- Accueil
- Volume 15 (2011)
- numéro 2
- Cell wall polysaccharides hydrolysis of malting barley (Hordeum vulgare L.): a review
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Cell wall polysaccharides hydrolysis of malting barley (Hordeum vulgare L.): a review

Notes de la rédaction
Received on April 22, 2010; accepted on August 24, 2010
Résumé
Revue bibliographique : l’hydrolyse des polysaccharides des parois cellulaires de l’orge brassicole (Hordeum vulgare L.). La qualité brassicole découle des différentes étapes du processus de maltage. Celui-ci utilise les modifications physiologiques internes du grain apparaissant lors de la germination, telles que la synthèse d’enzymes, afin d’obtenir des processus hydrolytiques efficaces et in fine les composés désirés. Parmi les trois processus hydrolytiques principaux, à savoir la dégradation de l’amidon, la dégradation des polysaccharides des parois cellulaires et l’hydrolyse des protéines, une dégradation intense des polysaccharides des parois cellulaires est un critère essentiel. En effet, dans un processus de maltage, la paroi cellulaire crée une barrière physique entre le contenu cellulaire et d’autres enzymes hydrolytiques. De plus, lors d’une mauvaise dégradation de ces polysaccharides des parois cellulaires, des problèmes de viscosité et de troubles du produit fini sont rencontrés au cours du processus industriel de brassage, conduisant à une augmentation de la durée et des couts de production. Comprendre l’organisation et connaitre les constituants des parois cellulaires et les acteurs impliqués dans leur dégradation est important pour mieux maitriser les processus d’hydrolyse. Les principaux constituants de la paroi cellulaire sont les (1-3,1-4)-β-glucanes et les arabinoxylanes. Les (1-3,1-4)-β-glucanes sont des polymères de résidus β-D-glucopyranoses avec des liaisons β-(1,3) et β-(1,4). Les arabinoxylanes ont un squelette d’unités D-xylanopyranosyles avec des liaisons β-(1-4), sur lesquels se connectent des L-arabinofuranoses par des liaisons α-(1→2) ou α-(1→3). Les enzymes qui interviennent dans la dégradation des (1-3,1-4)-β-glucanes sont les (1-3,1-4)-β-glucanases, β-glucosidases et les β-glucane exohydrolases. Les solubilases interviennent également en solubilisant le β-glucane, mais elles sont peu connues. Les (1-3)-β-glucanases pourraient en faire partie. Les arabinoxylanes sont principalement décomposés par les (1-4)-β-xylane endohydrolases, les arabinofuranosidases et les β-xylosidases.
Abstract
Malting quality results from the different steps of the malting process. Malting uses internal changes of the seed occurring during germination, such as enzymes synthesis, to obtain a good hydrolysis process and the components required. Among the three main hydrolytic events observed, that are namely starch degradation, cell wall breakdown and protein hydrolysis, an efficient cell wall polysaccharides hydrolysis is an essential condition for a final product of quality. Indeed, because of the physical barrier of the cell wall, cell wall polysaccharides hydrolysis is one of the first steps expected from the process to gain access to the cell components. Moreover, viscosity problem and haze formation in malting industry are related to their presence during the process when inefficient degradation occurs, leading to increased production time and cost. Understanding the key elements in cell wall degradation is important for a better control. (1-3,1-4)-β-glucans and arabinoxylans are the main constituents of cell wall. (1-3,1-4)-β-glucans are unbranched chains of β-D-glucopyranose residues with β-(1,3) linkages and β-(1,4) linkages. Arabinoxylan consists in a backbone of D-xylanopyranosyl units linked by β-(1-4) bonds connected to single L-arabinofuranose by α-(1→2) or α-(1→3)-linkages. Degradation of (1-3,1-4)-β-glucans is processed by the (1-3,1-4)-β-glucanases, the β-glucosidases and the β-glucane exohydrolases. It seems that the (1-3)-β-glucanases are also involved. Arabinoxylans are mainly decomposed by (1-4)-β-xylan endohydrolase, arabinofuranosidase and β-xylosidase.
Table des matières
1. Introduction
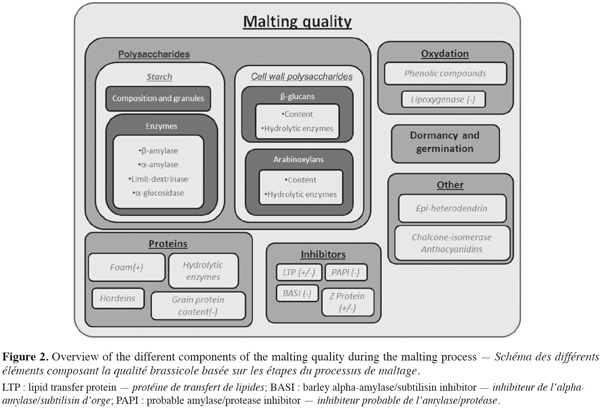
1Barley (Hordeum vulgare L.) is the primary cereal used in the worldwide malt production. Malting of barley consists in seed germination in which various enzymes are synthesised. These enzymes initiate the breakdown of the starchy endosperm and lipid reserves in the aleurone and embryo providing energy for growth. When growth is optimal and when useful elements are obtained, germination is stopped to avoid component consumption by the growing embryo (Briggs, 1978).
2Malting quality traits are based on the efficiency of these hydrolytic activities. Indeed, malting quality traits result from the different steps of the malting process. Potential of the grain is fully completed at the end of the malting process and brewing can only use this potential. It means that understanding and having under control the quality traits is essential to optimize germinated grain potential. Malting quality traits can therefore be identified on the basis of the process steps.
3Factors affecting germination such as dormancy of germination capacity are basic quality traits (Deymie, 1984; Prada et al., 2004). The content of starch and its degradation are essential to obtain a good yield in alcohol (Hayes et al., 2002; Clancy et al., 2003). Protein content, protein composition and hydrolysis can also influence the final quality by different ways (Agu et al., 1999; See et al., 2002; Borén et al., 2004; Holopainen et al., 2005). Hydrolysis of the cell wall polysaccharides is one of the key quality traits. In fact, it is needed to free the way to cell component for other enzymes; and it can influence the efficiency of other hydrolytic process. Moreover, presence of cell wall polysaccharides can lead to viscosity problems and haze formation in the beer and be synonymous of higher production costs (Izydorczyk et al., 2000; Swanston et al., 2002; Jin et al., 2004).
4This paper is focused on this particular trait because of its role in malting quality. The malting process and the different malting quality traits are first introduced. Importance of cell wall polysaccharides hydrolysis is then developed in the context of malting quality. The different phases of cell wall hydrolysis are then explained with regard to the enzymes implied in the process. Understanding the actors implied in cell wall polysaccharides hydrolysis and their importance leads to new pathways for improving particular malting quality parameters. Having a better control of it can help malters and brewers to increase yields, rapidity of the industrial transformations and to decrease the costs. By a better understanding of enzymes implied in cell wall degradation, possible routes for the selection of improved cell wall degradation potential may be defined.
