- Startpagina tijdschrift
- volume 16 (2012)
- numéro 2
- PAMPs, MAMPs, DAMPs and others: an update on the diversity of plant immunity elicitors
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
PAMPs, MAMPs, DAMPs and others: an update on the diversity of plant immunity elicitors

Nota's van de redactie
Received on July 25, 2001; accepted on November 8, 2011
Résumé
PAMPs, MAMPs, DAMPs et autres : mise à jour de la diversité des éliciteurs de l’immunité des plantes. Les plantes possèdent une large gamme de défenses qui peuvent être exprimées en réponse à la perception des organismes pathogènes ou parasites, mais aussi suite à la reconnaissance de certains micro-organismes saprophytes bénéfiques. Plus précisément, ce sont des composés dérivés de ces organismes et dénommés éliciteurs qui sont reconnus par la plante pour stimuler une résistance exprimée de manière locale ou systémique. La compréhension des bases physiologiques et biologiques des mécanismes de ces immunités a beaucoup progressé ces dernières années, mais une connaissance plus approfondie des mécanismes sous-jacents à la perception de ces éliciteurs est cependant essentielle pour développer de nouveaux moyens de contrôle des nuisibles. L’application de produits biologiques stimulateurs des défenses immunitaires des plantes dans l’agriculture conventionnelle est amenée à croître dans les prochaines années en tant que stratégie phytosanitaire. En raison de leur origine naturelle et étant donné qu’ils confèrent une protection sans modification génétique des plantes, les éliciteurs de l’immunité des plantes revêtent un énorme potentiel en tant que produits de lutte biologique. Au travers de cette revue, nous voulons illustrer la diversité des composés identifiés aujourd’hui comme pouvant stimuler les défenses immunitaires des plantes et les mécanismes par lesquels ils peuvent être perçus au niveau de la membrane plasmique.
Abstract
Plants possess a broad array of defenses that could be actively expressed in response of pathogenic organisms or parasites but also following beneficial saprophytic microorganisms recognition. Specifically, there are compounds derived from these organisms and called elicitors that are perceived by the plant to induce a locally or systemically expressed resistance. The understanding of the physiological and biological basis of these induced immunity mechanisms have greatly advanced over the past years but a deeper investigation of the mechanisms underlying the perception of elicitors is essential to develop novel strategies for pest control. The application of chemical and biological stimulators of plant immune defenses in conventional agriculture is expected to increase within the next years. Because of their organic origin and as they provide means for conferring plant protection in a non-transgenic manner, elicitors of plant immunity have a huge potential as biocontrol products. Through this review, we want to illustrate the diversity of compounds identified as stimulators of the plant immune system and describe the mechanisms by which they could be recognized at the plasma membrane level.
Inhoudstafel
I. Basic concepts of plant immunity
1As they are constantly exposed to pathogens but lack mobile defender cells and an adaptive immune system, plant defenses rely on the innate immunity of each cell and on systemic signals emanating from infection sites (Dangl et al., 2001; Ausubel, 2005). They have evolved a vast array of passive and active defense mechanisms that are manifested in the pest-colonized organ. Defense signals could be systemically emitted to activate a plethora of defense responses in the non-colonized organs of a plant locally infected by a microbe, infested by an herbivore or even stimulated by a chemical compound. Defense signals could also be primed for rapid activation after a localized perception of non-pathogenic fungi or bacterial strains.
2The presence of infectious agents is detected through the recognition of microbial signals. All signals that are perceived by plant cells and induce defense responses are considered as elicitors. Elicitors may be categorized in two classes: general (or non-specific) elicitors, which do not significantly differ in their effect on different cultivars within a plant species and may therefore be involved in general resistance, and specific elicitors, which are formed by specialized pathogen races or strains and function only in plant cultivars carrying the corresponding disease resistance gene (Montesano et al., 2003).
3General elicitors are designated Pathogen-Associated Molecular Patterns (PAMPs) when isolated from infectious agents but they may also correspond to endogen plant-host derived signals resulting from the action of the pathogen agent called DAMPs (Damage-Associated Molecular Patterns), to signals from non-pathogenic microorganisms referred here as MAMPs (Microbe-Associated Molecular Patterns) or to chemicals. The perception of general elicitors triggers a broad array of reactions, which culminate in the activation of the so-called basal resistance or PAMP-Triggered Immunity (PTI) (Nicaise et al., 2009) (Figure 1A). This defensive reaction may be strong enough to halt infection before the invader microbe becomes established. However, some successful pathogenic microorganisms may overcome basal resistance by delivering virulence effector proteins or DNA into host cells. These specific elicitors inhibit signalization pathways or the synthesis of defense compounds by the host plant and thus suppress this first type of immunity. Such signals are the specific elicitors and are likely the cause for susceptibility of many crops to virulent microbial pathogens. In response, plants have evolved a second line of defense through specific disease resistance (R) genes, the so-called effector-triggered-immunity (Pelletier et al., 2002; Jones et al., 2006) (Figure 1B). The recognized effector is termed an avirulence (Avr) protein. Because the effector-R protein relationship is highly specific, this R gene-mediated resistance appears to be similar to adaptative immunity in mammals. However, as R gene-mediated resistance is expressed through similar defense responses as those that are active in basal resistance, but on a much greater scale, ETI is considered as another form of plant innate immunity. Therefore, PTI and ETI are considered as primary and secondary innate immunity respectively.
