- Startpagina tijdschrift
- Volume 14 (2010)
- numéro 1
- Recent insights into Protein Phosphatase 2A structure and regulation: the reasons why PP2A is no longer considered as a lazy passive housekeeping enzyme
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Recent insights into Protein Phosphatase 2A structure and regulation: the reasons why PP2A is no longer considered as a lazy passive housekeeping enzyme

Résumé
Nouvelles avancées dans la structure et la régulation de la Protéine Phosphatase 2A : les raisons pour lesquelles PP2A ne doit plus être considérée comme une enzyme passive et non spécifique. La phosphorylation réversible de protéines régulatrices intervient dans virtuellement tous les processus biologiques chez les organismes supérieurs. La Protéine Phosphatase 2A (PP2A) est une phosphatase très abondante composée d'un noyau dimérique contenant une sous-unité catalytique (C) et une sous-unité structurale (A), auquel est associé une sous-unité régulatrice (B) variable. Bien que considérée dans le passé comme une enzyme constitutive non spécifique, PP2A est une phosphatase soumise à une régulation précise et qui est importante dans le contrôle des fonctions cellulaires impliquant la phosphorylation. Cette régulation est principalement accomplie par l'identité de la sous-unité régulatrice qui détermine la spécificité de substrat, la localisation cellulaire et l'activité catalytique de l'holoenzyme PP2A. Les nouvelles avancées sur le sujet, particulièrement sur la structure et la régulation basée sur des modifications post-traductionnelles de PP2A, soulignent bien l'importance de la composition de l'holoenzyme PP2A dans les multiples rôles de cette enzyme majeure.
Abstract
Although intracellular signal transduction is often portrayed as a protein kinase "domino effect", the counterbalancing function of phosphatases, and thus the control of phosphatase activity, is equally relevant to proper regulation of cellular function. Protein Phosphatase 2A (PP2A) is a widely expressed family of protein phosphatases made of a core dimer, composed of a catalytic (C) subunit and a structural (A) subunit, in association with a third variable regulatory (B) subunit. Although viewed as a constitutive housekeeping enzyme in the past, PP2A is a highly regulated phosphatase and is emerging as an important regulator of multiple cellular processes involving protein phosphorylation. The regulation of PP2A is mainly accomplished by the identity of the regulatory B-type subunit, which determines substrate specificity, subcellular localization and catalytic activity of the PP2A holoenzyme. In agreement with this, recent findings on the structure and post-translational modifications of PP2A emphasize the importance of PP2A holoenzyme composition in its regulation and pleiotropic activities.
Inhoudstafel
1. Introduction
1Reversible protein phosphorylation is an important regulatory mechanism that controls the activities of a myriad of proteins and is thus involved in virtually every major physiological process. In the past, most of the attention was focused primarily on protein kinases and on their regulation, mainly because phosphatases were then viewed as simple housekeeping enzymes. But advances in the understanding of protein phosphatases make now clear that these enzymes are precisely regulated and are as important as kinases in the regulation of cellular processes involving protein phosphorylation.
2Protein phosphatase 2A (PP2A) is a very abundant – it accounts for as much as 1% of total cellular proteins – ubiquitous and remarkably conserved enzyme. A large and still-growing number of PP2A substrates have been identified, which makes PP2A an important player in the regulation of a plethora of cellular processes.
3This article will review the recent advances in the structure and regulation of this fascinating enzyme.
2. Classification
4While proteins can be phosphorylated on nine amino acids, serine, threonine and tyrosine phosphorylation are by far the most predominant in eukaryotic cells. The enzymes that dephosphorylate these three amino acids are classified into four groups on the basis of specific catalytic signatures/domain sequences and substrate preference. Among the 150 individual members of the protein phosphatases superfamily, more than two thirds belong to the protein tyrosine phosphatase family (PTP), which dephosphorylates phosphotyrosine and, in some cases also phosphoserine and phosphothreonine. The majority of the remaining enzymes are specific for phosphorylated serine and threonine residues and are originally divided into two families (Cohen, 2002; Moorhead et al., 2009): the phosphoprotein phosphatases (PPP) and the Mg2+ or Mn2+-dependent protein phosphatases (PPM). Recently, the family of aspartate-based phosphatases was added to this classification. This group consists of serine and tyrosine-phosphatases with an aspartic acid signature (DXDXT/V) driving catalysis and includes the FCP/SCP [TFIIF (transcription initiation factor II)-associating C-terminal domain Phosphatase/small CTD Phosphatase] and HAD (haloacid dehalogenase) family of enzymes (Moorhead et al., 2009).
5The serine-threonine phosphatases share the common property of relying on the nucleophilic attack of the phosphorus atom by a metal-activated water molecule for their catalytic mechanism (Barford, 1996). The PPM family of phosphatases is mainly represented by the protein phosphatase type 2C (PP2C) whereas the PPP family is most diverse and contains 5 subfamilies. The PPP1 subfamily includes PP1 and the PPP2/4/6 subfamily comprises PP2A, PP4 and PP6. The PPP3 subfamily contains the Ca2+-activated PP2B. Two other minor families exist termed PPP5 and PPP7 which respectively comprise PP5 and PP7.
3. Structure of PP2A
6The native forms of PP2A holoenzymes are predominantly heterotrimers in which a core dimer, PP2AD, made of a structural A subunit (also known as PR65) and a catalytic C subunit, PP2AC, is associated with a third variable regulatory B-type subunit. In addition to the classical PP2A heterotrimer, studies demonstrated that independent PP2AD core dimers are found within cells (Kremmer et al., 1997; Janssens et al., 2001). In addition, some specific PP2A dimers, in which the PR65/A subunit is replaced by the α4 protein have been recently identified (Yang et al., 2007).