2. Relation between the malting process and malting quality
5Malting consists in barley seed transformation in malt, based on the physiological modifications inside the grain during germination. Three main kinds of components are hydrolyzed: starch, protein and cell wall polysaccharides (Hayes et al., 2002).
6Malting can be decomposed in five main steps. Calibration and cleaning is the first one. It helps to obtain homogeneous raw material and to eliminate dirt and broken seeds. Steeping is the second step. It uses immersed and dry phases. Its goal is to increase humidity to 42-46% for optimal imbibition of the seeds. It contributes to an homogenous germination (Briggs, 1978; Van Lierde, 2003).
7At the end of the steeping, seeds are transferred in germination rooms and the germination step begins. It is during this step that enzymes activities are developed (Figure 1). Main points are:
81. gibberellins are synthesized by germinating embryo and they migrate from scutellum to starchy endosperm;
92. gibberellins diffuse in the aleurone layer and induce hydrolytic enzymes synthesis (Sponsel, 2006);
103. hydrolytic enzymes initiate starch, proteins and cell wall polysaccharides degradation;
114. grain respiration uses sugar to liberate CO2 and water (Briggs, 1978; Van Lierde, 2003).
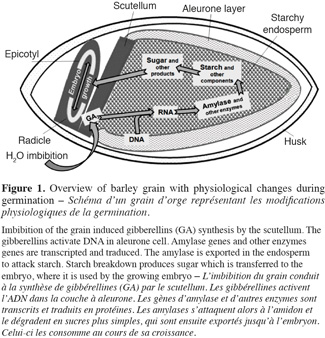
12When germination is optimum, it is stopped to avoid sugar consumption by the embryo. The fourth step is kilning. It uses two levels of temperature, mild figures to dry the seed and harder then stop germination and develop color and aroma of the malt. This step can also result in a loss of some enzyme activity. For example, a loss of 60% is observed for (1-3,1-4)-β-glucanase after kilning (Georg-Kraemer et al., 2004).
13The purpose of the fifth step is to eliminate the radicle.
14After the malting process all the expected enzymes are obtained. Brewing part cannot improve enzymes synthesis. Malting quality is directly related to the malting part of the process.
15Grain homogeneity, germination capacity, dormancy or pre-harvest sprouting are factors that affect the germination process (Deymie, 1984; Prada et al., 2004) (Figure 2). During brewing, yeast needs sugar to grow and to produce alcohol. Starch content and composition, starch hydrolysis and enzymes involved in the breakdown of the grain are traits which influence the amount of sugar available for subsequent transformation into alcohol (Hayes et al., 2002; Clancy et al., 2003). Yeast growth also requires proteins and nucleic acids, either partially or fully hydrolysed into amino acids and nucleotides (Agu et al., 1999; Kuhbeck et al., 2005). In addition, some proteins are needed for foam stability (Brandt et al., 1990; Roberts et al., 2003; Borén et al., 2004) while others may lead to haze formation (See et al., 2002). Moreover storage proteins such as hordeins can also modify endosperm structure and thus malting quality (Chandra et al., 1999; Echart-Almeida et al., 2001; Holopainen et al., 2005). Protein composition seems to be more important than total protein content (Wang et al., 2007).
16Breaking the cell wall by degrading the cell wall polysaccharides is one of the main steps in the hydrolysis process used in malting and brewing. It allows enzymes to access the cell content. Moreover cell wall polysaccharides that are not hydrolysed can lead to problems during brewing steps (e.g., slower filtration, haze formation) because of the higher viscosity they cause. These problems result in elevated cost and longer procedures for the industrial process (Izydorczyk et al., 2000; Swanston et al., 2002; Jin et al., 2004).
3. Importance of cell wall polysaccharides in malting quality and in other food industries
3.1. Malting and brewing industry
17Cell wall hydrolysis is one of the most important events in the process of malting and brewing because of its impact on malt, wort and beer.
18Degradation of the polysaccharides cell wall removes the physical barrier between hydrolytic enzymes released during germination and their substrates, stored in the starchy endosperm. It allows the enzymes to access cell components and increases the efficiency of other hydrolytic processes. This can explain why there is a decrease in malt extract, a key parameter to evaluate efficiency of transformation, if there is poor β-glucan and arabinoxylan degradation.
19Indeed, complete degradation of β-glucan and arabinoxylan can provide a supplement of 15-20% of fermentable carbohydrates used by yeast for conversion into alcohol (Ferre et al., 2000). However, as mentioned above, a high level of β-glucan in the wort or beer causes an increase of viscosity that may lead to filtration problems (Izydorczyk et al., 2000; Swanston et al., 2002; Jin et al., 2004).
20β-glucans, with proteins, polyphenols and pentosanes can lead to haze formation in wort and beer. High-molecular-weight (HMW) β-glucans precipitates readily after a freezing-and-thawing treatment, whereas the fractions with the lower MWs do not precipitate even after repeated freezing and thawing cycles (Jin et al., 2004).
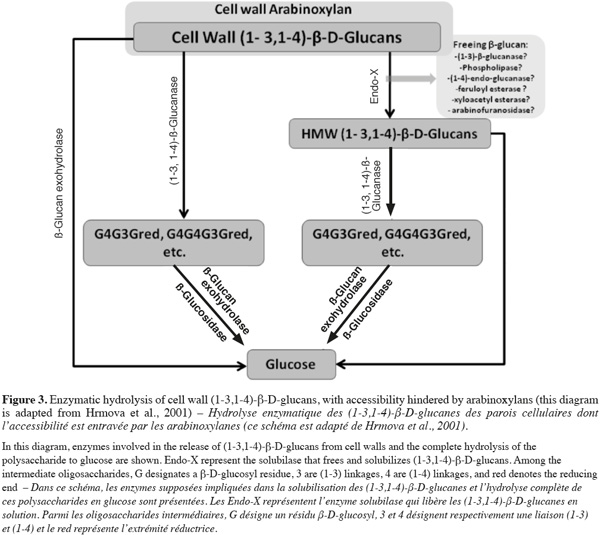
3.2. Other industries
21Feed. Currently, in chicken feed, barley is an unacceptable component because of its low metabolizable energy. Feeding barley diets to poultry leads to a limited uptake of nutrients, slows initial growth and results in sticky droppings adhering to the cloaca and down of the chicken as well as to the floors of the production cages (Wettstein et al., 2000).
22Food. Despite the problems it causes in brewing, barley β-glucan is used in food as a healthy component. It has effect on blood cholesterol and glucose reduction, it increases satiety that can lead to weight loss and it helps to have a better control in heart disease and type-2 diabetes (Wilson et al., 2004; Baik et al., 2008). Moreover, β-glucan has been recently demonstrated to be anti-cytotoxic, antimutagenic and anti-tumorogenic (Mantovani et al., 2008).