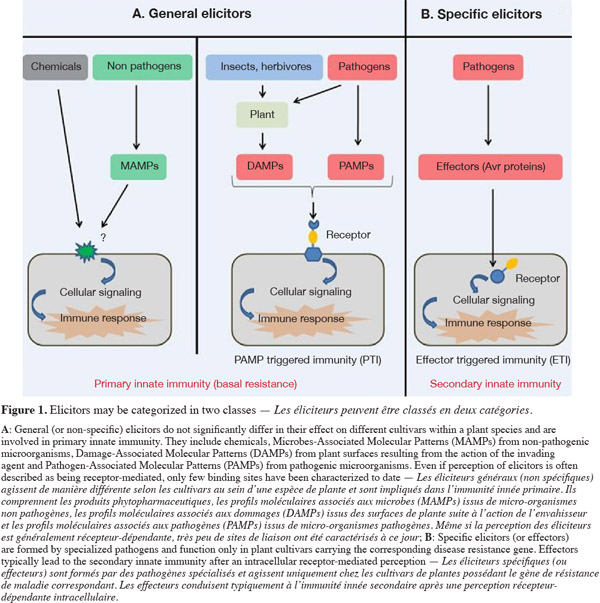
4In general, basal defense is considered to be less efficient than ETI in reducing plant disease. However, studies conducted on several plant-pathogen systems in the last decade have shown that basal defenses do actually play a significant role in pathogen restriction and disease resistance. Induction of primary innate immunity is now considered as a key component of biocontrol of pest in Integrated Pest Management. This will be further illustrated below with selected examples.
2. Systemic plant immunity
5When a resistance is established in the tissue surrounding the site of initial infection, it is called Localized Acquired Resistance (LAR) (Kombrink et al., 2001) (Figure 2A). However, via emission of molecular signals, defense mechanisms can also be induced in distal organs of a plant that is locally infected by a pathogen. Such systemic resistance reaction renders the host less susceptible to subsequent challenge by a pathogen or a parasite in distal tissues. This long-lasting phenomenon was termed systemic acquired resistance (Iriti et al., 2010) (Figure 2B) and has been extensively reviewed in the last years (Durrant et al., 2004). Recently, major advances have been made in identifying metabolites that are candidate systemic signals in plant defense against pathogens. Methyl salicylate, jasmonates, azelaic acid and a diterpenoid have been proposed as mobile signals involved in the activation of SAR which confers enhanced resistance against a broad spectrum of pathogens (Shah, 2009). Conceptually, SAR has been associated with the perception of elicitors from avirulent pathogens but a similar systemic defense may also be lighted on by DAMPs or by other compounds of biological but not microbial origins and by chemicals. Another form of induced resistance may also be triggered by molecular patterns isolated from beneficial non-pathogenic microorganisms (MAMPs), and is referred as induced systemic resistance (Mishra et al., 2009). Best characterized organisms inducing ISR are the so-called plant growth promoting rhizobacteria (PGPR) among which several species of Pseudomonas and Bacillus (Lugtenberg et al., 2009; Van Loon et al., 1998). ISR is also phenotypically similar to SAR and both are effective against a broad range of diseases caused by viruses, bacteria and fungi (Vallad et al., 2004) and therefore promising to control crop pests. Over the last 20 years, research on SAR and ISR has considerably improved our understanding of the molecular basis of systemic resistance. It appeared that, from a molecular point of view, ISR differs from SAR and it may explain why SAR is typically effective across a wide array of plant species, whereas there is some specificity in the ability of PGPR strains to elicit ISR in certain plant genotypes (Van Wees et al., 1997; Yan et al., 2002). Globally, local and systemic defense responses triggered by microorganisms are controlled by a signaling network in which the plant hormones salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) play important roles and the corresponding pathways crosscommunicate (Persello-Cartieaux et al., 2003). SAR triggered upon infection by necrosis-inducing pathogens is dependent on SA signaling (Park et al., 2008) while ISR triggered by beneficial rhizobacteria typically relies on the JA and ET signaling pathway (Pieterse et al., 2002). However, both SAR and ISR phenomena converge downstream since they are controlled by the same transcriptional regulator NPR1.
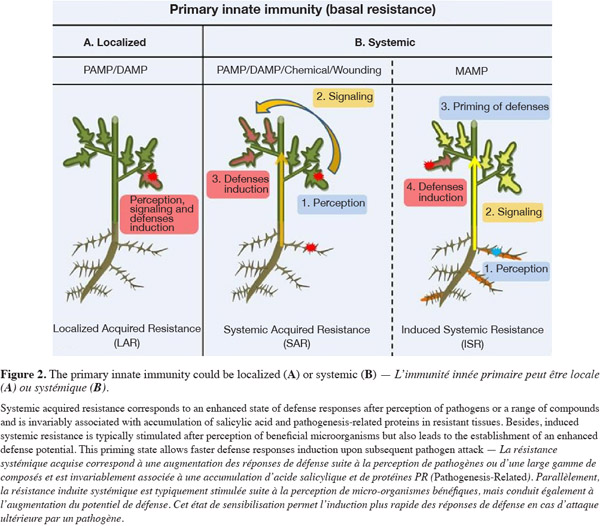
6The two main types of systemic resistance SAR and ISR can be both globally viewed as a three-step process involving sequentially:
7– the perception by plant cells of elicitors produced by the inducing agents that initiates the phenomenon,
8– signal transduction that is needed to propagate the induced state systemically through the plant,
9– expression of defense mechanisms sensu stricto that limit or inhibit further pathogen penetration into the host tissues.
10In this review, we focus on the early molecular dialogue and provide an overview of microbial elicitors of SAR, ISR but also LAR that are perceived by plants at the plasma membrane level. We will not consider those acting intracellularly as effectors of the ETI. Some excellent review papers are available to the reader for further updated information about these effectors (Sheen et al., 2007; Katagiri et al., 2010; Zhou et al., 2010).
3. The multiple PAMPs and their perception by plant cells
11PAMPs represent structures that are essential for microbial life and that are typically harbored by invading pathogens. These include cell surface constituents but may also be secreted enzymes or proteins normally located in the cytoplasm. A broad array of structurally diverse PAMPs has been described originating from fungal, oomycete and bacterial pathogens. Most of these PAMPs are oligosaccharides, glycopeptides, and peptides. Some of these patterns such as Pep-13, xylanase and cold-shock protein are only perceived by a narrow range of plant species belonging to only one plant family (Felix et al., 2003; Ron et al., 2004). A representative example is EF-Tu in the family Brassicaceae (Kunze et al., 2004). By contrast, other PAMPs such as chitin, LPS and flagellin trigger defense responses in many host species even if there is some degree of specificity and perception efficacy for a plant family or species as in the case of flagellin (Zipfel et al., 2006).