7The mammalian catalytic C subunit has two isoforms (α and β) which are 97% identical, ubiquitously expressed, highly conserved. While PP2ACα and PP2ACβ seem to be interchangeable in vitro (Zhou et al., 2003), studies in mice suggested that both isoforms are not functionally redundant in vivo (Gotz et al., 1998; 2003). Within the PP2A holoenzyme, the PR65/A subunit functions as a scaffold for the recruitment of the C and B-type subunits as well as additional proteins. The structural PR65/A subunit also exists in two isoforms, α and β, which are widely expressed and 86% identical in primary sequence (Hemmings et al., 1990). Interestingly, each PR65/A isoform shows differential ability to interact with B-type and C subunits (Zhou et al., 2003).
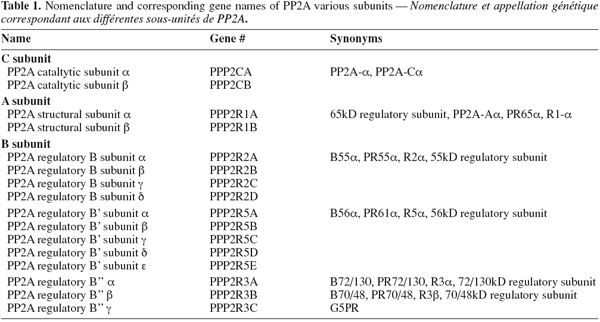
8By far, the most variable subunit of the PP2A holoenzyme is the B-type subunit. To date, about 20 different isoforms have been described that are encoded by distinct genes or result from alternative splicing of a single gene. The mammalian B-type subunits are classified into three subfamilies, called PR55/B, PR61/B′ and PR72/B″ (Table 1). While the PR55/B and PR61/B' families are quite evolutionary conserved, the PR72/B'' family consists of a less evolutionary conserved group of proteins, with some human gene products having no murine orthologue and vice versa (Zwaenepoel et al., 2008). Table 1 shows the nomenclature for human PR72/B'' genes. Each B-type subunit can potentially combine with any of the two isoforms of both the A and C subunits, generating over 75 potential trimeric PP2A holoenzymes (Janssens et al., 2001; 2008). This multiple combinatorial association is central to the mechanisms that regulate PP2A activity and ensure the pleiotropic roles of this important enzyme (Ruediger et al., 1992; Li et al., 2002).
9The structure of the PP2A holoenzyme has long remained elusive. The first structural information came from the isolated PR65/A scaffolding subunit, which consists entirely of 15 tandemly repeated motifs known as HEAT (huntingtin-elongation-A subunit of PP2A -TOR). Canonical HEAT motifs consist of two helixes which form a helical hairpin. In PR65/A, 15 HEAT motifs stack together to form an elongated, horseshoe-shaped molecule with a continuous hydrophobic core (Walter et al., 1989; Hemmings et al., 1990; Groves et al., 1999). However, it was more than 15 years later that the crystal structures of a PP2AD and a PP2AT61γ1 holoenzymes were solved (Xing et al., 2006; Xu et al., 2006; Cho et al., 2007b). The structural analysis of the PP2A core dimer showed that the catalytic subunit contains two catalytic metal ions at the active site and adopts a globular structure with an α/β fold, typical of the serine/threonine phosphoprotein phosphatase (PPP) family of phosphatases (Barford, 1996; Xing et al., 2006). Consistent with previous mutagenesis studies (Ruediger et al., 1992), structural data also revealed that the scaffolding subunit binds to the catalytic subunit via the intra-repeat loops of one end of its HEAT-repeats. Interestingly, these studies also pointed to a remarkable conformational flexibility of the PR65/A subunit, which undergoes pronounced conformational changes when incorporated into the PP2A core enzyme.
10The crystal structure of a trimeric PP2A holoenzyme containing a regulatory PR61/B'γ subunit was reported independently by two laboratories (Xu et al., 2006; Cho et al., 2007b). These studies revealed that, despite lacking canonical HEAT motifs, the PR61/B′γ subunit harbors a superhelical structure similar to that of PR65/A, with an apparent curvature that forms HEAT-like repeat motifs. In the PP2AT61γ trimer, the horseshoe-shaped PR65/A subunit undergoes additional conformational rearrangements, which brings the amino and carboxyl termini in close proximity. The C subunit and the convex side of the PR61/B' pseudo HEAT bind to the intra-repeat loops of HEAT repeats 2-7 and 11-15 respectively of the scaffold PR65/A subunit. PR61/B′γ also makes extensive discrete contacts with the C subunit by itself. In particular, the C-terminal tail of the C subunit docks on the interface of the PR65/A and B-type subunits, where it could regulate the recruitment of the B-type subunit.
11Crystal structure analysis gave valuable insights on how B-type subunits could regulate PP2A substrate specificities. Indeed, while the active site pocket of the PP2A catalytic subunit appears accessible to substrate, the binding of the PR61/B' subunit in the holoenzyme markedly changes the physicochemical environment near the active site and limits the accessible surface to the active site and provides novel potential substrate binding surfaces.
12Crystallisation data also provided structural basis for PP2A regulation by post-translational modifications of the catalytic subunit. Methylation of the C-terminus of PP2AC selectively affects the assembly of PP2A trimers in vivo (see below). Nevertheless, in vitro holoenzyme formation is independent of PP2AC methylation since a C-terminal truncated mutant or an unmethylated catalytic subunit can still stably form a PP2AT55 or PP2AT61 trimeric complex (Xu et al., 2006; Ikehara et al., 2007). In addition to methylation, tyrosine phosphorylation is another modification of PP2AC C-terminal tail that regulates PP2A activity. Structural data indicate that a hydrogen bond forms between the side chain of the targeted Tyr307 residue and a carbonyl group in the peptide backbone of PR61/B′. Tyrosine phosphorylation would therefore be detrimental to the assembly of PP2A holoenzyme containing a PR61/B' subunit. Direct interaction between phosphorylated tyrosine and the active site within the catalytic subunit could also explain why tyrosine phosphorylation of PP2AC inhibits PP2A activity (Cho et al., 2007b).