3.3. Technological criteria of malting quality and their relation to cell wall polysaccharides breakdown
23During malting and brewing, some criteria are used to evaluate technologically the extent of modification and the quality of the final product. The main ones are malt extract, kolbach index, friability, viscosity and diastasic power.
24Malt extract gives an idea of how successful the entire process has been in extracting the components of the grain into a form that is suitable for beer making. It is a key quality indicator (Ayoub et al., 2003). Malt extract consists in the percentage of solid material extracted from fine ground malt that solubilises during brewing. If hydrolytic enzymes cannot reach cell components because the cell wall polysaccharides degradation is not sufficient, they cannot hydrolyse their substrate and solubility of solid material will not be optimal.
25Kolbach index is the ratio of soluble nitrogen to the total nitrogen concentration in the malt. This trait is related with the protein trait in malting quality (Briggs, 1978).
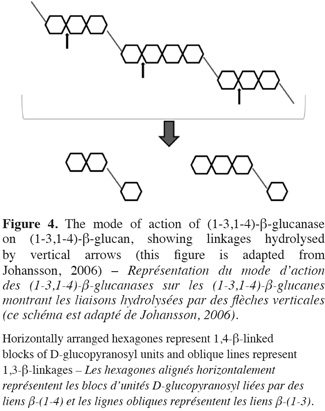
26Friability is an indicator of the degree of hydrolysis in the endosperm. High value is related to efficient degradations. Malt extract, poor degradation of β-glucan and arabinoxylan can influence friability (Briggs, 1978).
27Viscosity of the malt extract or the wort is related to β-glucan and indicates weak endosperm modification. Moreover, arabinoxylans effect on viscosity and filtrability are at least as important as β-glucan effect (Sadosky et al., 2002). β-glucan of the wort is also measured as technological criteria to evaluate cell wall breakdown efficiency.
28Diastasic power represents amylolytic enzyme activity (Clancy et al., 2003). In malting and brewing, a high level of diastatic power is generally sought. Starch is a cell component that can only be reached after cell wall breakdown. By this way, degradation of the cell wall is able to influence starch degradation.
4. Cell wall polysaccharides of barley
4.1. Cell wall composition
29Cell walls of the starchy endosperm of barley consist in 75% of (1-3,1-4)-β-D-glucans and 20% of arabinoxylan, with 2% of cellulose and 2% of glucomannan. The walls of aleurone cells consist of 71% of arabinoxylan and 26% of (1-3,1-4)-β-D-glucans, with 3% of cellulose and glucomannan (Fincher, 1992; Lazaridou et al., 2008). Gamlath et al. (2008) have shown that endosperm cell wall component may have significant impact on kernel hardness as well as water uptake. Large differences in the solubility and digestibility of endosperm cell wall polysaccharides are expected depending on the composition. Composition is affected by the environment of the growing barley grain (Lazaridou et al., 2008).
4.2. Barley β-glucans
30Barley β-glucans exist in two forms, according to their solubility or insolubility in water (Swanston et al., 2002).
31The (1-3,1-4)-β-glucans are unbranched chains of β-D-glucopyranose residues (glucose) with β-(1-4) linkages and β-(1-3) linkages in a ratio 3.2:1 to 6.6:1.Water-soluble fraction extracted at 65°C has higher ratio than water-soluble fraction extracted at 40°C. The lower solubility of 65°C water-soluble β-glucans is associated with their higher molecular weight (MW) and higher content of β-(1-4)-linkages. An average of 54% of barley β-glucan is water-extractable at 38°C (Aman et al., 1987). MW of β-glucan in barley, malt and beer has been reported to vary from 150 to 1 937 kDa, 800 to 1 220 kDa and 1 to 10 kDa respectively (Jin et al., 2004). Barley contains also a small amount of β-(1-3) glucan (Bacic et al., 1981).
32The β-glucan content and β-glucanase activity are influenced by the genotype and the environment (Wang et al., 2004; Swanston et al., 2006). Oscarsson et al. (1998) showed β-glucan content variation depending on the cultivars and the environmental conditions, with a more significant effect of the cultivar. Perez-Vendell et al. (1996) do not support the idea of higher cultivar effect and showed in their study a similar effect from cultivar and environment.
33Anker-Nilsen et al. (2008) identified variation of total β-glucan content of germinating grain from 4.0% to 7.4%, compared to Holtekjolen et al. (2006) who identified variation from 2.4% to 8.3%.
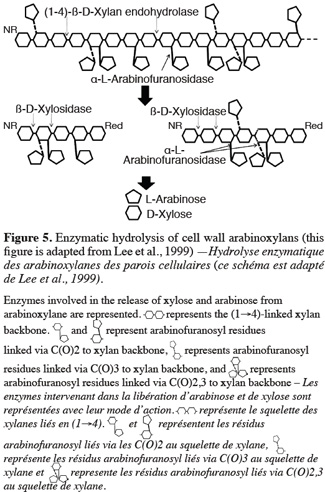
34Anker-Nilsen et al. (2008) demonstrated seasonal temperature effects during the synthesis of β-glucan on content, solubility, viscosity and MW. Waxy and high-amylose varieties showed higher β-glucan content and naked barley shows a higher content than covered barley. β-glucan content increases with the level of nitrogen fertilization (Oscarsson et al., 1998; Wood et al., 2003) and decreases with higher irrigation (Guler, 2003). Furthermore, β-glucan content is highly correlated with protein content, which is another important malting quality parameter (Guler, 2003).
4.3. Arabinoxylans
35Barley arabinoxylans consist in D-xylanopyranosyl units linked by β-(1-4) bonds to form a molecular backbone, connected to single L-arabinofuranose branches by α-(1→2) or α-(1→3) linkages. Typically, L-arabinufuranose or other side chains are carried on the main chain at non-reducing end groups (Lee et al., 2001; Egi et al., 2004). The degree of branching of arabinoxylan can be represented by the D- xylanopyranosyl units / L-arabinofuranose ratio. The endosperm tissue differs from the aleurone layer with an average ratio of 1.0-1.1 compared to 1.9-2.2, respectively. Unique feature is the presence of ferulic acid covalently linked via ester linkage to C(O)-5 of the arabinose residue (Li et al., 2005).
36The term “arabinoxylan” is recent and was previously referred to as pentosan, pentose gum or hemicellulose (Egi et al., 2004). Arabinoxylans are either water extractable or water unextractable. Water extractable arabinoxylans are thought to be loosely bound at the cell wall surface, while water unextractable arabinoxylans interact covalently and non covalently with other arabinoxylan molecular and/or cell wall constituents and are retained in the cell walls. The solubility of arabinoxylan depends on the D-xylanopyranosyl units/L-arabinofuranose ratio, their substitution pattern and the difference in MW. Long sequence of unsubstituted xylose residues increases arabinoxylan aggregation, decreasing solubility, whereas low MW increase solubility of arabinoxylan. Water unextractable arabinoxylans have a high water-binding capacity (Courtin et al., 2001; Izydorczyk et al., 2008).
