- Accueil
- Volume 15 (2011)
- numéro 3
- Predictive modelling of the combined effect of temperature and water activity on the in vitro growth of Erwinia spp. infecting potato tubers in Belgium
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Predictive modelling of the combined effect of temperature and water activity on the in vitro growth of Erwinia spp. infecting potato tubers in Belgium

Notes de la rédaction
Received on January 18, 2010; accepted on October 5, 2010
Résumé
Modélisation prédictive de l'effet combiné de la température et de l'activité de l'eau sur la croissance in vitro d'Erwinia spp. infectant les tubercules de pomme de terre en Belgique. Erwinia carotovora subsp. atroseptica (Eca), Erwinia carotovora subsp. carotovora (Ecc) et Erwinia chrysanthemi (Ech) sont responsables de la pourriture molle des tubercules de pomme de terre en stockage et de la jambe noire du plant de la pomme de terre au champ. Ces trois bactéries sont caractérisées par leur capacité à produire de larges variétés d'enzymes pectiques extra-cellulaires parmi lesquelles les Pectate Lyases (PEL) sont les plus importantes dans le développement de la maladie. Les paramètres écologiques tels que l'humidité et la température influencent énormément le développement de la maladie. L'objectif de ce travail est de déterminer in vitro l'effet combiné de l'activité de l'eau (0,960 ; 0,980 et 0,997) et de la température (10, 15 et 20 °C) sur le taux de croissance spécifique maximum (µmax) d'Eca, d'Ecc et d'Ech par l'utilisation de la densité optique (DO). Le taux de croissance spécifique maximum (µmax) a été calculé pour chaque combinaison aw-température pour les trois sous-espèces d'Erwinia. Les analyses statistiques ont montré un effet significatif de l'aw et de la température sur µmax. Nous avons observé que la croissance d'Eca et d'Ecc était plus rapide que celle d'Ech. Le deuxième objectif de ce travail était de suivre l'activité spécifique des PEL sous l'effet combiné de ces deux facteurs (aw-température). Nos résultats ont montré une augmentation de l'activité spécifique des PEL avec la température, quelles que soient les souches bactériennes. Mais contrairement à la croissance, ce travail n'a pas montré une augmentation de l'activité spécifique des PEL avec l'aw, excepté les traitements à 15 et 20 °C pour toutes les souches bactériennes. Selon les résultats obtenus sur la croissance et la production des PEL, nous avons conclu qu'Eca 03034/1 et Ecc 030033 ont le même comportement écologique comparé à Ech 03/016/1 dans la gamme des valeurs des deux facteurs (aw et la température) étudiés ici. À notre connaissance, ce travail est la première publication sur l'effet combiné de la température et de l'aw sur la croissance in vitro du genre Erwinia.
Abstract
Erwinia carotovora ssp. atroseptica (Eca), Erwinia carotovora ssp. carotovora (Ecc) and Erwinia chrysanthemi (Ech), are the main cause of potato tuber decay (soft rot) in storage and stem rot in the field (blackleg). The bacteria are characterized by the production of several extracellular pectic enzymes among them Pectate Lyase (PEL) activity is the most important key of pathogenesis. It has been reported that ecological parameters such as humidity and temperature, greatly influence the disease development. The objective of this work was to determine the in vitro effect of water activity (0.960, 0.980, 0.997) and temperature (10, 15 and 20°C) and their interactions on the growth parameters of Eca, Ecc and Ech using optical density (OD) measurement. The maximum specific growth rate (µmax) was calculated under each aw-temperature combinations for the three Erwinia species. Statistical analysis showed a significant effect of aw and temperature on µmax. We noticed that Eca and Ecc grow faster than Ech in our condition. A second aim of this work was to monitor the PEL specific activity under the combined effect of these two factors (aw-temperature). Our results showed an increase of PEL specific activity with the temperature whatever are the bacterial strains. But contrary to growth, this research did not show an increase of PEL specific activity with aw except the treatment at 15 and 20°C for all bacteria strains. According to our obtained results on growth and PEL production we concluded that Eca 03034/1 and Ecc 030033 had the same ecological behavior comparatively to Ech 03/016/1 in the range of the values of the two factors (aw and temperature) investigated here. To our knowledge, this research is the first publication which pointed out the combined in vitro effect of aw and temperature on the growth of Erwinia genius according to literature data.
