growth, but they had a highly significant effect on the growth of C. musea (Table 3). Given the strong decrease in growth for dilutions higher than 3 x 10-2 and given the sufficient growth of the studied fungi with a concentration of 104 conidia·ml-1, these parameters were selected for the subsequent experiments.
44The orange juice and banana juice allowed a quantifiable and sufficient growth of pathogens. Comparing natural and synthetic media, Kuhajek et al. (2003) showed that growth from zoospores was higher in a natural medium than the growth observed in a synthetic medium. However, when variability in batch-to-batch composition is observed in a natural medium, a completely synthetic, chemically defined medium should be considered.
4.2. Experiment 2: evaluation of the effect of methanol on the in vitro growth of fungal strains
45The effect of methanol concentration on fungal growth is shown in figure 4a (P. italicum), figure 4b (P. digitatum) and figure 4c (C. musea). A variability of fungal growth was observed depending on the type of blocks used. However, this variability was less important in Penicillium strains than in C. musea. Nevertheless, trends in average growth can be clearly defined. The sensitivity of each pathogen to methanol differed depending on concentration. High concentrations of methanol either partially or totally inhibited the growth of pathogens. This was the case for the highest concentration (33.0%), which partially inhibited P. italicum at 48.1% and P. digitatum at 46.8% and which totally inhibited C. musea after 168-192 h of incubation. The mixed model showed a very highly significant effect of the methanol concentration on the fungal OD for both periods (72-96 h and 168-192 h) and on the three fungi (Fmin = F6,42 = 6.87, p-value < 0.001) (Table 4). The loss of growth in the pathogens due to methanol was higher for the period 72-96 h than for the period 168-192 h. Although this loss of growth in the pathogens was significant for all concentrations (except 0.5%) during the period 72-96 h, there was no further loss of growth during the period 168-196 h (except in the case of the highest concentration and including 5%, 10% and 17% in the case of C. musea). This result shows that methanol caused a significant slowdown in the growth of pathogens but that it had no fungicidal effect (except in the highest concentration) given that this growth retardation was recovered at the end of the observation period, as shown by analysis of the results for the 168-192 h period. C. musea seems from this perspective to be more sensitive to alcohol. Indeed, growth retardation was still detectable at the end of the observation period for
46the majority of concentrations tested.
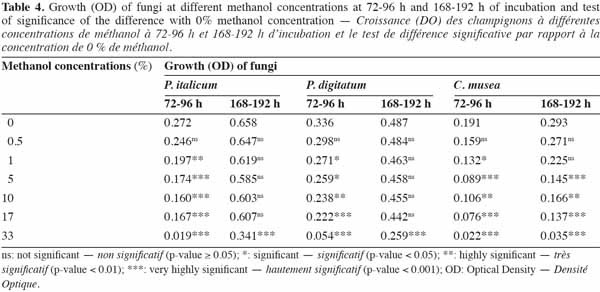
47For the six combinations of time period and fungi, only the 0.5% methanol concentration did not significantly affect growth compared to the control (0%).
4.3. Experiment 3: screening of essential oils
48The effect of essential oil concentration on fungal growth for 168-192 h of incubation is presented in figure 5. The thirty essential oils were firstly tested at 1,000 ppm for each pathogen. Only essential oils that inhibited the growth of pathogens for more than 70% of the reference growth were retained for the test at 500 ppm. The same growth limit was used to select the essential oils for the 100 ppm test from the 500 ppm results. It emerged that the most effective oils against the three pathogens at 1,000 ppm were those of Ormenis mixta, Cymbopogon martinii, Monarda fistulosa, Cymbopogon nardus, Thymus satureioides, Cinnamomum zeylanicum, Cinnamomum verum, Thymus vulgaris, Trachyspermum ammi, Eugenia caryophyllus, Corydothymus capitatus, Origanum compactum and Origanum heracleoticum. As can be seen in table 1, the main constituents of these essential oils are aldehydes, alcohols and phenols (forming the largest constituent). The high percentage inhibition of these most effective essential oils could be due to their high content of major compounds. This result is in agreement with those reported by Faid et al. (1996) and Dorman et al. (2000). These authors also observed a relationship between the high activity of essential oils and the presence of oxygenated compounds. Antimicrobial activity of major essential oil compounds is in the order: phenols > alcohols > aldehydes. In this study, despite the fact that some essential oils were rich in alcohol or aldehyde compounds, they did not show interesting antifungal activity. This was the case for Mentha piperita, Melaleuca alternifolia, Origanum majorana and Cymbopogon citratus.