13More recently, a study reported the crystal structure of a PP2A holoenzyme containing another family of regulatory subunit: the PR55/Bα family member (Xu et al., 2008). The sequence similarity between the various subfamilies of the regulatory subunits is very low, and in agreement with this, the structure of PR55/Bα subunit differs from the PR61/B' helical structure. Instead, the PR55/Bα subunit forms a seven-bladed β propeller, with each blade comprising four antiparallel β strands. In addition to the propeller core, PR55/Bα also contains additional secondary structure elements located above the top face which contribute to the formation of a putative substrate-binding groove in close proximity to the active site of the C subunit of PP2A. As observed for PR61/B', the regulatory PR55/Bα subunit recognizes the amino-terminal HEAT repeats of the PR65/A subunit. In contrast, PR55/Bα makes few interactions with the catalytic subunit, compared to the PR61/B' subunit, which leads to a relatively loose holoenzyme. The structural observations further suggest that the PR55/Bα subunit may form a relatively stable complex with the isolated A subunit, but do not seem to support the notion that the C subunit is required for interaction between the PR65/A and B-type subunits. Due to the distinct structure of the PR55/B and PR61/B' structural subunits, the conformation of the scaffold PR65/A subunit is different in the PP2AT55 holoenzyme compared to the PP2AT61. The intrinsic conformational plasticity of the PR65/A subunit might therefore be important in order to interact with structurally different regulatory subunits (Xu et al., 2008). Indeed, the third regulatory subunit family PR72/B″ is predicted to adopt yet a different structure and contains two calcium binding EF hands (Janssens et al., 2003).
14The recent characterizations of the structures of the PP2A holoenzyme are of prime interest because they constitute a new basis to improve the understanding of some aspects of PP2A assembly, function and regulation.
4. Regulation
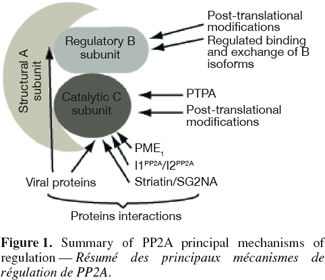
15PP2A has been historically regarded as a relatively non-specific and unregulated enzyme. This allegation is in direct contradiction with the discrepancy that exists between the relatively small number of Ser/Thr phosphatases and the plethora of proteins that are reversibly phosphorylated on serine or threonine residues. It is now clear that PP2A, and the other protein phosphatases, are subjected to finely tuned control mechanisms that allow cells to adequately orchestrate changes in protein phosphorylation during virtually every cellular process (Figure 1).
4.1. Holoenzyme composition
16The composition of the holoenzyme is the most impacting determinant in the regulation of PP2A pleiotropic functions. It is now well recognized that the identity of the variable B-type subunit incorporated in the holoenzyme has specific consequences on PP2A activity. In accordance with structural data, binding of specific B-type subunit modulates the catalytic activity of PP2A in vitro (Sontag, 2001) and probably in vivo. In addition, the nature of the B-type subunit also influences substrate selectivity (Imaoka et al., 1983; Agostinis et al., 1987; 1990; 1992; Mumby et al., 1987; Cegielska et al., 1994; Mayer-Jaekel et al., 1994a; Sontag et al., 1996). Lastly, subunit composition impacts PP2A localization within the cell by targeting the phosphatase to specific subcellular compartments (Sontag, 2001).
17Despite the lack of definite experimental evidence, recent data have lead to the model that the composition of the PP2A holoenzyme is not static in vivo and interconversions by dynamic exchange of regulatory subunits may represent a mechanism by which cells can quickly adapt to cellular demand at a given time. The observation that various viral proteins can replace specific regulatory subunits within PP2A holoenzyme in vivo provides a proof-of-principle that exchange between PP2A subunits is possible. In addition, B-type subunits can compete for binding to the PP2AD core complex in vitro (Kamibayashi et al., 1994) and suggest that the same phenomenon occurs within the cell. As detailed below, PP2A is subjected to diverse post-translational modifications which can have various impacts on B-type subunit binding. In this context, regulated specific post-translational modifications represent an attractive mechanism for controlling PP2A B-type subunit exchange. Alone or in combination, these post-translational modifications may constitute a "PP2A code" that dictates the formation of a specific holoenzyme or promotes the exchange between two subunits (Janssens et al., 2008).
4.2. Binding partners
18The number of proteins interacting with PP2A is large and still-growing. These proteins can interact with one or more subunits and sometimes associate with a specific PP2A holoenzyme. PP2A partners play critical roles in its function and regulation. For instance, some interactors have been shown to target PP2A to specific cellular domains and regulatory functions (Sontag et al., 1995; 1999; Kawabe et al., 1997; Takahashi et al., 1999; Turowski et al., 1999; Voorhoeve et al., 1999; Ito et al., 2000; Yan et al., 2000). Moreover, PP2A is part of multi-molecular signalling complexes through its binding to specific kinases (Westphal et al., 1998; 1999; Lebrin et al., 1999) or scaffolding proteins (Kikuchi, 1999).
19Among the vast array of PP2A partners are multiple viral proteins, such as the polyoma small t and middle T, as well as with the small DNA tumour viruses simian virus 40 small t (SV40 ST) (Janssens et al., 2001; Arroyo et al., 2005; Janssens et al., 2005). By directly binding to PP2A these viral antigens inhibit its phosphatase activity (Scheidtmann et al., 1991; Yang et al., 1991; Cayla et al., 1993; Kamibayashi et al., 1994) and/or displace the B-type subunit from the holoenzyme (Pallas et al., 1990; Mumby et al., 1991; Chen et al., 2004). This impairs the prevailing cellular functions of PP2A and might explain the transforming activities of these viral proteins.