Table des matières
1. Introduction
1Erwinia carotovora ssp. atroseptica (Eca), Erwinia carotovora ssp. carotovora (Ecc) and Erwinia chrysanthemi (Ech) belong to the soft rot Erwinia commonly associated with potato crops (Pérombelon et al., 1980). They cause soft rot in tubers in storage and, more or less frequently, a basal stem rot known as blackleg in the field (Pérombelon et al., 1987).
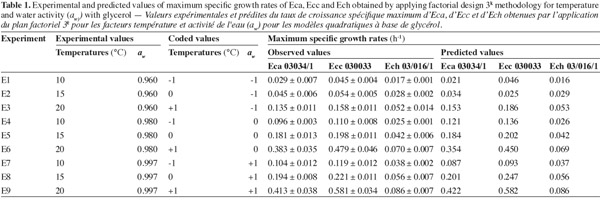
2These bacteria are Gram-, coliforms, non-spore-forming, facultative anaerobes, characterized by their capacity to produce a large variety of extracellular pectic enzymes such as pectate lyase (PEL), pectin lyase (PNL), pectin methyl esterase (PME) and polygalacturonase (PEH) (Toth et al., 2003). Among these enzymes, PEL are the most important in soft rot development (Smadja et al., 2004).
3Eca is mainly restricted to temperate regions, whereas Ecc is distributed in temperate and tropical zones worldwide and Ech is found in subtropical as well as in warm temperate regions. All Erwinia strains caused an economically important disease for which there is no chemical treatment (Toth et al., 2003).
4Two environmental factors such as temperature and free water play an essential role in disease development (Bacterial multiplication and PEL production) (Mildenhall et al., 1983; Lanham et al., 1991; Smadja et al., 2004). The role of free water with the developpement of potato soft rot suggests that the three bacteria (Eca, Ecc and Ech) were sensitive to dessication, and led us to check their water relation. The effects of temperature and water activity (aw) on soft rot Erwinia growth and enzyme production have been studied (Mildenhall et al., 1981; Hugouvieux-Cotte-Pattat et al., 1992; Smadja et al., 2004). Predictive modelling is a promising field of food microbiology. It has been extensively used mainly to evaluate how individual or combined environmental factors affect bacterial growth in foods (Membré et al., 2004; Braun et al., 2005; Juneja et al., 2009). To our knowledge, there is no available information on modelling the combined effect of temperature and aw on the growth of these plant pathogenic bacteria (Eca, Ecc and Ech). In this context, the goal of this work was to determine in vitro the effect of both parameters on the growth and the extracellular PEL specific activity of Eca, Ecc and Ech in order to integrate better environmental factors into the management of the risk of soft rot disease development.
2. Materials and methods
2.1. Bacterial strains
5The strains used in this study (Eca 03034/1, Ecc 030033 and Ech 03/016/1) were previously isolated and characterized by the Walloon Agricultural Research Centre (CRA-W) of Libramont (Belgium) and were stored frozen at -80°C in glycerol 25 % (v/v) for long-term storage. Strains were always replicated at least twice on solid ceria medium (Nachin et al., 2000) with the composition modified (15 g Agar, KH2PO4 8.5 g·l-1, (NH4)2SO4 1 g·l-1, MgSO4 0.1 g·l-1, MnSO4 10-6 M, FeSO4 10-6 M) and supplemented with polygalacturonic acid 4 g·l-1 (Potassium salt, Sigma P0853). The final pH of the medium was adjusted at 8.0 by addition of KOH before use.