5. Hydrolysis of cell wall polysaccharides
5.1. Cell wall hydrolytic enzymes classification
37Cell wall hydrolytic enzymes belong to the Glycosyl Hydrolase (GH) class of EC 3.2.1.XX. It is one of the two main classes of the carbohydrate active enzymes (CAZY). The classification is based on their amino acid sequence. GH enzymes are represented by around eighty-five families (Bourne et al., 2001). They have important roles in various physiological processes such as cell wall synthesis (by the control of the MW of synthesized polysaccharides), regulation of cell wall expansion and alteration, mobilization of storage reserves and also in plant defense for enzymes classified in Pathenogenesis Related protein (PR). GH enzymes have been located in different cell locations (cytoplasm, cell wall, membrane, vacuole, endoplasmic reticulum, peroxysome) but in Arabidopsis, the majority of these proteins were found to be in the cell wall, underlying their potential importance in cell wall biogenesis (Bourne et al., 2001; Boudart et al., 2007; Minic, 2008).
5.2. Hydrolysis of β-glucans
38During cell wall breakdown, the first step of β-glucan degradation is solubilisation. Two pathways coexist (Figure 3). β-glucan can be directly solubilized or it can be first solubilised by several enzymes that remove the outer layers of cell wall. The term “solubilase” is used to represent the enzyme involved in this process.
39Different enzymes implied in the solubilisation have been proposed such as (1-3)-β-glucanase, carboxipeptidase, phospholipases, (1-4)-endo-β-glucanase, feruloyl esterase, xyloacetyl esterase and arabinofuranosidase (Georg-Kraemer et al., 2004; Jin et al., 2004; Kuntz et al., 2007).
40The ability of esterases, xylanases, and arabinofuranosidase to solubilize glucan indicates that pentosan (arabinoxylan) component of cell wall can restrict the extraction of β-glucan, because of its location inside the wall, enwrapped in arabinoxylan to which is attached acetate and ferulate (Kanauchi et al., 2001; Kanauchi et al., 2008; Bamforth, 2009).
41The (1-4)-endo-β-glucanases have similar activity to the solubilases (Wilhelmi et al., 2001) and they are present in malt (de Sa et al., 2004), suggesting that they could be implicated in β-glucan solubilisation.
42Some authors (Yin et al., 1989; Wilhelmi et al., 2001) suggested that solubilase derives from fungi associated with the husk of the grain. But there is no hint of the existence of a unique enzyme solubilase for solubilising barley β-glucan. It seems that β-glucan solubilase activity derives from enzymes with different thermosensitivities and that the increase of temperature decreases solubilase activity. Moreover, (1-3,1-4)-β-glucanase might also be involved in β-glucan solubilisation. Solubilase activity was detected in the dry grain and during the first day of malting, but not (1-3,1-4)-β-glucanase activity (Gianinetti et al., 2007). To date, the solubilisation of cell wall polysaccharides is not well understood and there is no strong evidence for implication of one particular enzyme.
43The next step after solubilisation is the attack of the β-glucans by (1-3,1-4)-β-glucanase (EC 3.2.1.73) (Georg-Kraemer et al., 2004; Jin et al., 2004). (1-3,1-4)-β-glucanase are also called lichenase or (1-3,1-4)-β-glucan endohydrolase. The (1-3,1-4)-β-glucanases release mainly large fragments such (1-3,1-4)-β-D-tri- and tetrasaccharides as the major hydrolysis products, but they can also release oligosaccharides of up to ten or more units (Hrmova et al., 2001).
44The (1-3, 1-4)-β-D-oligoglucosides released by (1-3, 1-4)-β-glucanase can be further hydrolysed by several enzymes, including β-glucan exohydrolase and β-glucosidase. These enzymes remove glucose from the non-reducing termini of their substrates. They have an apparent preference for (1-3) linked substrates, but will nevertheless rapidly depolymerise oligopolysaccharides with (1-3) and (1-4) linkages, such as G4G3GR and G4G4G3GR, to glucose (Leah et al., 1995; Hrmova et al., 1996; Hrmova et al., 1997; Hrmova et al., 2001). Finally, a (1-3)-β-glucanase (EC 3.2.1.39) is also reported to be involved in β-glucan hydrolysis (Balance et al., 1978; Leah et al., 1995; de Sa et al., 2004).
45(1-3,1-4)-β-glucanases. The (1-3,1-4)-β-glucanases cleave specifically the (1-4)-linkages which are on the reducing terminal side of (1-3)-β-glucosyl residues (Figure 4) and release oligosaccharides that contain a variable number of (1,4)-β-glucosyl residues at their non-reducing end and a single (1,3)-β-glucosyl residue at their reducing end (Hrmova et al., 1999; Johansson, 2006).
46The (1-3,1-4)-β-glucanase exists as two isoforms referred to isozyme EI and EII. EI is encoded by the Glb 1 gene, located on chromosome 5(1H). The MW of EI is 28 kDa, its isoelectric point is 8.5 and the enzyme contains 0.7% of associated carbohydrate. EII is encoded by Glb 2 gene, located on chromosome 1(7H). EII has a MW of 33 kDa and an isoelectric point over 10, and it contains 4.0% of carbohydrates. EI is less thermostable than EII but shows a higher activity. Carbohydrates content was suggested to have an effect on the thermostability of the enzymes. Both genes would originate from a common ancestral gene in Hordeum (Litts et al., 1990; Georg-Kraemer et al., 2004).
47These isozymes are expressed in different tissues at different developmental stages and are under separate genetic control. EI is expressed in the scutellum, in young leaves and roots as well as in aleurone layer and EII only expressed in the aleurone layer (Slakeski et al., 1992; Jin et al., 2004). The two isozymes are also differentially switched on by the action of gibberellic acid (GA) and auxins (Hrmova et al., 1997; Georg-Kraemer et al., 2004). The activity of (1-3, 1-4)-β-glucanase is switched after two days of malting (Ginaninetti et al., 2007) and increases rapidly until the 4th day of the malting process. Nevertheless, the expression of these genes is first detected in the scutellum after one day and only in the epithelial layer. At this stage, no expression is apparent in the aleurone layer. After the second day, levels of mRNA decrease in the scutellar epithelium but increase in the aleurone (Georg-Kraemer et al., 2004).
48A loss of 60% of (1-3,1-4)-β-glucanase activity is observed after the kilning step of malting. It means that 40% was retained in the finished malt, for the brewery (Georg-Kraemer et al., 2004).
49(1-3)-β-glucanases. Endo-(1-3)-β-glucanases (EC 3.2.1.39) hydrolyze the internal (1-3)-β-glucosidic linkages in β-glucan. These enzymes are expressed at a high level as germination proceeds and also in young leaves. However, their functions are not clear. They might hydrolyze the (1-3)-β-glucan that is found in the isolated deposit in the sub-aleurone region of the starchy endosperm and they might as well partially hydrolyze (1-3,1-4)-β-D-glucan. They are also important representatives of the “Pathogenesis Related” proteins (PR2 subgroup), that play a role in plant protection because of their capacity to hydrolyze (1-3) and (1-3,1-6)-β-glucans commonly found in fungal cell walls (Hrmova et al., 1993; Burton et al., 1998). Some of the (1-3)-β-glucanases probably have also a function in processes such as dormancy break or wound callose, senescence and pollen formation during normal growth and development (Burton et al., 1998; Leubner-Metzger, 2003).