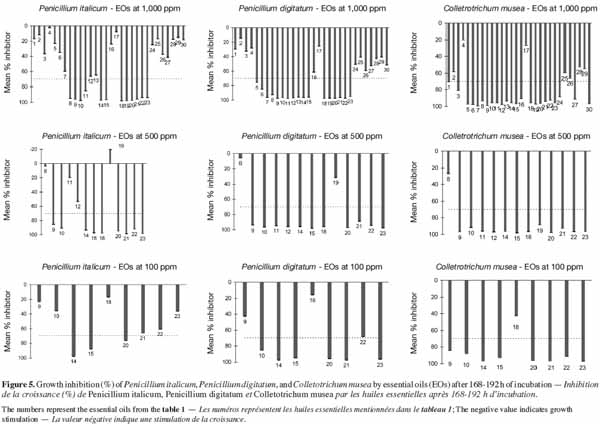
49Thus the relative antifungal activity of essential oils may not be easily correlated with the major compound but with a mixture of compounds acting in synergy.
50Penicillium digitatum and C. musea presented similar sensitivity towards essential oils. However P. italicum was more resistant to certain essential oils. This was the case, for example, with Cymbopogon nardus and Thymus satureioides, which inhibited completely the growth of P. digitatum and C. musea at 500 ppm, whereas inhibition was less than 60% for P. italicum.
51Among the eight essential oils still effective at 100 ppm, only Cinnamomum zeylanicum, Cinnamomum verum and Eugenia caryophyllus were active against the three pathogens (P. italicum, P. digitatum and C. musea). At such a concentration, essential oils are generally not dangerous or toxic for humans (Isman, 2000; Couderc, 2001). Furthermore, a high quantity of essential oils can be extracted from plants: 15 to 20% yield for Eugenia caryophyllus and 1 to 2% yield for the other two. Eugenia caryophyllus and Cinnamomum essential oils have been reported to inhibit the growth of P. digitatum (Yahyazadeh et al., 2008) and C. musea (Ranasinghe et al., 2002), respectively, in in vitro conditions. Using a solid culture medium (PDA) on Petri dishes and a technique based on the determination of mycelial dry weights respectively, these authors obtained higher values of inhibition concentration than ours.
5. Conclusion
52In this investigation, a microbioassay procedure was developed for evaluating the efficacy of antifungal essential oils against C. musea, P. italicum, and P. digitatum. The procedure reported here incorporates a standardized template for rapid in vitro screening. Due to the in vitro fungicidal effect of the essential oils C. zeylanicum, C. verum and E. caryophyllus, a possibility exists to develop an effective treatment system to control postharvest diseases of banana and citrus. However, the potential use of these essential oils requires a detailed examination of their biological activity and dispersion in fruit tissues.
53Acknowledgements
54We thank Pranarôm International S.A. for their collaboration and all those who provided technical assistance. The PhD scholarship of the first author was provided by the Ministry of Scientific Research of Côte d'Ivoire.
Bibliographie
Arthington-Skaggs B.A. et al., 2002. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob. Agents Chemother., 46, 2477-2481.
Aubertot J.N. et al., 2005. Pesticides, agriculture et environnement. Réduire l’utilisation des pesticides et limiter leurs impacts environnementaux. Synthèse du rapport. Paris : INRA/Cemagref.