2.2. Growth media
6Bacteria were grown in liquid ceria medium modified. To determine the influence of aw, glycerol was added to reach aw levels of 0.960 (120 ml·l-1), 0.980 (42 ml·l-1) and 0.997 (0 ml·l-1). Final aw values were always checked with AquaLab Model CX 3 (Decagon Devices Inc., USA, Pullman Washington).
2.3. Inoculation and incubation temperatures
7Batch cultures were grown in Erlenmeyer flasks (100 ml) at 10, 15, or 20°C with agitation 120 rpm in the incubator (New Brunswick, USA, New Jersey). Bacterial concentration was adjusted by optical density at 600 nm to reach a concentration of 106 cfu·ml-1 in the media. Three independent replicates were made for each object with Prim light spectrophotometer 230 V (Secomam, France).
2.4. Monitoring of bacterial growth
8The evolution of bacterial population was monitored in triplicate 5 times a day for each object by optical density (OD) measurements at wavelength of 600 nm. A correspondence between OD measurements and cfu·ml-1 was established with a calibration curve according to the formula cfu·ml-1 = 2*108*OD600 - 107 for Eca, Ecc and Ech.
2.5. Monitoring of Pectate Lyase (PEL) specific activity
9To monitor PEL specific activity (total PEL production) 1.5 ml of culture supernatant were collected during early stationary phase (~108 cfu·ml-1) of growth by centrifugated at 12,000 rpm at 4°C during 10 min. The culture supernatant was sterilized by filtration through a 0.2 µm pore size of Acrodisc Syringe Filters with Supor Membrane Sterile (Pall Corporation Newquay Cornwall, UK). PEL activity was further evaluated as described previously by Smadja et al. (2004) by monitoring the increase of C4-C5 unsaturated products spectrophotometrically at 235 nm. Briefly, this method is as follows: 500 µl of a 0.1 mol·l-1 Tris–HCl (pH 9.0) buffer containing 0.5 mmol·l-1 CaCl2 was quickly added in a 1.5 ml cuvette with 370 µl of distilled water, 100 µl of a polygalacturonic acid solution 10% (w/v), and 30 µl of filtred supernatants. The reaction mix was incubated at 30°C. One unit is defined as the amount of enzyme which produces 1 µmol of unsaturated product per min. PEL specific activity was expressed in micromoles of unsaturated product liberated per minute per unit of optical density at 600 nm. Three samples were performed independently for each combination.
2.6. Experimental design
10For each strain the design schema corresponded to 9 experiments with three replicates over time. This design was applied in triplicate combination with a single block. Response surface methodology (RSM) with a 3k full factorial design was performed using Minitab version 15. Temperature (10, 15 and 20°C) and aw (0.960, 0.980 and 0.997) were investigated (Table 1). Data modelling was carried out by multiple regression analysis.
2.7. Modeling
11Primary model fitting. The growth [log10(cfu·ml-1)] for each treatment was plotted according to time, and a non linear regression procedure of Excel solver (Microsoft Office Excel 2007) was employed to estimate the maximum specific growth rate (µmax). The primary model of Gompertz (1825) was used as the fitting equation (Zwietering et al., 1990):
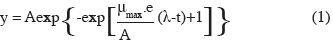
12where y is defined as the logarithm of the relative population size, the asymptote A is the maximal value reached, µmax (h-1) is defined as the tangent in the inflection point; e which is exp(1), λ is the lag phase (h), which is defined as the t-axis intercept of this tangent; and t is time (h).
13Secondary model. The average estimates of µmax from primary modeling were fitted by secondary models to describe the single and combined effects of temperature and aw on Eca, Ecc and Ech growth:
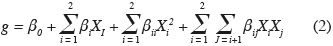
14where g is the response (µmax), β0 is a constant coefficient, Xi are coded variables ranging from -1 to +1, βi represent linear coefficients, βii are the quadratic coefficients, and βij are the second order interaction coefficients. All values of model coefficients were calculated by multiple regression analysis. Interpretation of the data was based on the signs (positive or negative effect on the response) and statistical significance of coefficients (P < 0.05).