50At least seven different (1-3)-β-glucanase isoformes are known, represented by seven genes, clustered on the long arm of chromosome 3(3H). They are identified as GI to GVII. All are single copy except GIV. However it seems likely that the endo-(1-3)-β-glucanase genes and the (1-3,1-4)-β-glucanase genes evolved from a common ancestor which has subsequently functionally differentiated in Hordeum (Burton et al., 1998; Finnie et al., 2006).
51β-glucosidases. Based on their ability to hydrolyse the synthetic β-D-glucoside, 4-nitrophenyl β-D-glucoside (4NPG), Leah et al. (1995) classified β-glucosidases as EC 3.2.1.21. Barley β-glucosidase hydrolyzes aryl-β-glucosides. The increased hydrolytic rate with oligosaccharides is a characteristic often observed with polysaccharides exohydrolase but β-glucosidases are unable to hydrolyze polymeric (1-4)-β-glucans. Based on this peculiarity, β-glucosidases could be either classified as a polysaccharide exohydrolase of the (1-4)-β-glucan glucohydrolase group (EC 3.2.1.74). Problems have been encountered when classifying these enzymes into Enzyme Commission classes (Hrmova et al., 1998). The barley β-glucosidases exhibit a marked preference for (1-4)-β-D-oligoglucosides (cellodextrins) of increasing chain length, suggesting that it has an extended substrate-binding region (Hrmova et al., 1998; Hrmova et al., 2001).
52Two isozymes of β-glucosidase, called βI and βII have been purified. Their molecular weights are both 62 kDa and they have isoelectric points of 8.9 and 9.0, respectively. Hrmova et al. (1998) reported that the two isozymes differed only by one amino acid in the protein sequence. The sequence of the gene named bgq60 found by Leah et al. (1995) could be a single copy coding for the βII isozyme.
53Three main functions have been suggested for these enzymes. First, they might be related to auxin-mediated cell elongation in growing coleoptile where they can be implicated in cell wall loosening. Secondly, they might be implicated in the complete conversion of the cell wall (1-3,1-4)-β-glucan to glucose in germinated barley. Implication in the protection of germinating grain from pathogen attack might be the third function (Leah et al., 1995; Hrmova et al., 1996). For Hrmova et al. (1998), their most likely role seems to be cell wall hydrolysis during germination because β-glucosidase is synthesized in the starchy endosperm of developing grains, but β-glucosidase content is not increased after germination.
54β-glucan exo-hydrolases. As for β-glucosidases, classification is difficult for these enzymes. They are considered as member of the family 3 of GH. The β-glucan exo-hydrolases exhibit a relatively broad specificity with respect to linkage position in their substrates. Even if they have a preference for (1-3)-β-glucan, they can also hydrolyze a range of β-D-glucan and β-D-oligoglucosides with (1-2), (1-4) and (1-6) linkage. These enzymes release glucose as major product from (1-3,1-4)-β-glucan (Hrmova et al., 1998; Hrmova et al., 2001).
55Two isozymes of β-glucan exo-hydrolase, ExoI and ExoII, have been identified and characterized with isoelectric points of 7.8 and 8.0, respectively, and apparent MW of 69 to 71 kDa, respectively. They hydrolyze (1-3)-β-glucan, laminarin, β-oligoglucoside and (1-3,1-4)-β-glucan (Hrmova et al., 1996; Jin et al., 2004). They share the same potential functions than the β-glucosidase (Hrmova et al., 1996). The genes encoding the exohydrolases are transcribed in the scutellum of germinated grain, but mRNA is also abundant in elongating coleoptile. As for β-glucosidase, the barley β-glucan exo-hydrolase may exhibit multiple functions (Hrmova et al., 1999).
5.3. Hydrolysis of arabinoxylans
56The changes in arabinoxylan content during malting can be divided into three categories. The water soluble arabinoxylans already present in the grain are first degraded. Then, water soluble arabinoxylans just released through enzymatic degradation of the endosperm wall during the germination are also degraded. Finally, the embryo itself synthesizes arabinoxylan. Endo β-(1-4)-xylanase, exoxylanase, β-xylosidase and α-arabinofurnosidase are believed to be involved in arabinoxylan degradation (Hrmova et al., 1997). The combined action of theses enzymes is required to complete arabinoxylan degradation (Figure 5). Arabinoxylan degradation is at least as important as β-glucan. In beer, water extractable arabinoxylan can reach level of 4 g.l-1. It is more than the maximum of 2 g.l-1 for β-glucan content (Sungurtas et al., 2004). Li et al. (2005) reported that arabinoxylan content is ten times more elevated in beer than the β-glucan content. According to Allosio-Ouarnier et al. (2007), arabinoxylan degradation occurs mainly during brewing whereas arabinoxylans are not extensively degraded during malting.
57Arabinofuranosidases. The α-arabinofuranosidases (EC 3.2.1.55) catalyse the breakdown of α-(1-2) and α-(1-3) linked arabinofuranose units. They have a broad range specificity for various (1-2)-, (1-3)- and (1-5)-α-arabinofuranosyl linkages of arabinan and arabinoxylan than other members of the family to which they belong. Three functions can be related with the action of the enzyme. The first suggested function is to lock the (1-4)-β-xylan backbone into cell wall through hydrogen bonding to unsubstituted regions of other (1-4)-β-xylan chains, with cellulose or with other wall polysaccharides. Arabinofuranosidases can also help the turnover of cell wall polysaccharides in elongating coleoptiles. They also have a role in the depolymerisation of the germinated grains walls.
58They are most likely active in the hydrolysis of oligosaccharides released through endoxylanase activity. The α-arabinofuranosidases are secreted from the aleurone layer. Two isoforms, ARA-I and ARA-II (also called AXAHI and AXAHII), have a MW of 68 and 69 kDa, and isoelectric points of 4.8 and 5.6, respectively. The two mature proteins consist of 626 amino acid residues (Lee et al., 2001; Egi et al., 2004). ARAI is considered as a bifunctional α-arabinofuranosidase/β-D-xylosidase enzyme (Lee et al., 2003). Sungurtas et al. (2004) showed that α-arabinofuranosidase activity increases rapidly from the day 1 of stepping to the day 7 of germination.
59(1-4)-β-xylan endohydrolases. The (1-4)-β-xylan endohydrolases (EC 3.2.1.8) decrease the viscosity of the water soluble arabinoxylan. They catalyse the hydrolysis of β-(1-4) xylosidic linkages in the arabinoxylan polymer and the removal of arabinofuranosyl residues (Egi et al., 2004). They have a molecular weight of 41 kDa and an isoelectric point of 5.2. The gene family encoding (1-4)-β-xylan endohydrolases contains 3 genes clustered on chromosome 7(5HL). Two isoforms, called X-I and X-II, have been described (Banik et al., 1997; Hrmova et al., 1997). X-I is the major endo-β-(1,4)-xylanase released from the aleurone layer of germinating barley.