20Structure of the SV40 ST/PP2A complex was recently solved and provides the basis to explain PP2A inhibition by viral proteins. One domain of SV40 ST is in a position to directly interact with the PP2A catalytic C subunit, near its active site. Therefore, it is likely that binding of SV40 ST alters PP2A phosphatase activity through direct competition with substrate for access to the catalytic site. In addition, two distinct SV40 ST domains interact with a specific region of the structural PR65/A subunit that is also recognized by PR55/B and PR61/B'. This observation could explain the competition that exists between SV40 ST and regulatory subunits for binding to the core enzyme (Chen et al., 2007; Cho et al., 2007a). However, SV40 ST has surprising little affinity for PP2A and does not efficiently displace PR55/B, PR61/B' or PR72/B'' from their respective holoenzymes in vitro (Chen et al., 2007; Cho et al., 2007a). It is thus likely that modulation of PP2A holoenzyme assembly through displacement of structural subunits is only a minor contributor in the inhibition of PP2A activity by SV40 ST.
21The PP2AD core dimer also forms stable complexes with two calmodulin-binding scaffolding proteins, Striatin and the S/G2 nuclear autoantigen (SG2NA), which suggests that species of PP2A could be recruited to Ca2+-dependent signal transduction cascades (Moreno et al., 2000). Striatin and SG2NA share some homology with PR61/B' isoforms and have sometimes been considered as a fourth regulatory subunit family. These PP2A interactors illustrate the fact that the distinction between a bona fide regulatory subunit and a binding partner is sometimes difficult. A proposition would be to consider a protein as a regulatory subunit only if it contains the canonincal PR65/A subunit-binding domain conserved in the existing regulatory subunits (Janssens et al., 2008).
22Two intracellular heat stable inhibitors of PP2A, named I1PP2A/Phap and I2PP2A/SET have been identified. Both proteins inhibit specifically all holoenzyme forms of PP2A, probably by binding to the catalytic subunit (Li et al., 1996a; 1996b).
4.3. Post-translational modifications
23The catalytic subunit of the phosphoprotein phosphatase (PPP) family members is very conserved both in sequence and structure. The most distinctive feature of this subunit consists in a unique C-terminal tail which extends away from the globular structure and is crucially located at the interface between the two other subunits (Xing et al., 2006; Xu et al., 2006; Cho et al., 2007b). Consistent with an important functional role for this domain, a highly conserved Thr304-Pro-Asp-Tyr-Phe-Leu309 motif is heavily post-translationally modified by methylation, tyrosine and threonine phosphorylation. These modifications are crucial for PP2A regulation and holoenzyme formation.
24Leu309 residue is subjected to carboxymethylation by the S-adenosylmethionine-dependent LCMT1 (leucine carboxyl methyltransferase 1) (Lee et al., 1993; De Baere et al., 1999). The reverse demethylation is achieved through the action of a specific phosphatase methylesterase, PME-1 (Lee et al., 1996). Carboxymethylation of PP2AC has been directly implicated in the regulation of PP2A holoenzyme assembly. Indeed, several studies have shown that methylation enhances the affinity of the PP2A core enzyme for some but not all regulatory subunits. More specifically, C-terminal PP2AC methylation seems to selectively affect the assembly of PP2A trimers containing a PR55/B subunit (Ogris et al., 1997; Bryant et al., 1999; Tolstykh et al., 2000; Wu et al., 2000; Wei et al., 2001; Yu et al., 2001; Gentry et al., 2005; Longin et al., 2007; Nunbhakdi-Craig et al., 2007). In contrast, methylation of the C subunit seems to have little impact on the recruitment of other regulatory subunits (Wei et al., 2001; Gentry et al., 2005; Longin et al., 2007; Nunbhakdi-Craig et al., 2007). One indication of this selectivity relies on the observation that PP2AT61 and PP2AT72 can recruit a mixture of methylated and demethylated PP2AC, whereas PP2AT55 exclusively associates with methylated PP2AC. Recent insights on PP2A structure suggest a plausible mechanism for how methylation could affect PP2A holoenzyme assembly. Indeed, crystal structure of a PP2AT61 heterotrimeric PP2A holoenzyme has shown that the C-terminal PP2AC residue Leu309 does not mediate direct contact with the Aα or PR61/B′γ1 subunits but is located in a highly negatively charged environment formed by the side chains of Glu62, Asp63, Glu64 and Glu101 of the PR65/A subunit (Cho et al., 2007b). Although methylation is not strictly required for PP2AT61γ1 assembly, neutralization of the PP2AC C-terminal negative charge by carboxymethylation would promote docking of the tail in this area and, therefore, binding of PR61/B′γ1 to PP2AD. Methylation of the catalytic subunit is thus a crucial determinant in PP2A holoenzyme composition and could participate in the regulation of its diverse functions in vivo. Surprisingly, several studies argue that methylation of the C subunit is not required for the in vitro assembly of PP2A holoenzymes involving the PR55/B and PR61/B′ regulatory subunits (Xu et al., 2006; Ikehara et al., 2007). It is important to note that most of in vitro studies use an inactive form of PP2AC harboring a mutation which is known to alter the affinity of PP2AC for interacting partners (Janssens et al., 2008). Nonetheless, methylation may facilitate the assembly of the holoenzyme through enhanced binding affinity between the PP2A core enzyme and the regulatory subunit, this slight advantage being sufficient to tip the balance for holoenzyme assembly in cells but not in vitro. PP2AC carboxy-methylation in cells could also promote assembly of PP2A holoenzymes by recruiting assembly factors or by targeting the catalytic subunit to a specific cellular compartment where the assembly takes place.