15Evaluation of model performance. The following mathematical and statistical indices were calculated to evaluate the performance of the predictive models, i.e. their ability to describe the observed experimental data adequately: root mean square error (RMSE), regression coefficient (r2), bias factor, and accuracy factor (Dantigny et al., 2005; Lahlali et al., 2007; Ding et al., 2010). The RMSE and the bias and accuracy factors were calculated as follows:
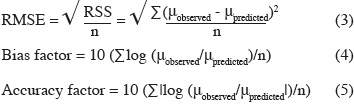
3. Results and discussion
3.1. Effects of water activity and temperature on maximum specific growth rate
16The observed and predicted average of maximum specific growth rates (µmax) for each combination aw-temperature are shown in table 1. No great difference was observed between the observed and predicted values for three bacterial strains, except for Ecc where the predicted value tend to be too low than the observed value at the combination aw of 0.960 and temperature of 15°C. The minimal and maximal growth for all Erwinia species were observed respectively at the combination aw of 0.960 and temperature of 10°C, and at the combination aw of 0.997 and temperature of 20°C. At the combination aw of 0.960 and temperature of 10°C the minimal growth of Eca and Ecc was at least 2 times higher than minimal growth of Ech. But at the combination aw of 0.997 and temperature of 20°C the maximal growth of Eca and Ecc was at least 5 times higher than maximal growth of Ech. These results led us to say that Eca and Ecc grow faster than Ech in our condition. From table 1 it appears that an increase in aw from 0.960 to 0.997 resulted in an increase in the µmax for the three Erwinia species. The same results for Eca, Ecc and Ech were noticed when temperature increase from 10°C to 20°C, except for Ecc due to the poor prediction of the model at 0.960 aw and 15°C. A lack of information was reported on combined effect of temperature and aw on the growth of phytopathogenic bacteria in literature data. The effect of aw upon growth of Ech was studied by Mildenhall et al. (1981). Their results indicate that minimal aw for growth of Ech on yeast extract-salts medium supplemented with glucose and adjusted to various aw with NaCl or mannose was 0.970 at 30°C. Also, they reported an increase in µmax when aw increases from 0.970 to 0.998 for Ech. Smadja et al. (2004) investigated the effects of various temperatures (8°C to 28°C) on maximum specific growth rate of Eca and Ecc. They noticed no growth for Ecc and low growth for Eca at 8°C and observed an optimum growth for Eca at 24°C and above 28°C for Ecc. Laurent et al. (2001) observed that the growth of Ecc MFCL0 was optimal at 28°C. In our work, maximun specific growth rates were calculated based on turbidemetric measurement (absorbance) of the three Erwinia after a correspondence between OD and CFU with a calibration curve. Previously, several researches used absorbance data transformed into equivalent viable counts to develop growth models for bacteria (McClure et al., 1993; Dalgaard et al., 1994; Chorin et al., 1997; Valero et al., 2006). This method has the advantage of being rapid, non-destructive, inexpensive and relatively easy to automate as compared to many other techniques such as classical viable counts methods which is a very time consuming labor and rather expensive method (Swinnen et al., 2004). Dalgaard et al. (2001) compared turbidemetric and viable counts methods to estimate μmax of bacterial growth rates. They concluded that turbidimetric measurements may be used reliably for estimation of μmax.
17Figure 1 presents contour plots of the predicted effects of aw and temperature on the growth of Eca, Ecc, and Ech. Whatever the strain, the contour plots showed that the growth rates were very sensitive to both parameters. The results in table 2 show a highly significant effect of each single factor and interaction (P < 0.0001) for Eca, Ecc and Ech, except quadratic effect of temperature, aw and interaction effect of aw-t for Ech. The higher is the absolute value of a linear coefficient (β1 or β2), the greater is the influence of the corresponding factor (temperature or aw) on the growth rate. Thus, in all cases, the influence of temperature was greater than that of aw. A positive linear, quadratic, and interaction effect of temperature and aw was noticed on the growth rate of Eca, Ecc and Ech except quadratic effect of aw for Eca and Ecc. To our knowledge, this is the first predictive modelisation of growth in the Erwinia genus based on combined effect of temperature and water activity (aw). Previous researches were focused on effect of dynamic conditions of temperatures on growth kinetics of Erwinia carotovora (Leporq et al., 2001), on the effect of temperature on the maximum specific growth rate of Eca and Ecc (Smadja et al., 2004) or on temperature as contribution of the risk of Erwinia carotovora spoilage of vegetable juice (Shorten et al., 2006).