12PAMPs are perceived at the plant cell surface by high-affinity receptors typically consisting in an extracellular ligand-binding domain with leucine-rich repeats (LRR), a single transmembrane domain and an intracellular serine/threonine kinase-signaling domain. They are referred to as receptor-like kinases (RLK). Receptor-like proteins (RLPs) are similarly structured, but lack the cytoplasmic kinase domain. In Arabidopsis, 610 RLKs and 56 RLPs have been identified (Shiu et al., 2001; Fritz-Laylin et al., 2005). A large number of genes encoding RLKs and RLPs are transcriptionally induced upon PAMP treatment, illustrating the large diversity of such perception systems and suggesting their potential role in defense (Zipfel et al., 2004; Zipfel et al., 2006).
4. Indirect perception of pathogens via DAMPs
13In a more indirect way, plants can also detect the presence of pathogens through the perception of endogenous compounds that have been released from structural barriers or from other macromolecules by lytic enzymes produced by the invader or by the host itself. Such DAMPs typically appear in the apoplast and may thus, like PAMPs, play the role of signal for danger to induce innate immunity. For instance, oligogalacturonides are released by microbial enzymes and putatively recognized by the receptor WAK1 (D'Ovidio et al., 2004). Emission of these endogenous signals allows disrupted or injured cells to communicate their damage to the tissue or systemically to all organs. Systemin is formed in damaged tomato leaves and is further perceived as primary signal for systemic defense induction (Ryan et al., 2003). Similarly, the 23-residue peptide AtPep1 is released from precursor proteins in response to wounding and triggers an innate immune response in Arabidopsis via recognition by the PEPR1 receptor (Yamaguchi et al., 2006).
5. A panoply of MAMPs to render plants more resistant
5.1. Elicitors from beneficial rhizobacteria
14Compared to PAMPs from pathogens, less information are available on the determinants from non-pathogenic rhizobacteria that trigger ISR. Nevertheless, the characterization of compounds and/or sub-structures of rhizobacteria recognized by plant cells has considerably improved these last decades allowing a better understanding of the molecular talks occurring in this kind of interaction (De Vleesschauwer et al., 2009).
15It has been demonstrated that flagellin from the plant beneficial rhizobacterium Pseudomonas putida strain WCS358 can act as elicitor of systemic resistance in Arabidopsis against P. syringae (Meziane et al., 2005). However, additional experiments with other bacterial isolates and on multiple pathosystems are required to accurately evaluate to what extend flagellins may be considered as general determinants of the rhizobacteria-mediated ISR. Lipopolysaccharides (LPS) are cell surface components of Gram- bacteria associated with the outer membrane of the cell envelope. These compounds have also been occasionally reported as PAMPs. They are tripartite amphipathic molecules comprising a lipid A moiety which is embedded in the outer leaflet of the phospholipid/protein bilayer, a core oligosaccharide and a O-antigen side chain. This last part is immunologically dominant and can show considerable structural variation. Involvement of LPS in the elicitation of ISR by beneficial bacteria was reported in various pathosystems with P. fluorescens (Vanpeer et al., 1992; Leeman et al., 1996; Duijff et al., 1997; Tang et al., 2005) and P. putida strains (Meziane et al., 2005) but also with Burkholderia cepacia in the tobacco/Phytophthora nicotianeae pathosystem and Rhizobium elti G12 on cyst nematode-infected potato (Reitz et al., 2002). It was evidenced by testing purified LPS, heat-killed cells, crude cell envelope extracts or mutants with modified LPS. In many cases, mutants that lack the O-antigen side chain are not inducers, suggesting a crucial role of this sub-structure. Therefore the observed degree of specificity should be related to the composition of pseudomonad LPS that are almost strain-specific regarding the structure of the O-side chain and their eliciting activity seems to be dependent on the isolate studied.
16To ensure their growth in iron-limited environments, microorganisms have evolved powerful Fe3+-acquisition systems based on the excretion of high-affinity iron-chelating molecules termed siderophores (Loper et al., 1991). Pyoverdines are siderophores typically synthesized by fluorescent Pseudomonas (Budzikiewicz, 2004) and from experiments involving pyoverdin-non-producing mutants or addition of pure pyoverdines, these compounds were also demonstrated as potential ISR elicitors (Hofte et al., 2007; De Vleesschauwer et al., 2009). For instance, WCS358 can elicit ISR in several plants such as Arabidopsis, bean, tomato and Eucalyptus through its siderophore (Bakker et al., 2003; Meziane et al., 2005; Ran et al., 2005). SA is produced by some of the rhizobacteria that induce systemic resistance under iron-limited conditions. Its role in the ISR elicitation process was demonstrated in the case of Pseudomonas aeruginosa KMPCH (Demeyer et al., 1997; De Meyer et al., 1999). Nevertheless, several reports showed that SA production by other strains was not associated with ISR (Leeman et al., 1996; Press et al., 1997). SA is also an intermediate in the biosynthesis of other siderophores such as pyochelin in Pseudomonas aeruginosa (Serino et al., 1997) and a role for pyochelin was proposed in ISR triggered in tomato by P. aeruginosa 7NSK2 (Audenaert et al., 2002).
17In our laboratory, searching for molecular determinants of P. putida BTP1 responsible for ISR elicitation led to the isolation of an excreted compound consisting of a tri-N-alkylated benzylamine derivative (NABD) (Ongena et al., 2005). The elicitor properties were mainly established on the basis of treatment of bean roots with the pure compound NABD that mimicked the protective effect of the producing strain and by showing that a BTP1 derivative affected in NABD synthesis was also impaired in its efficacy to stimulate ISR.
18Some Pseudomonas products known for their antibiotic activities such as pyocyanine and 2,4-diacetylphloroglucinol (DAPG) may also act as elicitors of systemic resistance (Iavicoli et al., 2003; Siddiqui et al., 2003b). The phenazine-type molecule pyocyanine was proposed to act synergistically with pyochelin to trigger ISR in tomato treated with P. aeruginosa 7NSK2 (Audenaert et al., 2002). DAPG is another antibiotic produced by P. fluorescens CHA0 that also retains some ability to stimulate defense-related reactions in the host plant as it is an essential component of the ISR-mediated disease reduction by this strain in Arabidopsis and tomato plants infected respectively by Peronospora parasitica and the nematode Meloidogyne javanica (Iavicoli et al., 2003; Siddiqui et al., 2003a).