60Both isoenzymes are expressed during germination but, in contrast to X-I, the X-II transcripts accumulate in the developing shoot and root of the seedling embryo and not in the aleurone layer surrounding the endosperm. During subsequent stages of development, up to anthesis, X-II remains expressed in various organs. In developing grains, X-II gene is expressed in the early stages of grain filling whereas X-I transcription is switched on during the later stages (Van Campenhout et al., 2005). The mRNA level is strongly enhanced by GA3, and abscisic acid abolishes GA induction of gene expression. Their synthesis also depends on Ca++ (Banik et al., 1997; Hrmova et al., 1997; Egi et al., 2004).
61Previous reports indicated that cytosolic enzyme X-I is synthesized as an inactive precursor which is proteolytically processed to active forms, depending on its programmed cell death. However, Van Campenhout et al. (2007) showed that the precursor form of X-I is an endo-acting enzyme which has the striking ability to release xylose from both polymeric xylan as well as from small xylo-oligosaccharides.
62β-xylosidases. The β-xylosidases (EC 3.2.1.37) catalyse the hydrolysis of β-(1-4) xylosidic linkage within xylo-oligosaccharides. There are two isoforms, having the same regulation factors as endoxylanase (i.e., expression increased by GA3 and Ca++, expression decreased by abscisic acid) (Egi et al., 2004; Hrmova et al., 1997). One of the isoformes, designated XYL, has a MW of 67 kDa and an isoelectric point of 6.7 and the cDNA code a protein of 748 amino acids. It is a member of the family 3 GH (Lee et al., 2003). Sungurtas et al. (2004) showed that β-D-xylanopyranosidase activity increases rapidly from the day 1 of stepping to the day 7 of germination.
63Other enzymes involved in hydrolysis process. Arabinoxylan hydrolysis can also be influenced by other enzymes. Acetyl esterase (Humberstone et al., 2000a) and ferulic acid esterase (Humberstone et al., 2000b) may influence degradation by altering the accessibility and/or solubility of arabinoxylan. Moreover, endoxylanase inhibitors can also modify arabinoxylan hydrolysis. Inhibitors activity can be relatively high in barley (Goesaert et al., 2004).
6. Conclusion
64Malting quality rests on multiple factors. Each one contributes to the good development of the malting and brewing process.
65Cell wall polysaccharides hydrolysis is a key malting quality parameter because it is the first step in the transformation of the germinating grain. Without this step, other enzymes synthesized during germination such as amylases cannot access cell component. Moreover, viscosity problems caused by low hydrolysis of β-glucan and arabinoxylan slow the procedure and increase the production costs. Haze formation in the final product is also an undesirable consequence of poor cell wall degradation.
66Understanding cell wall hydrolysis may help in the selection of malting varieties. Indeed, properties of the enzymes involved can be used as tool in a breeding program. Different traits can be measured in grain, malt or wort such as β-glucan and arabinoxylan content to characterize the hydrolysis. Because of the decrease of β-glucan content during the malting (50% loss during malting by the activity of β-glucanase), grain content is not the best trait to illustrate quality. There is poor correlation between cultivar grain β-glucan content and malting quality (Edney et al., 1998). This indicates that it is more relevant to improve hydrolytic enzymes activities than to modify arabinoxylan and β-glucan content in grain in breeding process.
67Despite the fact that the first studies were mainly focused on β-glucan hydrolysis, new interest for arabinoxylan degradation seems to show the relative significance it could have in the problems mentioned above (e.g., access to cell components, viscosity and haze formation).
68Moreover, recent models focused on cell wall structure suggest that the accessibility to β-glucan is hindered by arabinoxylan and, to an extent, by the attached ferulic acid and acetic acid moieties (Bamforth et al., 2001). It highlights the potential role of arabinoxylan hydrolysis in malting quality.
69Selecting barley varieties with superior cell wall polysaccharides degradation could assist brewers and malters to develop more efficient, faster and cheaper processes. This review should contribute to the identification of new selection tools, helpful is the breeding of barley malting cultivars.
70Abbreviations
71GA: Gibberellic Acid
72GH: Glycosyl Hydrolase
73HMW: High Molecular Weight
74MW: Molecular Weight
Bibliographie
Agu R. & Palmer G.H., 1999. Comparative development of soluble nitrogen in the malts of barley and sorghum. Process Biochem., 35, 497-502.
Allosio-Ouarnier N., Saulnier L., Guillon F. & Boivin P., 2007. Beta-glucan and arabinoxylan distribution in barley and malt. In: Proceedings of the 31st EBC Congress, 6-10 May, Venice, Italy.
Aman P. & Graham H., 1987. Analysis of total and insoluble mixed-linked (1-3),(1-4)-β-D-glucans in barley and oats. J. Agric. Food Chem., 35(5), 704-709.
Anker-Nilssen K., Sahlstrøm S., Knutsen S.H. & Holtekjølen A.K., 2008. Influence of growth temperature on content, viscosity and relative molecular weight of water-soluble β-glucans in barley (Hordeum vulgare L.). J. Cereal Sci., 48, 670-677.
Ayoub M. et al., 2003. Marker-based selection in barley for QTL region affecting α-amylase activity of malt. Crop Sci., 43, 556-561.
Bacic A. & Stone B.A., 1981. Chemistry and organisation of aleurone cell wall components from wheat and barley. Aust. J. Plant Physiol., 8, 475-495.
Baik B.K. & Ullrich S.E., 2008. Barley for food: characteristics, improvement, and renewed interest. J. Cereal Sci., 48, 233-242.
Balance G.M. & Manners D.J., 1978. Structural analysis and enzymic solubilization of barley endosperm cell walls. Carbohydr. Res., 61(1), 107-118.
Bamforth C.W., 2009. Current perspectives on the role of enzymes in brewing. J. Cereal Sci., 50(3), 353-357.
Banik M., Li C.D., Langridge P. & Fincher G.B., 1997. Structure, hormonal regulation, and chromosomal location of genes encoding barley (1-4)-β-xylan endohydrolase. Mol. Gen. Genet., 253, 599-608.
Borèn M., Larsson H., Falk A. & Jansson C., 2004. The barley starch granule proteome-internalized granule polypeptides of the mature endosperm. Plant Sci., 166, 617-626.
Boudart G. et al., 2007. Cell wall proteome. In: Samaj J. & Thelen J., eds. Plant Proteomics. Berlin, Deutschland: Springer, 169-184.
Bourne Y. & Henrissat B., 2001. Glycosyl hydrolases and glycosyltransferases: families and functional modules. Curr. Opin. Struct. Biol., 11, 593-600.
Brandt A., Svendsen I. & Hejgaard J., 1990. A plant serpin gene: structure, organization and expression of the gene encoding barley Z4. Eur. J. Biochem., 194, 499-505.