25Due to its importance in PP2A selective composition, regulation of PP2AC carboxy-methylation attracted a lot of attention these past few years. Methylation of PP2AC changes during cell cycle, suggesting a critical role in cell-cycle regulation (Janssens et al., 2001; Lee et al., 2007). In addition, differences in subcellular localizations of LCMT1 and PME-1 suggest that methylation and demethylation might be spatially controlled (Longin et al., 2008). Interestingly, structure of PME-1 in complex with PP2A reveals that PME-1 directly binds to the active site of PP2AC, what is supposed to lead to the eviction of the metal ions required for the catalytic activity of PP2A (Longin et al., 2004; Xing et al., 2008). These findings indicate that, in addition to removing the methyl group from Leu309, PME-1 could directly control the phosphatase activity of PP2A. The interaction also results in the activation of PME-1 by structural rearrangement, which ensures the specificity of the methylesterase activity towards PP2A (Xing et al., 2008).
26In addition to methylation at Leu309, the PP2AC tail is also subjected to phosphorylation on Tyr307 and possibly on Thr304. Tyr307 phosphorylation seems to have two striking consequences. First, it could inhibit the interaction of PP2AC with PR61/B' (Longin et al., 2007; Nunbhakdi-Craig et al., 2007) by annihilating an hydrogen bound between Tyr307 of the catalytic subunit and the carbonyl group of Val257 in the peptide backbone of the PR61/B′γ1 subunit (Cho et al., 2007b). On the other hand, Tyr307 could indirectly affect the assembly of the PP2A holoenzyme containing PR55/B by preventing methylation of Leu309. Indeed, it has been suggested that Tyr307 phosphorylation might impair access to the LCMT1 cavity (Ogris et al., 1997; Yu et al., 2001; Longin et al., 2007; Nunbhakdi-Craig et al., 2007).
27It should be emphasized that the above observations result from mutagenesis analysis and need to be physiologically confirmed. Mutagenesis studies have also pointed out a role for threonine phosphorylation in B-type subunit selection. Phosphorylation of Thr304 induces the selective inhibition of PR55/B subunit recruitment (Ogris et al., 1997; Wei et al., 2001; Gentry et al., 2005; Longin et al., 2007; Nunbhakdi-Craig et al., 2007) without affecting Leu309 methylation (Yu et al., 2001; Longin et al., 2007).
28Evidence suggests that regulatory subunits, and in particular PR61/B' could also be subjected to phosphorylation. Phosphorylation of PR61/B′ could have opposing effects depending on the physiological context. While phosphorylation of a conserved Ser/Pro motif by extracellular signal-regulated kinase ERK would promote dissociation of PR61/B′ from the catalytic subunit (Letourneux et al., 2006; Cho et al., 2007b), phosphorylation of Ser37 by Chk1 enhances holoenzyme formation (Margolis et al., 2006).
29It is now well-admitted that post-translational modifications of PP2A subunits have important roles in various aspects of holoenzyme regulation. Particularly, each B-type subunit is associated with a combination of specific post-translational modifications on PP2AC. Leu309 methylation specifically favors formation of PR55/B subunit-containing PP2A holoenzyme. In contrast, Tyr307 phosphorylation is defavourable to association with PR55/B and PR61/B' and Thr304 selectively inhibits incorporation of PR55/B. This has lead to the notion of a "PP2A code" on the C-terminal tail that dictates the formation of specific PP2A holoenzymes (Janssens et al., 2008).
4.4. Substrate specificity
30The reversible protein phosphorylation on proline-directed Ser/Thr motifs (Ser/Thr-Pro) is a key regulatory mechanism for the control of various cellular processes. Pro can exist in two conformations, cis and trans, in this motif. PP2A is considered as a major Pro-directed phosphatase which dephosphorylates phospho-Ser/Thr-Pro substrates. Studies of several PP2A substrates including Tau, Cdc25C, Myc and Raf1 substrates have lead to the hypothesis that a trans configuration of the proline residue adjacent to the phosphorylated residue is more favorable to dephosphorylation by PP2A. Pin1 is a peptidyl-prolyl isomerase (PPIase) which catalyses cis-to-trans isomerisation of specific pSer/Thr-Pro motifs. Studies have suggested that isomerisation of the Ser-Pro bound by Pin1 would be required to promote dephosphorylation of substrates by PP2A (Zhou et al., 2000; Stukenberg et al., 2001; Yeh et al., 2004; Dougherty et al., 2005). Nevertheless, some observation that specific PP2A holoenzyme (especially PP2AT55) could dephosphorylate these motifs without Pin1 requirement are not compatible with this model (Agostinis et al., 1992; Mayer-Jaekel et al., 1994b).
31On the other hand, PP2A itself seems to subjected to proline isomerization. Indeed, PTPA (phosphotyrosyl phosphatase activator, newly renamed phosphatase two A phosphatase activator) can activate the classical Ser-Thr phosphatase activity of a native inactive PP2A form (Longin et al., 2004) through an isomerase activity. Isomerization induces a conformational change in PP2A which correlates with its activation (Jordens et al., 2006; Leulliot et al., 2006).
5. Conclusion
32Genetic deletion of PP2A catalytic subunit is lethal in yeast (Sneddon et al., 1990), demonstrating the prevailing place of PP2A in homeostasis. In accordance with this and early observations (Bialojan et al., 1988), dysregulation of PP2A-regulated signalling pathways can contribute to cancer (Arroyo et al., 2005; Janssens et al., 2005; Eichhorn et al., 2009). Initial understanding of PP2A as a tumor suppressor was mainly based on the tumor-promoting activities of okadaic acid, the most famous naturally occurring PP2A inhibitor. But this loss of function approach does not discriminate between specific holoenzyme contribution and it now appears that the description of PP2A as a tumor suppressor is oversymplistic and needs more investigation (Eichhorn et al., 2009). Moreover, studies employing general inhibitory strategy, like okadaic acid, have pointed to a role for PP2A in multiple pathologies besides cancer. In this context, in order to improve our knowledge of this clinically relevant target protein, it seems important to dissect PP2A-controlled signalling pathways and, to achieve this, to precisely delineate specific cellular functions and context of each holoenzyme. Further studies on the precise role of individual PP2A B-type regulatory subunits within these signalling cascades is thus a challenging question for the future.