19Another class of compounds that recently emerged as ISR elicitors are biosurfactants such as rhamnolipids and lipopeptides. The potential of LPs as plant resistance inducers was demonstrated in 2007 for two different molecules synthesized by Pseudomonas and Bacillus. Tran et al. (2007) showed that massetolide A produced by Pseudomonas fluorescens retains ISR-eliciting activity in tomato plants for the control of Phytophthora infestans, the causal agent of late blight. Pure fengycins and surfactins from Bacillus subtilis provided a significant induced protective effect similar to the one induced by living cells of the producing strain. In a complementary approach, experiments conducted on bean and tomato showed that overexpression of both surfactin and fengycin biosynthetic genes in the naturally poor producer B. subtilis strain 168 was associated with a significant increase in the potential of the derivatives to induce resistance (Ongena et al., 2007). Until recently, volatile organic compounds and more particularly 2,3-butendiol were the sole determinants for elicitation identified from Bacillus spp. (Ryu et al., 2004).
20Some other molecules from beneficial rhizobacteria retain plant defense eliciting activity such as exopolysaccharides (Ipper et al., 2008) or quorum sensing signal molecules (N-acyl-L-homoserine lactone) (Schuhegger et al., 2006) again illustrating the variety in structure and nature of that kind of MAMPs.
5.2. Elicitors from beneficial fungi and from yeast
21MAMPs involved in systemic resistance triggered by beneficial fungi are not so well characterized compared to rhizobacteria. Djonovic et al. (2006) recently demonstrated that the hydrophobin-like elicitor Sm1 of the beneficial soil-borne fungus Trichoderma virens induces systemic resistance in maize. Maize plants grown with SM1-deletion strains or SM1-overexpressing strains displayed decreased or enhanced levels of systemic disease protection, respectively, demonstrating its role in triggering host defense. Peptaibols are linear peptide antibiotics produced by Trichoderma and other fungal genera. In the biocontrol agent and inducer of plant defense responses Trichoderma virens, enzymes forming peptaibols are encoded by tex1 and disruption of these genes led to a significantly reduced systemic resistance response in cucumber plants against the leaf pathogen Pseudomonas syringae pv. lachrymans as compared with plants grown in presence of the wild-type (Viterbo et al., 2007). Two synthetic 18-amino-acid peptaibol isoforms induce systemic protection when applied to cucumber seedlings suggesting that these peptides are critical in the chemical communication between Trichoderma and plants as triggers of defense responses. However, the peptaibol alamethicin induced a form of active cell death in Arabidopsis thaliana cell cultures and caused lesions in leaves of plants after a few days showing that these molecules may also retain some phytotoxicity on certain plant species (Rippa et al., 2010). It has also recently been demonstrated that some other secondary metabolites of plant beneficial Trichoderma spp. such as harzianolide and pentyl-pyranone may have a role in activation of plant defense responses (Vinale et al., 2008).
5.3. Still searching for receptors
22The molecular basis of defense activation following PAMPs, DAMPs, MAMPs and chemicals perception remain elusive but some aspects have just recently been disclosed (Conrath, 2011). It has been speculated that MAMPs of beneficial microbes and PAMPs from pathogens are recognized in a similar way, ultimately resulting in an enhanced defensive capacity of the plant. However there should be differences in the molecular talk since the host plant tolerates the non-pathogenic associated microbes while it tries to antagonize pathogen populations. Also, in plant – beneficial microbe interactions, MAMP-triggered immunity relies on priming for enhanced defense with almost no transcriptional re-programming and fitness cost prior to infection. This is contrasting with other forms of systemic resistance involving direct activation of the defense arsenal. So, mechanistically, plant perception of MAMPs should retain some specificity. Intriguingly, no specific proteinaceous binding sites have been identified for MAMPs perception while a few plasma membrane receptors for PAMPs have been characterized (Gressent et al., 1999; Fliegmann et al., 2004; Kunze et al., 2004; Ron et al., 2004; Chinchilla et al., 2006; Kaku et al., 2006; Lee et al., 2009).
23Recognition of different parts in the lipopolysaccharide structure may allow plant cells to discriminate symbiotic and infectious Gram- bacteria and this strongly suggests that a somewhat specialized perception system is involved at the plant cell wall level. However, this has yet to be demonstrated.
24The strain-specific effect of pyoverdins in ISR may be explained as far as the peptide chain is involved in the perception process by plant cells because of the structural differences between naturally occuring pyoverdines. Actually, there is no partial sequence shared by three active pyoverdins from WCS358, WCS374 and CHA0. Testing a wider range of heterogeneous pyoverdins on the same plant is required to evaluate whether some amino acid sequences may represent epitopes perceived by specific receptors in the membrane of root cells. An alternative to direct recognition of pyoverdins by the plant is the indirect perception of rhizobacterially induced alterations in the plant’s immediate environment i.e. the rhizosphere. Given the scarcity of bioavailable iron and the high affinity of pyoverdin for the ferric ion, pyoverdin-producing rhizobacteria are thought to interfere with the iron acquisition by other soil organisms, including the host plant (Vansuyt et al., 2007). A model implying pyoverdin-induced iron stress on the roots as a primary event in the activation of rhizobacteria mediated resistance has been proposed (De Vleesschauwer et al., 2009).
25Structural similarities are neither obvious in other bacterial products identified so far as ISR determinants like NABD, SA, DAPG, pyocyanin or volatile 2,3-butanediol. Results obtained by comparing the activity of pure benzylamine with that of NABD in ISR assays with bean and cucumber suggest that the aromatic amino part of the molecule is important for its biological activity (Ongena et al., 2008b). SA and DAPG also contain an aromatic phenolic group and thus such phenyl-derived moieties could constitute a general motif widely recognized by specific plant cell receptors. Additional experiments are required to appreciate the relative importance of such structural traits by testing multiple naturally co-produced or chemically synthesized derivatives.