Briggs D.E., 1978. Barley. London: Chapman & Hall.
Burton R., Qi Z., Roulin S. & Fincher G.B., 1998. Gene structure and a possible cytoplasmic location for (1-.3)-b-glucanase isoenzyme GI from barley (Hordeum vulgare). Plant Sci., 135, 39-47.
Chandra G.S., Proudlove M.O. & Baxter E.D., 1999. The structure of barley endosperm: an important determinant of malt modification. J. Sci. Food Agric., 79, 37-46.
Clancy J.A., Han F. & Ullrich S.E., 2003. Comparative mapping of β-amylase activity QTLs among three barley crosses. Crop Sci., 43, 1043-1052.
Courtin C.M. & Delcour J.A., 2001. Relative activity of endoxylanase towards water-extractable and water-unextractable arabinoxylan. J. Cereal Sci., 33, 301-312.
de Sa R.M. & Palmer G.H., 2004. Assessment of enzymatic endosperm modification of malting barley using individual grain analysis. J. Ins. Brew., 110(1), 43-50.
Deymie B., 1984. Pratical problems posed by dormancy of malting barley. C.R. Séances Acad. Agric. Fr., 70(5), 699-707.
Echart-Almeida C. & Cavalli-Molina S., 2001. Hordein polypeptide patterns in relation to malting quality in Brazilian barley varieties. Pesqui. Agropecu. Bras., 36(2), 211-217.
Edney M.J., LaBerge D.E. & Langrell D.E., 1998. Relationships among the β-glucan content of barley, malt, malt congress extract, and beer. J. Am. Soc. Brew. Chem., 56(4), 164-168.
Egi A., Speers R.A. & Schwarz P.B., 2004. Arabinoxylans and their behaviour during malting and brewing. Tech. Q. Master Brew. Assoc. Am., 41(3), 248-267.
Ferre H., Broberg A., Duus J.O. & Thomsen K.K., 2000. A novel type of arabinoxylan arabinofuranohydrolase isolated from germinated barley. Eur. J. Biochem., 267, 6633-6641.
Fincher G.B., 1992. Cell wall metabolism in barley. In: Shewry P.R., ed. Barley: genetics, biochemistry, molecular biology and biotechnology. Wallingford, UK: CAB international, 413-437.
Finnie Ch. et al., 2006. Differential appearance of isoforms and cultivar variation in protein temporal profiles revealed in the maturing barley grain proteome. Plant Sci., 170, 808-821.
Gamlath J., Aldred G.P. & Panozzo J.F., 2008. Barley (1→3; 1→4)-β-glucan and arabinoxylan content are related to kernel hardness and water uptake. J. Cereal Sci., 47, 365-371.
Georg-Kraemer J.E. et al., 2004. The (1-3, 1-4)-β-glucanases in malting barley: enzyme survival and genetic and environmental effects. J. Ins. Brew., 110(4), 303-308.
Gianinetti A., Ferrari B., Frigeri P. & Stanca A.M., 2007. In vivo modeling of β-glucan degradation in contrasting barley (Hordeum vulgare L.) genotypes. J. Agric. Food. Chem., 55, 3158-3166.
Goesaert H. et al., 2004. Occurrence of proteinaceous endoxylanase inhibitors in cereals. Biochim. Biophys. Acta, 1696, 193-202.
Guler M., 2003. Barley grain β-glucan content as affected by nitrogen and irrigation. Field Crop Res., 84(3), 335-340.
Hayes P.M. et al., 2002. Genetic diversity for quantitatively inherited agronomic and malting quality traits. In: Von Bothmer R., Knuppfer H., van Hintum T. & Sato K., eds. Diversity barley. Amsterdam, The Netherlands: Elsevier Science Publisher, 201-226.
Holopainen U.R.M. et al., 2005. Endosperm structure affects the malting quality of barley (Hordeum vulgare L.). J. Agric. Food Chem., 53(18), 7279-7287.
Holtekjølen A.K. et al., 2006. Contents of starch and non-starch polysaccharides in barley varieties of different origin. Food Chem., 94, 348-358.
Hrmova M. & Fincher G.B., 1993. Purification and properties of three (1-3)-β-D-glucanase isoenzymes from young leaves of barley (Hordeum vulgare). Biochem. J., 289, 453-461.
Hrmova M. et al., 1996. Barley β-D-glucan exohydrolases with β-D-glucosidase activity. J. Biol. Chem., 271(9), 5277-5286.
Hrmova M. et al., 1997. Polysaccharides hydrolases in germinated barley and their role in the depolymerisation of plant and fungal cell walls. Int. J. Biol. Macromol., 21, 67-72.
Hrmova M. & Fincher G.B., 1998. Barley β-D-glucan exohydrolases. Substrate specificity and kinetic properties. Carbohydr. Res., 305, 209-221.
Hrmova M. et al., 1999. Cell wall degrading enzymes in barley. In: Second European symposium on enzymes in grain processing, Technical Research Centre of Finland (VTT), 1999, Helsinki, Finland, 63-74.
Hrmova M. & Fincher G.B., 2001. Structure-function relationships of β-D-glucan endo- and exohydrolases from higher plants. Plant Mol. Biol., 47, 73-91.
Humberstone F.J. & Briggs D.E., 2000a. Extraction and assay of acetic acid esterase from malted barley. J. Inst. Brew., 106, 31-37.
Humberstone F.J. & Briggs D.E., 2000b. Extraction and assay of ferulic acid esterase from malted barley. J. Inst. Brew., 106, 21-29.
Izydorczyk M.S. & MacGregor A.W., 2000. Evidence of intermolecular interactions of β-glucans and arabinoxylans. Carbohydr. Polym., 41, 417-420.
Izydorczyk M.S. & Dexter J.E., 2008. Barley β-glucans and arabinoxylans: molecular structure, physicochemical properties, and uses in food products: a review. Food. Res. Int., 41(9), 850-868.
Jin Y.L., Speers R.A., Paulson A.T. & Stewart R.J., 2004. Barley β-glucan and their degradation during malting and brewing. Tech. Q. Master Brew. Assoc. Am., 41(3), 231-240.
Johansson L., 2006. Structural analyses of (1→3),(1→4)-β-D-glucan of oats and barley. Academic dissertation: University of Helsinki, Department of Applied Chemistry and Microbiology, General Chemistry Division (Finland).
Kanauchi M. & Bamforth Ch.W., 2001. Release of β-glucan from cell walls of starchy endosperm of barley. Cereal Chem., 78, 121-124.
Kanauchi M. & Bamforth Ch.W., 2008. The relevance of different enzymes for the hydrolysis of β-glucans in malting and mashing. J. Ins. Brew., 114(3), 224-229.
Kuhbeck F. et al., 2005. Effect of masjing parameters on mash β-glucan, FAN and soluble extract levels. J. Ins. Brew., 111(3), 316-327.