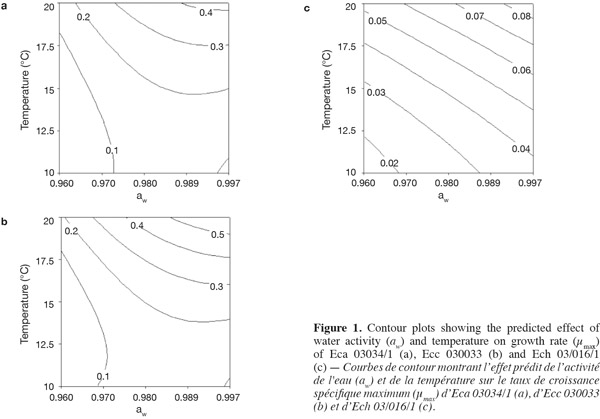
3.2. Evaluation of model performance
18Various methodologies were used to evaluate the model performance. The obtained results are shown in table 3. The coefficient r2 values of maximum specific growth rates showed that aw and temperature account for 97.29%, 97.76%, and 95.09% of the maximum specific growth rate variation observed for Eca, Ecc and Ech respectively. RMSE value was known to be the most simple and most informative measure of goodness of fit of model. The smaller is the RMSE value, the better is the performance of the model (Dantigny et al., 2005; Lalhali, 2007). Our Erwinia models displayed a small RMSE value, ranging between 0.005 and 0.021. The bias and accuracy factors are the most widely used method for evaluating performance of predictive models (Ross, 1996). In this work, the bias factors of models were ranged between 1.00 and 1.07. These values close to 1 indicated that the models were fail-safe for Eca 03034/1, Ecc 030033 and Ech 03/016/1. The average differences between observed and predicted maximum specific growth rates (accuracy factors) were 18%, 23% and 9% for Eca, Ecc and Ech models, respectively. The values of bias and accuracy factors obtained in this work were similar to those noticed in literature data by Neumeyer et al. (1997). It can be concluded that models based on µmax are, within the limits investigated in this work, able to predict the effect of aw and temperature on the growth of the three Erwinia strains.
3.3. Effects of water activity and temperature on PEL specific activity
19Figure 2 shows the mean of PEL specific activity of Eca 03034/1, Ecc 030033 and Ech 03/016/1 plotted as function of aw and temperature. The sampling period was selected according to Laurent et al. (2001) who stated that the PEL activity reaches a maximum and remains constant during early stationary phase. Temperature and aw had a high effect on the PEL specific activity of the three Erwinia strains. The maximum and the minimum PEL specific activity of all Erwinia strains were produced respectively at the combination aw-temperature equal to (0.997-20°C) and (0.960-10°C). Besides, we also noticed low PEL specific activity at a combination 0.960-15°C for Ech. Mildenhall et al. (1981) observed at 30°C no PEL production for Ech in glucose-yeast extract-salts medium adjusted at aw equal to 0.980 with mannose. In North-Western Europe the optimal temperature for pathogenicity is estimated to be between 15 and 20°C for E. carotovora subsp. atroseptica. (Pérombelon et al., 1995; Priou et al., 1996). Pectate lyase production by Ecc MFCL0 was optimal at 14°C as observed in polygalacturonate medium (PGA) and celeriac medium (CM) by Laurent et al. (2001). Smadja et al. (2004) obtained optimal temperature for Ecc PEL production around 15 and 17°C. Some authors noticed that optimal temperature for Ecc pathogenicity is estimated around 25°C in North-Western Europe (Pérombelon et al., 1995; Priou et al., 1996). Whatever the Erwinia species, our results showed an increase of PEL specific activity in supernatant with the temperature. The same result was pointed out with the growth of the three phytopathogenic bacteria (Eca, Ecc and Ecc). This suggests that PEL specific activity of Erwinia species is related to the multiplication of the bacterial strains. But contrary to growth, this research did not show an increase of PEL specific activity with aw except the treatment at 15 and 20°C for all bacteria strains. Some authors observed an increased of PEL specific activity for Ech in glucose-yeast extract-salts medium adjusted at various aw values (0.980 to 0.998) with mannose (Mildenhall et al., 1981). These results are in accordance with the role of temperature in soft rot development (Pérombelon, 2002; Toth et al., 2003).