33Acknowledgement
34This work was supported by the Belgian National Fund for Scientific Research (FNRS) and the Interuniversity Attraction Poles Programme, Belgian Science Policy (PAI6/28). Franck Dequiedt is Research Associate, Maud Martin is Research Fellow and Richard Kettmann is Research Director of the FNRS.
Bibliographie
Agostinis P. et al., 1987. Dephosphorylation of phosphoproteins and synthetic phosphopeptides. Study of the specificity of the polycation-stimulated and MgATP-dependent phosphorylase phosphatases. J. Biol. Chem., 262(3), 1060-1064.
Agostinis P. et al., 1990. Synthetic peptides as model substrates for the study of the specificity of the polycation-stimulated protein phosphatases. Eur. J. Biochem., 189(2), 235-241.
Agostinis P. et al., 1992. Specificity of the polycation-stimulated (type-2A) and ATP,Mg-dependent (type-1) protein phosphatases toward substrates phosphorylated by P34cdc2 kinase. Eur. J. Biochem., 205(1), 241-248.
Arroyo J.D. & Hahn W.C., 2005. Involvement of PP2A in viral and cellular transformation. Oncogene, 24(52), 7746-7755.
Barford D., 1996. Molecular mechanisms of the protein serine/threonine phosphatases. Trends Biochem. Sci., 21(11), 407-412.
Bialojan C. & Takai A., 1988. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem. J., 256(1), 283-290.
Bryant J.C., Westphal R.S. & Wadzinski B.E., 1999. Methylated C-terminal leucine residue of PP2A catalytic subunit is important for binding of regulatory Balpha subunit. Biochem. J., 339(Pt 2), 241-246.
Cayla X.K., Ballmer-Hofer K., Merlevede W. & Goris J., 1993. Phosphatase 2A associated with polyomavirus small-T or middle-T antigen is an okadaic acid-sensitive tyrosyl phosphatase. Eur. J. Biochem., 214(1), 281-286.
Cegielska A. et al., 1994. Different oligomeric forms of protein phosphatase 2A activate and inhibit simian virus 40 DNA replication. Mol. Cell. Biol., 14(7), 4616-4623.
Chen W. et al., 2004. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell, 5(2), 127-136.
Chen Y. et al., 2007. Structural and biochemical insights into the regulation of protein phosphatase 2A by small t antigen of SV40. Nat. Struct. Mol. Biol., 14(6), 527-534.
Cho U.S. et al., 2007a. Structural basis of PP2A inhibition by small t antigen. PLoS Biol., 5(8), e202.
Cho U.S. & Xu W., 2007b. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature, 445(7123), 53-57.
Cohen P.T.W., 2002. Protein phosphatase 1 - targeted in many directions. J. Cell Sci., 115, 241-256.
De Baere et al., 1999. Purification of porcine brain protein phosphatase 2A leucine carboxyl methyltransferase and cloning of the human homologue. Biochemistry, 38(50), 16539-16547.
Dougherty M.K. et al., 2005. Regulation of Raf-1 by direct feedback phosphorylation. Mol. Cell, 17(2), 215-224.
Eichhorn P.J., Creyghton M.P. & Bernards R., 2009. Protein phosphatase 2A regulatory subunits and cancer. Biochim. Biophys. Acta, 1795(1), 1-15.
Gentry M.S. et al., 2005. A novel assay for protein phosphatase 2A (PP2A) complexes in vivo reveals differential effects of covalent modifications on different Saccharomyces cerevisiae PP2A heterotrimers. Eukaryotic Cell, 4(6), 1029-1040.
Gotz J. & Schild A., 2003. Transgenic and knockout models of PP2A. Methods Enzymol., 366, 390-403.
Gotz J.R. et al., 1998. Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit C. Proc. Natl Acad. Sci. USA, 95(21), 12370-12375.
Groves M.R. et al., 1999. The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of Its 15 tandemly repeated HEAT Motifs. Cell, 96(1), 99-110.
Hemmings B.A. et al., 1990. Alpha- and beta-forms of the 65-kDa subunit of protein phosphatase 2A have a similar 39 amino acid repeating structure. Biochemistry, 29(13), 3166-3173.
Ikehara T. et al., 2007. Methylation of the C-terminal leucine residue of the PP2A catalytic subunit is unnecessary for the catalytic activity and the binding of regulatory subunit (PR55/B). Biochem. Biophys. Res. Commun., 354(4), 1052-1057.
Imaoka T. et al., 1983. Resolution and reassociation of three distinct components from pig heart phosphoprotein phosphatase. J. Biol. Chem., 258(3), 1526-1535.
Ito A. et al., 2000. A truncated isoform of the PP2A B56 subunit promotes cell motility through paxillin phosphorylation. Embo J., 19(4), 562-571.
Janssens V. & Goris J., 2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J., 353(Pt 3), 417-439.
Janssens V. et al., 2003. Identification and functional analysis of two Ca2+-binding EF-hand motifs in the B/PR72 subunit of protein phosphatase 2A. J. Biol. Chem., 278(12), 10697-10706.
Janssens V., Goris J. & Van Hoof C., 2005. PP2A: the expected tumor suppressor. Curr. Opin. Genet. Dev., 15(1), 34-41.
Janssens V., Longin S. & Goris J., 2008. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail). Trends Biochem. Sci., 33(3), 113-121.