Kuntz R.J. & Bamforth Ch.W., 2007. Time course for the development of enzymes in barley. J. Ins. Brew., 113(2), 196-205.
Lazaridou A., Chornick T., Biliaderis C.G. & Izydorczyk M.S., 2008. Composition and molecular structure of polysaccharides released from barley endosperm cell walls by sequential extraction with water, malt enzymes, and alkali. J. Cereal Sci., 48, 304-318.
Leah R., Kigel J., Svendsen I. & Mundy J., 1995. Biochemical and molecular characterization of a barley seed β-glucosidase. J. Biol. Chem., 270(26), 15789-15797.
Lee R.C., Hrmova M. & Fincher G.B., 1999. Biochemistry and molecular biology of arabinoxylan metabolism in germinated barley. In: Proceedings of the 9th Australian Barley Technical Symposium. Melbourne, Victoria ABTS, http://www.regional.org.au/au/abts/1999/lee.htm#TopOfPage, (05/01/2010).
Lee R.C. et al., 2003. Bifunctional family 3 glycoside hydrolases from barley with α-l-arabinofuranosidase and β-d-xylosidase activity. J. Biol. Chem., 278, 5377-5387.
Leubner-Metzger G., 2003. Function and regulation of β-1,3-glucanase during seed germination, dormancy release and after-ripening. Seed Sci. Res., 13(1), 17-34.
Li Y. et al., 2005. Studies on water-extractable arabinoxylans during malting and brewing. Food Chem., 93, 33-38.
Litts J.C. et al., 1990. The isolation and characterization of a barley 1,3-1,4-β-glucanase gene. Eur. J. Biochem., 194, 831-838.
Mantovani M.S. et al., 2008. β-glucan in promoting health: prevention against mutation and cancer. Mutat. Res., 658, 154-161.
Minic Z., 2008. Physiological roles of plant glycoside hydrolases. Planta, 227, 723-740.
Oscarsson M., Andersson R., Aman P. & Jonsson A., 1998. Effects of cultivar, nitrogen fertilization rate and environment on yield and grain quality of barley. J. Sci. Food Agric., 78, 359-366.
Perez-Vendell A.M. et al., 1996. Effects of cultivar and environment on β-(1,3)-(1,4)- D-glucan content and acid extract viscosity of Spanish barleys. J. Cereal Sci., 23, 285-292.
Prada D. et al., 2004. Genetic control of dormancy in a triumph/morex cross in barley. Theor. Appl. Genet., 109, 62-70.
Roberts T.H., Marttila S., Rasmussen S. & Hejgaard J., 2003. Differential gene expression for suicide-substrat serine proteinase inhibitors (serpins) in vegetative and grain tissues of barley. J. Exp. Bot., 54, 2251-2263.
Sadosky P., Schwarz P.B. & Horsley R.D., 2002. Effect of arabinoxylans, β-glucans, and dextrins on the viscosity and membrane filterability of a beer model solution. J. Am. Soc. Brew. Chem., 60(4), 153-162.
See D., Kanazin V., Kephart K. & Blake T., 2002. Mapping genes controlling variation in barley grain protein concentration. Crop Sci., 42, 680-685.
Slakeski N. & Fincher G.B., 1992. Developmental regulation of (1-3, 1-4)-β-glucanase gene expression in barley. Plant Physiol., 99, 1226-1231.
Sponsel V., 2006. Gibberellins: regulators of plant height, topic 20.1: structures of some important gibberellins, their precursors and derivatives, and inhibitors of gibberellin biosynthesis. In: Cruiziat P. & Richter H. Plant Physiology. Essay 4.2: the cohesion‑tension theory at work. 4th ed., http://www.plantphys.net, (02.09.09).
Sungurtas J., Swanston J.S., Davies H.V. & McDougall G.J., 2004. Xylan-degrading enzymes and arabinoxylan solubilisation in barley cultivars of differing malting quality. J. Cereal Sci., 39, 273-281.
Swanston J.S. & Ellis R.P., 2002. Genetics and breeding of malting quality attributes. In: Slafer G.A. et al., eds. Barley science: recent advances from molecular biology to agronomy of yield and quality. New York, USA: Food Product Press, 85-114.
Swanston J.S., Newton A.C., Hoad S.P. & Spoor W., 2006. Variation across environments in patterns of water uptake and endosperm modification in barley varieties and variety mixtures. J. Sci. Food Agric., 86(5), 826-833.
Van Campenhout S. & Volckaert G., 2005. Differential expression of endo-β-1,4-xylanase isoenzymes X-I and X-II at various stages throughout barley development. Plant Sci., 169(3), 512-522.
Van Campenhout S. et al., 2007. Unprocessed barley aleurone endo-β-1,4-xylanase X-I is an active enzyme. Biochem. Biophys. Res. Commun., 356(3), 799-804.
Van Lierde G., 2003. Cours élémentaire « Bière ». Bruxelles : Édition des Brasseurs Belges.
Wang J., Zhang G., Chen J. & Wu F., 2004. The change of β-glucan content and β-glucanase activity in barley before and after malting and their relationships to malt qualities. Food Chem., 86, 223-228.
Wang J.M. et al., 2007. Protein fractions in barley grains as affected by some agronomic factors and their relationships to malt quality. Cereal Res. Commun., 35(1), 129-140.
Wettstein D., Mikhaylenko G., Froseth J. & Kannangara C.G., 2000. Improved barley broiler feed with transgenic malt containing heat-stable (1,3–1,4)-b-glucanase. Proc. Natl. Acad. Sci. USA, 97(25), 13512-13517.
Wilhelmi Ch. & Morgan K., 2001. The hydrolysis of barley β-glucan by the cellulose EC 3.2.1.4 under dilute conditions is identical to that of barley solubilase. Carbohydr. Res., 330, 373-380.
Wilson T.A. et al., 2004. Reduce and high molecular weight barley β-glucans decrease plasma total and non-HDL-cholesterol in hypercholesterolemic Syrian golden hamsters. J. Nutr., 134, 2617-2622.
Wood P.J. et al., 2003. Structure of (1→3)(1→4)-β-D-glucan in waxy and non-waxy barley. Cereal Chem., 80, 329-332.
Yin X.S. & MacGregor A.W., 1989. Substrate specificity and nature of action of barley β-glucan solubilase. J. Ins. Brew., 95(2), 105-109.
Pour citer cet article
A propos de : Catherine Jamar
Univ. Liège - Gembloux Agro-Bio Tech. Unité de Biologie végétale. Passage des Déportés, 2. B-5030 Gembloux (Belgique).
A propos de : Patrick du Jardin
Univ. Liège - Gembloux Agro-Bio Tech. Unité de Biologie végétale. Passage des Déportés, 2. B-5030 Gembloux (Belgique).
A propos de : Marie-Laure Fauconnier
Univ. Liège - Gembloux Agro-Bio Tech. Unité de Biologie végétale. Passage des Déportés, 2. B-5030 Gembloux (Belgique). E-mail : marie-laure.fauconnier@ulg.ac.be