26Lipopeptides may be sensitized by plant tissues via a less specific mechanism than high-affinity proteic receptor. Due to their amphiphilic nature and their putative surfactant properties, these molecules readily insert into phospholipid bilayer thereby creating some disturbance or channeling in the plasma membrane. This may, in turn, activate a cascade of molecular events leading to defensive responses. It is noteworthy that such membrane perturbation should remain limited since the pure compounds had no toxic effect on plant health at the concentration used (Jourdan et al., 2009).
6. Non-microbial elicitors
27As stated above, some compounds that are not from microbial origin have also been reported as efficient plant defense inducers. Biotic elicitors were isolated from algae or shrimp and crab walls. The linear hepta-β-glucoside laminarin elicitor from the brown alga Laminaria digitata elicits defense responses in various plants and a binding site has been identified in membranes of the model legumes Medicago truncatula and Lotus japonicas as well as in membrane fractions from soybean (Fliegmann et al., 2004; Klarzynski et al., 2000). Apparent Kd values range from 5 to 200 nM and elicitor effects observed in tobacco are specific to linear β-1,3 linkages, with laminaripentaose being the smallest elicitor-active structure. But contrary to branched glucans, no receptor has been yet isolated. Chitosan is a deacylated derivative of chitin usually prepared from crab shells and serves as a molecular pattern which stimulates the innate immune systems of plants (Nurnberger et al., 2004). Although a plasma membrane receptor for chitin fragments has been characterized (Iriti et al., 2010), the signal transduction pathway activated by chitosan is not well defined.
28Besides pathogenic microbes, there are certain chemicals which upon application to plants mimic the host-pathogen interaction leading to SAR (Gullino et al., 2000; Oostendorp et al., 2001). Natural signaling molecules like SA, JA and systemin are components of the biological induction and are able to induce a systemic protection (Cohen et al., 1993; Holley et al., 2003; Mayers et al., 2005). Chemical elicitors like DL-β-aminobutyric acid (BABA) (Hong et al., 1999), oxalic acid (Mucharromah et al., 1991), 2,6-dichloro isonicotinic acid (INA) and its derivatives (Qian et al., 2006), benzo[1,2,3]thiadiazole (BTH) (Kunz et al., 1997) and derivatives S-methyl benzo[1,2,3]thiadiazole-7-carbothiate (acibenzolar-S-methyl) (Cools et al., 2002) have also been shown to be effective elicitors for inducing the biosynthesis of plant secondary metabolites. However, plants exposed to high concentrations of BTH or INA may also exhibit signs of phytotoxicity, but this effect seems to be independent of the induced resistance response (Louws et al., 2001).
7. Practical applications of plant immunity triggering compounds
29Recent progresses in our understanding of principles of plant systemic immunity has been the driving force to set up field and greenhouse crop protection experiments based on these phenomena. Based on the promising results obtained with beneficial ISR-inducing microorganisms, the development of microbial formulations was promoted for application in conventional agriculture. Rhizobacterial- or fungal-mediated ISR does not confer a total protection against pathogen infection but as the phenomenon is long-lasting (Van Loon et al., 1998), effective against a broad range of diseases and in multiple plant species (see below) and not conducive for development of pathogen resistance (multiplicity and variety of induced defense strategies, see below), ISR-based biocontrol strategies are promising and some trials were successfully performed under field conditions (Wei et al., 1996; Zehnder et al., 2001). Also, some chemicals are strong inducers of a SAR-like response. Compounds such as 2,6-dichloro isonicotinic acid, benzothiadiazole and its derivative acibenzolar-S-methyl, or β-amino butyric acid, are nowadays successfully employed to control diseases of various crop plants (Vallad et al., 2004).
30On another hand, the continuous discovery of new PAMPs and MAMPs contributes to enlarge our reservoir of very efficient structural patterns for boosting plant immunity. The most active of these compounds may be produced biotechnologically and purified to the required level for commercialization. Alternatively they may serve as molecular basis for the development of new structural derivatives with higher activity and/or lower susceptibility to degradation and/or lower lateral toxicity. Even if neither SAR nor ISR will become a stand-alone method for pest control, it is now clear that they will be further integrated into pest management systems.
31Interfering with the molecular dialogue between PAMPs and their cognate plasma membrane, sensing systems may also be the basis of novel strategies to engineer durable plant disease resistance. For instance, enhancement of the potential of plant to recognize a broader range of PAMPs and therefore resist to a broader range of pathogens has been successfully achieved via heterologuous expression or overexpression of PRR/LRR-RK receptors in some plants (Gust et al., 2007). Another approach to improve disease resistance is overexpression of antibodies fused with antimicrobial peptides and that recognize specific pathogen surface components (Li et al., 2008). Enhancing the expression of key regulators of systemic resistance such as NPR1 which controls immunity-associated genes is also an alternative strategy to boost the defense reaction in its entirety (Makandar et al., 2006).
8. Conclusion
32Considering the large and still increasing number of molecular patterns and effectors harbored by pathogens, it is clear that plants have evolved to mount very efficient non-self recognition systems. As they also develop performing and specific receptors to detect endogenous DAMPs, the concept of “stranger” recognition could be extended to “danger” recognition. The perception of all these signals appears to trigger the same stereotypical defense program even if it varies in terms of kinetic and strength of the response between PTI and ETI (Boller et al., 2009).
33Elicitors of SAR and ISR could potentially revolutionize pest management in conventional agriculture. A lot of new molecules acting as PAMPs and MAMPs will most probably be discovered in the coming years but we are still far from a detailed understanding of the fascinating mechanisms by which non-pathogenic microorganisms induce resistance in plants. In support to the complexity of this interaction is the plethora of structurally distinct elicitors active at triggering plant defense responses. Such diversity is reminiscent of the large variety of pathogen-derived elicitors with immune-stimulating activity (Schreiber et al., 2008). Some high-affinity proteic receptor may be involved in the recognition of those ISR elicitors active at concentrations in the pico- to nanomolar range without any dose-response relationship (flagellin, SA, pyocyanin, DAPG). However, the perception of those compounds acting at micromolar concentrations or even more should rely on another less specific mechanism based either on low-affinity receptor or on some interaction with other cell membrane components such as lipid bilayer as was suggested for lipopeptides (Ongena et al., 2008a). It is becoming clearer that the lipid fraction of eukaryotic plasma membranes may act as very efficient sensor of various abiotic and biotic external signals. It may thus represent an alternative mode of microbe sensing and intimately cooperate together with specialized proteic receptors to optimize non-self recognition by plants.