Jordens J. et al., 2006. The protein phosphatase 2A phosphatase activator is a novel peptidyl-prolyl cis/trans-isomerase. J. Biol. Chem., 281(10), 6349-6357.
Kamibayashi C. et al., 1994. Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J. Biol. Chem., 269(31), 20139-20148.
Kawabe T., Muslin A.J. & Korsmeyer S.J., 1997. HOX11 interacts with protein phosphatases PP2A and PP1 and disrupts a G2/M cell-cycle checkpoint. Nature, 385(6615), 454-458.
Kikuchi A., 1999. Roles of axin in the wnt signalling pathway. Cell. Signalling, 11(11), 777-788.
Kremmer E.K. et al., 1997. Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: abundant expression of both forms in cells. Mol. Cell. Biol., 17(3), 1692-1701.
Lebrin F. et al., 1999. CK2alpha-protein phosphatase 2A molecular complex: possible interaction with the MAP kinase pathway. Mol. Cell. Biochem., 191(1-2), 207-212.
Lee J. & Stock J., 1993. Protein phosphatase 2A catalytic subunit is methyl-esterified at its carboxyl terminus by a novel methyltransferase. J. Biol. Chem., 268(26), 19192-19195.
Lee J., Chen Y., Tolstykh T. & Stock J., 1996. A specific protein carboxyl methylesterase that demethylates phosphoprotein phosphatase 2A in bovine brain. Proc. Natl Acad. Sci. USA, 93(12), 6043-6047.
Lee J.A. & Pallas D.C., 2007. Leucine carboxyl methyltransferase-1 is necessary for normal progression through mitosis in mammalian cells. J. Biol. Chem., 282(42), 30974-30984.
Letourneux C., Rocher G. & Porteu F., 2006. B56-containing PP2A dephosphorylate ERK and their activity is controlled by the early gene IEX-1 and ERK. Embo J., 25(4), 727-738.
Leulliot N. et al., 2006. Crystal structure of the PP2A phosphatase activator: implications for its PP2A-specific PPIase activity. Mol. Cell, 23(3), 413-424.
Li M., Makkinje A. & Damuni Z., 1996a. Molecular identification of I1PP2A, a novel potent heat-stable inhibitor protein of protein phosphatase 2A. Biochemistry, 35(22), 6998-7002.
Li M., Makkinje A. & Damuni Z., 1996b. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J. Biol. Chem., 271(19), 11059-11062.
Li X. & Virshup D.M., 2002. Two conserved domains in regulatory B subunits mediate binding to the A subunit of protein phosphatase 2A. Eur. J. Biochem., 269(2), 546-552.
Longin S. et al., 2004. An inactive protein phosphatase 2A population is associated with methylesterase and can be re-activated by the phosphotyrosyl phosphatase activator. Biochem. J., 380(Pt 1), 111-119.
Longin S. et al., 2007. Selection of protein phosphatase 2A regulatory subunits is mediated by the C terminus of the catalytic subunit. J. Biol. Chem., 282(37), 26971-26980.
Longin S. et al., 2008. Spatial control of protein phosphatase 2A (de)methylation. Exp. Cell Res., 314(1), 68-81.
Margolis S.S., Perry J.A. & Forester C.M., 2006. Role for the PP2A/B56[delta] phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell, 127(4), 759-773.
Mayer-Jaekel R.E. & Hemmings B.A., 1994a. Protein phosphatase 2A -- a "ménage à trois". Trends Cell Biol., 4(8), 287-291.
Mayer-Jaekel R.E. et al., 1994b. Drosophila mutants in the 55 kDa regulatory subunit of protein phosphatase 2A show strongly reduced ability to dephosphorylate substrates of p34cdc2. J. Cell Sci., 107(Pt 9), 2609-2616.
Moorhead G.B., De Wever V., Templeton G. & Kerk D., 2009. Evolution of protein phosphatases in plants and animals. Biochem. J., 417(2), 401-409.
Moreno C.S. et al., 2000. WD40 repeat proteins striatin and S/G(2) nuclear autoantigen are members of a novel family of calmodulin-binding proteins that associate with protein phosphatase 2A. J. Biol. Chem., 275(8), 5257-5263.
Mumby M.C., Russell K.L., Garrard L.J. & Green D.D., 1987. Cardiac contractile protein phosphatases. Purification of two enzyme forms and their characterization with subunit-specific antibodies. J. Biol. Chem., 262(13), 6257-6265.
Mumby M.C. & Walter G., 1991. Protein phosphatases and DNA tumor viruses: transformation through the back door? Cell Regul., 2(8), 589-598.
Nunbhakdi-Craig V. et al., 2007. Expression of protein phosphatase 2A mutants and silencing of the regulatory B alpha subunit induce a selective loss of acetylated and detyrosinated microtubules. J. Neurochem., 101(4), 959-971.
Ogris E., Gibson D.M. & Pallas D.C., 1997. Protein phosphatase 2A subunit assembly: the catalytic subunit carboxy terminus is important for binding cellular B subunit but not polyomavirus middle tumor antigen. Oncogene, 15(8), 911-917.
Pallas D.C. et al., 1990. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell, 60(1), 167-176.
Ruediger R. et al., 1992. Identification of binding sites on the regulatory A subunit of protein phosphatase 2A for the catalytic C subunit and for tumor antigens of simian virus 40 and polyomavirus. Mol. Cell. Biol., 12(11), 4872-4882.
Scheidtmann K.H., Mumby M.C., Rundell K. & Walter G., 1991. Dephosphorylation of simian virus 40 large-T antigen and p53 protein by protein phosphatase 2A: inhibition by small-t antigen. Mol. Cell. Biol., 11(4), 1996-2003.
Sneddon A.A., Cohen P.T.W. & Stark M.J.R., 1990. Saccharomyces cerevisiae protein phosphatase 2A performs an essential cellular function and is encoded by two genes. Embo J., 9(13), 4339-4346.