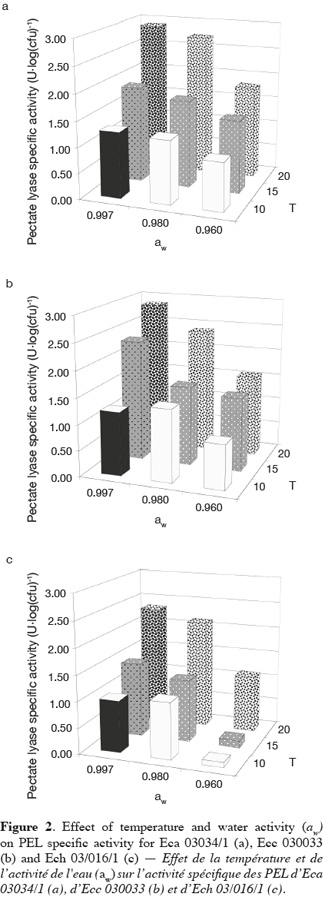
20In a general way we observed that PEL specific activity of Ech at the quantitative level was lower than this one of Eca and Ecc for all treatments. These results suggest that Eca and Ecc produced more PEL than Ech in our condition. In this study we monitored total PEL specific activity of Eca, Ecc and Ech. The lowest value of PEL specific activity observed at 10 and 15°C with 0.960 aw for Ech could be explained by the different pools of isoenzymes of Pel in Erwinia species. Eca, Ecc and Ech produce at least three and as many as six extracellular Pel isoenzymes according to the strain (Kotoujansky, 1987; Hugouvieux-Cotte-Pattat et al., 1996). For Ecc, Laurent et al. (2000, 2001) showed that activities of some isoenzymes of PEL such PelI and PelIII were strongly dependent on the incubation temperature and maximum amounts of both were produced at the same temperature (14°C), whereas PelII was expressed at almost the same level at many temperatures (4 to 28°C) in polygalacturonate medium (PGA). The presence of several isoenzymic forms of PEL may explain the differential temperatures of pathogenicity observed between the three Erwinia subspecies (Eca, Ecc and Ech) infecting potato (Smadja et al., 2004). Our results showed that the combined effect of the two environmental factors such as aw and temperature greatly influences the PEL specific activity of the three Erwinia species.
4. Conclusion
21Our research constitutes a preliminary and original investigation interested to evaluate the response behavior of Erwinia species to environmental factors. Based on in vitro experiments, we developed for the first time a predictive model of Eca 03034/1, Ecc 030033 and Ech 03/016/1 growth parameter (µmax) depending on temperature and aw. The results showed a significant effect of aw and temperature on the maximum specific growth rate of Eca 03034/1, Ecc 030033 and Ech 03/016/1. The maximum and minimal growths of Eca and Ecc were higher than those of Ech, and PEL specific activity of Eca and Ecc were also higher than those of Ech at each combination. Based on these observations we underlined that Eca and Ecc had the same ecological behavior comparatively to Ech in the studied conditions. Ongoing research will be focused on the effect of relative humidity (which is directly related to aw) and temperature on soft rot Erwinia population dynamic and disease development in planta.