34Abbreviations
35BABA: DL-β-aminobutyric acid
36BTH: Benzo[1,2,3]thiadiazole
37DAMP: Damage-Associated Molecular Pattern
38DAPG: 2,4-diacetylphloroglucinol
39ET: Ethylene
40ETI: Effector-Triggered Immunity
41ISR: Induced Systemic Resistance
42JA: Jasmonic Acid
43LAR: Localized Acquired Resistance
44LPS: Lipopolysaccharides
45LRR: Leucine-Rich Repeats
46MAMP: Microbe-Associated Molecular Pattern
47PAMP: Pathogen-Associated Molecular Pattern
48PGPR: Plant Growth Promoting Rhizobacteria
49PTI: PAMP-Triggered Immunity
50RLK: Receptor-Like Kinase
51RLP: Receptor-Like Protein
52SA: Salicylic Acid
53SAR: Systemic Acquired Resistance
54Acknowledgements
55Guillaume Henry is beneficial of a grant from the Formation à la Recherche dans l’Industrie et l’Agriculture (F.R.I.A.) and Marc Ongena is research associate at the Fonds National de la Recherche Scientifique (F.R.S-FNRS).
Bibliographie
Audenaert K., Pattery T., Cornelis P. & Hofte M., 2002. Induction of systemic resistance to Botrytis cinerea in tomato by Pseudomonas aeruginosa 7NSK2: role of salicylic acid, pyochelin, and pyocyanin. Mol. Plant-Microbe Interact., 15, 1147-1156.
Ausubel F.M., 2005. Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol., 6, 973-979.
Bakker P., Ran L.X., Pieterse C.M.J. & Van Loon L.C., 2003. Understanding the involvement of rhizobacteria-mediated induction of systemic resistance in biocontrol of plant diseases. Can. J. Plant Pathol.-Rev. Can. Phytopathol., 25, 5-9.
Boller T. & Felix G., 2009. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol., 60, 379-406.
Budzikiewicz H., 2004. Bacterial catecholate siderophores. Mini-Rev. Org. Chem., 1, 163-168.
Chinchilla D. et al., 2006. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell, 18, 465-476.
Cohen Y., Gisi U. & Niderman T., 1993. Local and systemic protection against Phytophthora infestans induced in potato and tomato plants by jasmonic acid and jasmonic methyl-ester. Phytopathology, 83, 1054-1062.
Conrath U., 2011. Molecular aspects of defence priming. Trends Plant Sci., 16, 524-531.
Cools H.J. & Ishii H., 2002. Pre-treatment of cucumber plants with acibenzolar-S-methyl systemically primes a phenylalanine ammonia lyase gene (PAL1) for enhanced expression upon attack with a pathogenic fungus. Physiol. Mol. Plant Pathol., 61, 273-280.
Dangl J.L. & Jones J.D.G., 2001. Plant pathogens and integrated defence responses to infection. Nature, 411, 826-833.
De Meyer G. et al., 1999. Nanogram amounts of salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 activate the systemic acquired resistance pathway in bean. Mol. Plant-Microbe Interact., 12, 450-458.
Demeyer G. & Hofte M., 1997. Salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 induces resistance to leaf infection by Botrytis cinerea on bean. Phytopathology, 87, 588-593.
De Vleesschauwer D. & Hofte M., 2009. Rhizobacteria-Induced Systemic Resistance. Adv. Bot. Res., 51, 223-281.
Djonovic S. et al., 2006. Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol. Plant-Microbe Interact., 19, 838-853.
D'Ovidio R., Mattei B., Roberti S. & Bellincampi D., 2004. Polygalacturonases, polygalacturonase-inhibiting proteins and pectic oligomers in plant-pathogen interactions. Biochim. Biophys. Acta Proteins Proteomics, 1696, 237-244.
Duijff B.J., Gianinazzi-Pearson V. & Lemanceau P., 1997. Involvement of the outer membrane lipopolysaccharides in the endophytic colonization of tomato roots by biocontrol Pseudomonas fluorescens strain WCS417r. New Phytol., 135, 325-334.
Durrant W.E. & Dong X., 2004. Systemic acquired resistance. Annu. Rev. Phytopathol., 42, 185-209.
Felix G. & Boller T., 2003. Molecular sensing of bacteria in plants. The highly conserved RNA-binding motif RNP-1 of bacterial cold shock proteins is recognized as an elicitor signal in tobacco. J. Biol. Chem., 278, 6201-6208.
Fliegmann J., Mithofer A., Wanner G. & Ebel J., 2004. An ancient enzyme domain hidden in the putative beta-glucan elicitor receptor of soybean may play an active part in the perception of pathogen-associated molecular patterns during broad host resistance. J. Biol. Chem., 279, 1132-1140.
Fritz-Laylin L.K. et al., 2005. Phylogenomic analysis of the receptor-like proteins of rice and Arabidopsis. Plant Physiol., 138, 611-623.
Gressent F. et al., 1999. Ligand specificity of a high-affinity binding site for lipo-chitooligosaccharidic Nod factors in Medicago cell suspension cultures. Proc. Natl. Acad. Sci U.S.A., 96, 4704-4709.
Gullino M.L., Leroux P. & Smith C.M., 2000. Uses and challenges of novel compounds for plant disease control. Crop Prot., 19, 1-11.
Gust A.A. et al., 2007. Bacteria-derived peptidoglycans constitute pathogen-associated molecular patterns triggering innate immunity in Arabidopsis. J. Biol. Chem., 282, 32338-32348.