Sontag E., 2001. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell. Signalling, 13(1), 7-16.
Sontag E., Nunbhakdi-Craig V., Bloom G.S. & Mumby M.C., 1995. A novel pool of protein phosphatase 2A is associated with microtubules and is regulated during the cell cycle. J. Cell Biol., 128(6), 1131-1144.
Sontag E. et al., 1996. Regulation of the phosphorylation state and microtubule-binding activity of tau by protein phosphatase 2A. Neuron, 17(6), 1201-1207.
Sontag E. et al., 1999. Molecular interactions among protein phosphatase 2A, tau, and microtubules. Implications for the regulation of tau phosphorylation and the development of tauopathies. J. Biol. Chem., 274(36), 25490-25498.
Stukenberg P.T. & Kirschner M.W., 2001. Pin1 acts catalytically to promote a conformational change in Cdc25. Mol. Cell, 7(5), 1071-1083.
Takahashi M. et al., 1999. Characterization of a novel giant scaffolding protein, CG-NAP, that anchors multiple signaling enzymes to centrosome and the golgi apparatus. J. Biol. Chem., 274(24), 17267-17274.
Tolstykh T., Lee J., Vafai S. & Stock J.B., 2000. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. Embo J., 19(21), 5682-5691.
Turowski P. et al., 1999. Vimentin dephosphorylation by protein phosphatase 2A is modulated by the targeting subunit B55. Mol. Biol. Cell, 10(6), 1997-2015.
Voorhoeve P.M., Hijmans E.M. & Bernards R., 1999. Functional interaction between a novel protein phosphatase 2A regulatory subunit, PR59, and the retinoblastoma-related p107 protein. Oncogene, 18(2), 515-524.
Walter G., Ferre F., Espiritu O. & Carbone-Wiley A., 1989. Molecular cloning and sequence of cDNA encoding polyoma medium tumor antigen-associated 61-kDa protein. Proc. Natl Acad. Sci. USA, 86(22), 8669-8672.
Wei H. et al., 2001. Carboxymethylation of the PP2A catalytic subunit in Saccharomyces cerevisiae is required for efficient interaction with the B-type subunits Cdc55p and Rts1p. J. Biol. Chem., 276(2), 1570-1577.
Westphal R.S., Anderson K.A., Means A.R. & Wadzinski B.E., 1998. A signaling complex of Ca2+-calmodulin-dependent protein kinase IV and protein phosphatase 2A. Science, 280(5367), 1258-1261.
Westphal R.S. et al., 1999. Identification of kinase-phosphatase signaling modules composed of p70 S6 kinase-protein phosphatase 2A (PP2A) and p21-activated kinase-PP2A. J. Biol. Chem., 274(2), 687-692.
Wu J. et al., 2000. Carboxyl methylation of the phosphoprotein phosphatase 2A catalytic subunit promotes its functional association with regulatory subunits in vivo. Embo J., 19(21), 5672-5681.
Xing Y. et al., 2006. Structure of protein phosphatase 2A core enzyme bound to tumor-inducing toxins. Cell, 127(2), 341-353.
Xing Y. et al., 2008. Structural mechanism of demethylation and inactivation of protein phosphatase 2A. Cell, 133(1), 154-163.
Xu Y. et al., 2006. Structure of the protein phosphatase 2A holoenzyme. Cell, 127(6), 1239-1251.
Xu Y. et al., 2008. Structure of a protein phosphatase 2A holoenzyme: insights into B55-mediated tau dephosphorylation. Mol. Cell, 31(6), 873-885.
Yan Z., Fedorov S.A., Mumby M.C. & Williams R.S., 2000. PR48, a novel regulatory subunit of protein phosphatase 2A, interacts with Cdc6 and modulates DNA replication in human cells. Mol. Cell. Biol., 20(3), 1021-1029.
Yang J. et al., 2007. The structure of Tap42/alpha4 reveals a tetratricopeptide repeat-like fold and provides insights into PP2A regulation. Biochemistry, 46(30), 8807-8815.
Yang S.I. et al., 1991. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol. Cell. Biol., 11(4), 1988-1995.
Yeh E. et al., 2004. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat. Cell Biol., 6(4), 308-318.
Yu X. et al., 2001. Methylation of the protein phosphatase 2A catalytic subunit is essential for association of Balpha regulatory subunit but not SG2NA, striatin, or polyomavirus middle tumor antigen. Mol. Biol. Cell, 12(1), 185-199.
Zhou J., Pham H.T., Ruediger R. & Walter G., 2003. Characterization of the Aalpha and Abeta subunit isoforms of protein phosphatase 2A: differences in expression, subunit interaction, and evolution. J. Biol. Chem., 369(2), 387-398.
Zhou X.Z. et al., 2000. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol. Cell, 6(4), 873-883.
Zwaenepoel K., Louis J.V., Goris J. & Janssens V., 2008. Diversity in genomic organisation, developmental regulation and distribution of the murine PR72/B subunits of protein phosphatase 2A. BMC Genomics, 9, 393.
Om dit artikel te citeren:
Over : Maud Martin
ULg - Gembloux Agro-Bio Tech. Cellular and Molecular Biology Unit. Avenue Maréchal Juin, 13. B-5030 Gembloux (Belgium). E-mail: Maud.Martin@ulg.ac.be
Over : Richard Kettmann
ULg - Gembloux Agro-Bio Tech. Cellular and Molecular Biology Unit. Avenue Maréchal Juin, 13. B-5030 Gembloux (Belgium).
Over : Franck Dequiedt
ULg - Gembloux Agro-Bio Tech. Cellular and Molecular Biology Unit. Avenue Maréchal Juin, 13. B-5030 Gembloux (Belgium).