22Acknowledgements
23We thank Mr J.L. Rolot from Walloon Agricultural Research Centre (CRA-W) of Libramont (Belgium) for having supplied the strains of Erwinia ssp. We also thank Mr Yves Brostaux from the Unity of Applied Statistics, Computer Science and Mathematics (SIMa) of University of Liege, Gembloux Agro-Bio Tech (GxABT) for his excellent assistance in statistical analysis. Finally, the first author thanks the Government of Côte d'Ivoire for the scholarship.
Bibliographie
Braun P. & Sutherland J.P., 2005. Predictive modelling of growth and measurement of enzymatic synthesis and activity by a cocktail of selected Enterobacteriaceae and Aeromonas hydrophila. Int. J. Food Microbiol., 105, 257-266.
Chorin E., Thuault D., Cléret J.-J. & Bourgeois C.M., 1997. Modelling Bacillus cereus growth. Int. J. Food Microbiol., 38, 229-234.
Dalgaard P. et al., 1994. Estimation of bacterial growth rates from turbidimetric and viable count data. Int. J. Food Microbiol., 23, 391-404.
Dalgaard P. & Koutsoumanis K., 2001. Comparison of maximum specific growth rates and lag times estimated from absorbance and viable count data by different mathematical models. J. Microbiol. Methods, 43, 183-196.
Dantigny P. et al., 2005. Modelling the effect of ethanol on growth rate of food spoilage moulds. Int. J. Food Microbiol., 98, 261-269.
Ding T., Rahman S.M.E., Purev U. & Oh D.-H., 2010. Modelling of Escherichia coli O157:H7 growth at various storage temperatures on beef treated with electrolyzed oxidizing water. J. Food Eng., 97, 497-503.
Gompertz B., 1825. On the nature of the function expressive of the law of human mortality and on a nex mode of determining the value of life contingencies. Phil. Trans. R. Soc. London, 115, 513-585.
Hugouvieux-Cotte-Pattat N., Dominguez H. & Robert-Baudouy J., 1992. Environmental conditions affect transcription of the pectinase genes of Erwinia chrysanthemi 3937. J. Bacteriol., 174, 7807-7818.
Hugouvieux-Cotte-Pattat N., Condemine G., Nasser W. & Reverchon S., 1996. Regulation of pectinolysis in Erwinia chrysanthemi. Annu. Rev. Microbiol., 50, 213-257.
Juneja V.K. et al., 2009. Mathematical modeling of growth of Salmonella in raw ground beef under isothermal conditions from 10 to 45°C. Int. J. Food Microbiol., 131, 106-111.
Kotoujansky A., 1987. Molecular genetics of pathogenesis by softrot Erwinias. Annu. Rev. Phytopathol., 25, 405-430.
Lahlali R., Serrhini M.N., Friel D. & Jijakli M.H., 2007. Predictive modelling of temperature and water activity (solutes) on the in vitro radial growth of Botrytis cinerea Pers. Int. J. Food Microbiol., 114, 1-9.
Lanham P.G., McIlravey K.I. & Pérombelon M.C.M., 1991. Production of cell wall dissolving enzymes by Erwinia carotovora subsp. atrospetica in vitro at 27°C and 30.5°C. J. Appl. Bacteriol., 70, 20-24.
Laurent P., Buchon L., Guespin-Michel J.F. & Orange N., 2000. Production of pectate lyases and cellulases by the bacterium Chryseomonas luteola strain MFCL0 depends on the growth temperature and the nature of the culture medium: evidence for two critical temperatures. Appl. Environ. Microbiol., 66, 1538-1543.
Laurent P., Buchon L., Burini J.F. & Orange N., 2001. Low pH and cold temperature combine to limit growth and pectate lyase production by psychrotrophic bacterium Erwinia carotovora ssp. carotovora MFCL0. Biotechnol. Lett., 23, 753-756.
Leporq B. et al., 2001. Validation of predictive models in dynamic conditions: microbial proliferation of Erwinia carotovora spp. at low changing temperatures. Acta Hortic., 566, 137-142.
McClure P.J., Cole M.B., Davies K.W. & Anderson W.A., 1993. The use of automated turbidimetric data for the construction of kinetic models. J. Ind. Microbiol., 12, 277-285.