Hofte M. & Bakker P.A.H.M., 2007. Competition for iron and induced systemic resistance by siderophores of plant growth promoting rhizobacteria. Soil Biol., 12, 121-133.
Holley S.R. et al., 2003. Convergence of signaling pathways induced by systemin, oligosaccharide elicitors, and ultraviolet-B radiation at the level of mitogen-activated protein kinases in Lycopersicon peruvianum suspension-cultured cells. Plant Physiol., 132, 1728-1738.
Hong J.K., Hwang B.K. & Kim C.H., 1999. Induction of local and systemic resistance to Colletotrichum coccodes in pepper plants by DL-beta-amino-n-butyric acid. J. Phytopathol., 147, 193-198.
Iavicoli A., Boutet E., Buchala A. & Metraux J.P., 2003. Induced systemic resistance in Arabidopsis thaliana in response to root inoculation with Pseudomonas fluorescens CHA0. Mol. Plant-Microbe Interact., 16, 851-858.
Ipper N.S. et al., 2008. Antiviral activity of the exopolysaccharide produced by Serratia sp. strain Gsm01 against cucumber mosaic virus. J. Microbiol. Biotechnol., 18, 67-73.
Iriti M. et al., 2010. Chitosan-induced ethylene-independent resistance does not reduce crop yield in bean. Biol. Control, 54, 241-247.
Jones J.D.G. & Dangl J.L., 2006. The plant immune system. Nature, 444, 323-329.
Jourdan E. et al., 2009. Insights into the defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Mol. Plant Microbe Interact., 22, 456-468.
Kaku H. et al., 2006. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc. Natl. Acad. Sci. U.S.A., 103, 11086-11091.
Katagiri F. & Tsuda K., 2010. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol., 13, 459-465.
Klarzynski O. et al., 2000. Linear beta-1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol., 124, 1027-1037.
Kombrink E. & Schmelzer E., 2001. The hypersensitive response and its role in local and systemic disease resistance. Eur. J. Plant Pathol., 107, 69-78.
Kunz W., Schurter R. & Maetzke T., 1997. The chemistry of benzothiadiazole plant activators. Pestic. Sci., 50, 275-282.
Kunze G. et al., 2004. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell, 16, 3496-3507.
Lee S.W. et al., 2009. A type I-secreted, sulfated peptide triggers XA21-mediated innate immunity. Science, 326, 850-853.
Leeman M. et al., 1996. Iron availability affects induction of systemic resistance to Fusarium wilt of radish by Pseudomonas fluorescens. Phytopathology, 86, 149-155.
Li H.P. et al., 2008. Engineering Fusarium head blight resistance in wheat by expression of a fusion protein containing a Fusarium-specific antibody and an antifungal peptide. Mol. Plant-Microbe Interact., 21, 1242-1248.
Loper J.E. & Buyer J.S., 1991. Siderophores in microbial interactions on plant surfaces. Mol. Plant-Microbe Interact., 4, 5-13.
Louws F.J. et al., 2001. Field control of bacterial spot and bacterial speck of tomato using a plant activator. Plant Dis., 85, 481-488.
Lugtenberg B. & Kamilova F., 2009. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol., 63, 541-556.
Makandar R. et al., 2006. Genetically engineered resistance to Fusarium head blight in wheat by expression of Arabidopsis NPR1. Mol. Plant-Microbe Interact., 19, 123-129.
Mayers C.N. et al., 2005. Salicylic acid-induced resistance to cucumber mosaic virus in squash and Arabidopsis thaliana: contrasting mechanisms of induction and antiviral action. Mol. Plant-Microbe Interact., 18, 428-434.
Meziane H. et al., 2005. Determinants of Pseudomonas putida WCS358 involved in inducing systemic resistance in plants. Mol. Plant Pathol., 6, 177-185.
Mishra A.K., Sharma K. & Misra R.S., 2009. Purification and characterization of elicitor protein from Phytophthora colocasiae and basic resistance in Colocasia esculenta. Microbiol. Res., 164, 688-693.
Montesano M., Brader G. & Palva E.T., 2003. Pathogen derived elicitors: searching for receptors in plants. Mol. Plant Pathol., 4, 73-79.
Mucharromah E. & Kuc J., 1991. Oxalate and phosphates induce systemic resistance against diseases caused by fungi, bacteria and viruses in cucumber. Crop Prot., 10, 265-270.
Nicaise V., Roux M. & Zipfel C., 2009. Recent advances in PAMP-Triggered Immunity against bacteria: pattern recognition receptors watch over and raise the alarm. Plant Physiol., 150, 1638-1647.
Nurnberger T., Brunner F., Kemmerling B. & Piater L., 2004. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol. Rev., 198, 249-266.
Ongena M. et al., 2005. Isolation of an n-alkylated benzylamine derivative from Pseudomonas putida BTP1 as elicitor of induced systemic resistance in bean. Mol. Plant-Microbe Interact., 18, 562-569.
Ongena M. et al., 2007. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol., 9, 1084-1090.
Ongena M. & Jacques P., 2008a. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol., 16, 115-125.
Ongena M. et al., 2008b. Amino acids, iron, and growth rate as key factors influencing production of the Pseudomonas putida BTP1 benzylamine derivative involved in systemic resistance induction in different plants. Microb. Ecol., 55, 280-292.
Oostendorp M., Kunz W., Dietrich B. & Staub T., 2001. Induced disease resistance in plants by chemicals. Eur. J. Plant Pathol., 107, 19-28.
Park S.W., Vlot A.C. & Klessig D.F., 2008. Systemic acquired resistance: the elusive signal(s). Curr. Opin. Plant Biol., 11, 436-442.
Pelletier I. et al., 2002. Study by infrared spectroscopy of ultrathin films of behenic acid methyl ester on solid substrates and at the air/water interface. J. Phys. Chem. B., 106, 1968-1976.
Persello-Cartieaux F., Nussaume L. & Robaglia C., 2003. Tales from the underground: molecular plant-rhizobacteria interactions. Plant Cell Environ., 26, 189-199.