Bakkali F., Averbeck S., Averbeck D. & Idaomar M., 2008. Biological effects of essential oils. Food Chem. Toxicol., 46, 446-475.
Banerjee U.C., Chisti Y. & Moo-Young M., 1993. Spectrophometric determination of mycelial biomass. Biotechnol. Techn., 7, 313-316.
Blasco C., Font G. & Pico Y., 2006. Evaluation of ten pesticide residues in oranges and tangerines from Valencia (Spain). Food Control, 17, 841-846.
Cos P., Vlietinck A.J., Vanden Berghe D. & Maes L., 2006. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol., 106, 290-302.
Couderc L.V., 2001. Toxicité des huiles essentielles. Thèse de doctorat : Université Paul Sabatier, Toulouse (France).
Datry A. et al., 1995. Analyse critique des tests de sensibilité in vitro aux antifongiques. Med. Mal. Infectieuses, 25, 6-13.
De Lapeyre de Bellaire L. & Dubois C., 1997. Distribution of thiabendazole-resistant Colletotrichum musea isolates from Guadeloupe banana plantations. Plant Dis., 81, 1378-1382.
Dogheim S.M. et al., 2002. Monitoring of pesticide residues in Egyptian fruits and vegetables during 1997. Food Addit. Contam., 19, 1015-1027.
Dorman H.J.D. & Deans S.G., 2000. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol., 88, 308-316.
Faid M., Charai M. & Mosaddak M., 1996. Chemical composition and antimicrobial activities of two aromatic plants: Origanum majorana L. and O. compactum Benth. J. Essent. Oil Res., 8, 657-664.
Fernández-Torres B., Pereiro Jr. M. & Guarro J., 2002. Comparison of two methods for antifungal susceptibility testing of Trichophyton rubrum. Eur. J. Clin. Microbiol. Infectious Dis., 21, 70-71.
Ghosopha J.M., Schmidt L.S., Margosan D.A. & Smilanick J.L., 2007. Imazalil resistance linked to a unique insertion sequence in the PdCYP51 promoter region of Penicillium digitatum. Postharvest Biol. Technol., 44, 9-18.
Isman M.B., 2000. Plant essential oils for pest and disease management. Crop Prot., 19, 603-608.
Kuhajek J.M., Jeffers S.N., Slattery M. & Wedge D.E., 2003. A rapid microbioassay for discovery of novel fungicides for Phytophthora spp. Phytopathology, 93, 46-53.
Muirhead I.F. & Jones D.R., 2000. Fungal diseases of banana fruit: postharvest diseases. In: Jones D.R., ed. Diseases of banana, Abacá and Enset. Wallingford, UK: CABI Publishing, 190-211.
R Development Core Team, 2008. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, URL http://www.R-project.org, (01/02/2010).
Ranasinghe L., Jayawardena B. & Abeywickrama K., 2002. Fungicidal activity of essential oils of Cinnamomum zeylanicum (L.) and Syzygium aromaticum (L.) Merr et L.M. Perry against crown rot and anthracnose pathogens isolated from banana. Lett. Appl. Microbiol., 35, 208-211.
Timmer L.W., Garnsey S.M. & Broadbent P., 2003. Diseases of Citrus. In: Ploetz R.C., ed. Diseases of tropical fruit crops. Wallingford, UK: Cabi Publishing, 163-195.
Unnikrishnan V. & Nath B.S., 2002. Hazardous chemicals in foods. Indian J. Dairy Biosci., 11, 155-158.
Wilson C.L., Solar J.M., EL-Ghaouth A. & Wisniewski M.E., 1997. Rapid evaluation of plant extracts and essential oils for antifungal activity against Botrytis cinerea. Plant Dis., 81, 204-210.
Yahyazadeh M., Omidbaigi R., Zare R. & Taheri H., 2008. Effect of some essential oils on mycelial growth of Penicillium digitatum Sacc. World J. Microbiol. Biotechnol., 24, 1445-1450.