Membré J.-M., Kubaczka M., Dubois J. & Chèné C., 2004. Temperature effect on Listeria monocytogenes growth in the event of contamination of cooked pork products. J. Food Prot., 67, 463-469.
Mildenhall J.P., Prior B.A. & Trollope L.A., 1981. Water relations of Erwinia chrysanthemi: growth and extracellular pectic acid lyase production. J. Gen. Microbiol., 127, 27-34.
Mildenhall J.P. & Prior B.A., 1983. Water relations of Erwinia chrysanthemi: intracellular and extracellular pectate lyase production. J. Gen. Microbiol., 129, 3019-3025.
Nachin L. & Barras F., 2000. External pH: an environmental signal that helps to rationalize pel gene duplication in Erwinia chrysanthemi. Mol. Plant-Microbe Interact., 13, 882-886.
Neumeyer K., Ross T., Thomson G. & McMeekin T.A., 1997. Validation of a model describing the effects of temperature and water activity on the growth of Pseudomonas. Int. J. Food Microbiol., 38, 55-63.
Pérombelon M.C.M. & Kelman A., 1980. Ecology of the soft rot Erwinias. Ann. Rev. Phytopathol., 18, 361-387.
Pérombelon M.C.M. & Kelman A., 1987. Blackleg and other potato diseases caused by soft rot Erwinias: a proposal for a revision of the terminology. Plant Dis., 71, 283-285.
Pérombelon M.C.M. & Salmond G., 1995. Bacterial soft rot. In: Singh U.S., Singh R.P. & Kohmoto K., eds. Pathogenesis and specificity in plant disease. Histopathological, biochemical, genetic and molecular basis. Oxford, UK: Pergamon, 1-19.
Pérombelon M.C.M., 2002. Potato diseases caused by soft rot erwinias: an overview of pathogenesis. Plant Pathol., 51, 1-12.
Priou S. & Jouan B., 1996. Les maladies provoquées par les bactéries pathogènes du genre Erwinia. In: Rousselle P., Robert Y. & Crosnier J.C., eds. La pomme de terre. Paris : INRA, 260-265.
Ross T., 1996. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol., 81, 501-508.
Shorten P.R., Soboleva T.K., Pleasants A.B. & Membré J.M., 2006. A risk assessment approach applied to the growth of Erwinia carotovora in vegetable juice for variable temperature conditions. Int. J. Food Microbiol., 109, 60-70.
Smadja B. et al., 2004. Thermodependance of growth and enzymatic activities implicated in pathogenicity of two Erwinia carotovora subspecies (Pectobacterium spp.). Can. J. Microbiol., 50, 19-27.
Swinnen I.A.M. et al., 2004. Predictive modelling of the microbial lag phase: a review. Int. J. Food Microbiol., 94, 137-159.
Toth I.K., Bell K., Holeva M.C. & Birch P.R.J., 2003. Soft rot Erwinia: from genes to genomes. Mol. Plant Pathol., 4, 17-30.
Valero A. et al., 2006. Modeling the growth rate of Listeria monocytogenes using absorbance measurements and calibration curves. J. Food Sci., 71, 257-264.
Zwietering M.H., Jongenburger I., Rombouts F.M. & Van’t Riet K., 1990. Modeling of the bacterial growth curve. Appl. Environ. Microbiol., 56, 1875-1881.
Pour citer cet article
A propos de : Ahoussi Augustin Moh
Univ. Liege. Gembloux Agro-Bio Tech. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
A propos de : Sébastien Massart
Univ. Liege. Gembloux Agro-Bio Tech. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
A propos de : Rachid Lahlali
Univ. Liege. Gembloux Agro-Bio Tech. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
A propos de : Mohamed Haïssam Jijakli
Univ. Liege. Gembloux Agro-Bio Tech. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
A propos de : Philippe Lepoivre
Univ. Liege. Gembloux Agro-Bio Tech. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: philippe.lepoivre@ulg.ac.be