Pieterse C.M.J. et al., 2002. Signalling in rhizobacteria-induced systemic resistance in Arabidopsis thaliana. Plant Biol., 4, 535-544.
Press C.M., Wilson M., Tuzun S. & Kloepper J.W., 1997. Salicylic acid produced by Serratia marcescens 90-166 is not the primary determinant of induced systemic resistance in cucumber or tobacco. Mol. Plant-Microbe Interact., 10, 761-768.
Qian Z.G. et al., 2006. Novel synthetic 2,6-dichloroisonicotinate derivatives as effective elicitors for inducing the biosynthesis of plant secondary metabolites. Appl. Microbiol. Biotechnol., 71, 164-167.
Ran L.X. et al., 2005. Induction of systemic resistance against bacterial wilt in Eucalyptus urophylla by fluorescent Pseudomonas spp. Eur. J. Plant Pathol., 113, 59-70.
Reitz M. et al., 2002. Importance of the O-antigen, core-region and lipid A of rhizobial lipopolysaccharides for the induction of systemic resistance in potato to Globodera pallida. Nematology, 4, 73-79.
Rippa S. et al., 2010. Hypersensitive-like response to the pore-former peptaibol alamethicin in Arabidopsis thaliana. ChemBioChem, 11, 2042-2049.
Ron M. & Avni A., 2004. The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell, 16, 1604-1615.
Ryan C.A. & Pearce G., 2003. Systemins: a functionally defined family of peptide signal that regulate defensive genes in Solanaceae species. Proc. Natl. Acad. Sci. U. S. A., 100, 14577-14580.
Ryu C.M. et al., 2004. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol., 134, 1017-1026.
Schreiber K. & Desveaux D., 2008. Message in a bottle: chemical biology of induced disease resistance in plants. Plant Pathol. J., 24, 245-268.
Schuhegger R. et al., 2006. Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ., 29, 909-918.
Serino L. et al., 1997. Biosynthesis of pyochelin and dihydroaeruginoic acid requires the iron-regulated pchDCBA operon in Pseudomonas aeruginosa. J. Bacteriol., 179, 248-257.
Shah J., 2009. Plants under attack: systemic signals in defence. Curr. Opin. Plant Biol., 12, 459-464.
Sheen J., He P. & Shan L., 2007. Elicitation and suppression of microbe-associated molecular pattern-triggered immunity in plant-microbe interactions. Cell Microbiol., 9, 1385-1396.
Shiu S.H. & Bleecker A.B., 2001. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. U. S. A., 98, 10763-10768.
Siddiqui I.A., Shaukat S.S., Khan G.H. & Ali N.I., 2003a. Suppression of Meloidogyne javanica by Pseudomonas aeruginosa IE-6S(+) in tomato: the influence of NaCl, oxygen and iron levels. Soil Biol. Biochem., 35, 1625-1634.
Siddiqui M.A. & Shaukat S.S., 2003b. Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato: importance of bacterial secondary metabolite, 2,4-diacetylpholoroglucinol. Soil Biol. Biochem., 35, 1615-1623.
Tang X.R., Marciano D.L., Leeman S.E. & Amar S., 2005. LPS induces the interaction of a transcription factor, LPS-induced TNF-alpha factor, and STAT6(B) with effects on multiple cytokines. Proc. Natl. Acad. Sci. U.S.A., 102, 5132-5137.
Tran H. et al., 2007. Role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens. New Phytol., 175, 731-742.
Vallad G.E. & Goodman R.M., 2004. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci., 44, 1920-1934.
Van Loon L.C., Bakker P.A.H.M. & Pieterse C.M.J., 1998. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol., 36, 453-483.
Vanpeer R. & Schippers B., 1992. Lipopolysaccharides of plant-growth-promoting Pseudomonas sp. strain WCS417R induce resistance in carnation to Fusarium-wilt. Neth. J. Plant Pathol., 98, 129-139.
Vansuyt G. et al., 2007. Iron acquisition from Fe-pyoverdine by Arabidopsis thaliana. Mol. Plant-Microbe Interact., 20, 441-447.
Van Wees S.C.M. et al., 1997. Differential induction of systemic resistance in Arabidopsis by biocontrol bacteria. Mol. Plant-Microbe Interact., 10, 716-724.
Vinale F. et al., 2008. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol., 72, 80-86.
Viterbo A. et al., 2007. The 18mer peptaibols from Trichoderma virens elicit plant defence responses. Mol. Plant Pathol., 8, 737-746.
Wei G., Kloepper J.W. & Tuzun S., 1996. Induced systemic resistance to cucumber diseases and increased plant growth by plant growth-promoting rhizobacteria under field conditions. Phytopathology, 86, 221-224.
Yamaguchi Y., Pearce G. & Ryan C.A., 2006. The cell surface leucine-rich repeat receptor for AtPep1, an endoaenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc. Natl. Acad. Sci. U.S.A., 103, 10104-10109.
Yan Z.N. et al., 2002. Induced systemic protection against tomato late blight elicited by plant growth-promoting rhizobacteria. Phytopathology, 92, 1329-1333.
Zehnder G.W., Murphy J.F., Sikora E.J. & Kloepper J.W., 2001. Application of rhizobacteria for induced resistance. Eur. J. Plant Pathol., 107, 39-50.
Zhou J.M. et al., 2010. Effector-triggered and pathogen-associated molecular pattern-triggered immunity differentially contribute to basal resistance to Pseudomonas syringae. Mol. Plant-Microbe Interact., 23, 940-948.
Zipfel C. et al., 2004. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature, 428, 764-767.
Zipfel C. et al., 2006. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell, 125, 749-760.
Om dit artikel te citeren:
Over : Guillaume Henry
Univ. Liege - Gembloux Agro-Bio Tech. Walloon Center for Industrial Biology. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : Philippe Thonart
Univ. Liege - Gembloux Agro-Bio Tech. Walloon Center for Industrial Biology. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : Marc Ongena
Univ. Liege - Gembloux Agro-Bio Tech. Walloon Center for Industrial Biology. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: Marc.Ongena@ulg.ac.be






