- Startpagina tijdschrift
- volume 13 (2009)
- numéro 3
- Insight into the role of catalases in salt stress in potato (Solanum tuberosum L.)
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Insight into the role of catalases in salt stress in potato (Solanum tuberosum L.)

Nota's van de redactie
Received 5 May 2008; accepted 9 October 2008
Résumé
Aperçu sur le rôle des catalases dans le stress salin chez la pomme de terre (Solanum tuberosum L.). Dans le but d'étudier d'éventuelles relations entre l'activité catalase (CAT) et la tolérance à la salinité, une étude de comportement in vitro et in vivo de lignées transgéniques de pomme de terre (cv. 'Désirée') sous conditions de stress salin a été effectuée. Trois groupes de lignées transgéniques et un témoin non transformé (DWT) ont été utilisés dans cette étude : des lignées sur-exprimant une catalase bactérienne et des lignées réprimées en activité CAT par des stratégies anti-sens ou de co-suppression. Différentes concentrations de NaCl ont été testées : in vitro 0, 25, 50 et 75 mM et in vivo 25, 50 et 75 mM. Les résultats de ce travail montrent que la modification génétique de l'activité CAT affecte le taux de multiplication des vitroplants, ainsi que des paramètres physiologiques et de croissance sous conditions de stress salin. A des concentrations de 25, 50 et 75 mM de NaCl, la sur-expression (lignée KatE16) augmente le taux de multiplication des vitroplants et la répression le réduit. Des différences entre les lignées transgéniques et le type sauvage (non transformé) dans le rendement en tubercules et le contenu en chlorophylle des feuilles ont été observées. Ces paramètres ont augmenté significativement chez les lignées sur-exprimant la CAT et ont légèrement diminué chez la lignée SU3 partiellement réprimée en CAT sous 25 mM de stress salin. Une stabilité dans le rendement quantique (Fv/Fm) a été observée chez les lignées sur-exprimant la CAT aux concentrations de 25, 50 et 75 mM de NaCl. La répression de la CAT a été associée à une diminution de la valeur Fv/Fm au niveau 50 mM de NaCl. Ces résultats montrent que la CAT contribue aux mécanismes de tolérance à la salinité chez la pomme de terre.
Abstract
In order to investigate a possible link between catalase (CAT) activity and salinity tolerance, an in vitro and in vivo study of the behavior of transgenic lines of potato (cv. 'Désirée') under salt stress conditions was carried out. Three groups of transgenic lines and non transformed control (DWT) were used in this study: lines expressing a bacterial catalase gene and lines repressing catalase activity by either co-suppression or anti-sense strategies. Various concentrations of NaCl were tested: in vitro 0, 25, 50 and 75 mM and in vivo 25, 50 and 75 mM. The results of this work show that the genetic modification of CAT activity affects the multiplication rate of vitroplants, as well as vegetative and physiological growth parameters under salt stress conditions. At 25, 50 and 75 mM of NaCl, over-expression (line KatE16) and repression of CAT increased and reduced respectively the multiplication rate of vitroplants. Differences between the transgenic lines and the wild type were evident in tuber yield and leaf chlorophyll content. These parameters were significantly increased in CAT over-expressing and slightly decreased in SU3 line repressed in CAT under 25 mM of salt stress. A stability of the potential quantum yield (Fv/Fm) was observed in the lines over-expressing the CAT at 25, 50 and 75 mM of NaCl. The repression of CAT was associated with a decrease of Fv/Fm value at 50 mM of NaCl. These results show that catalases contribute to salinity tolerance mechanisms in potato.
Inhoudstafel
1. Introduction
1Water salinity is a complex and harmful threat faced by plants; due to disruption of ionic, osmotic, and cell-water homeostasis (Munns, 1993). At the cellular level, salinity in general and NaCl in particular cause membrane damage, nutrient imbalance, altered levels of growth regulators, enzymatic inhibition and metabolic dysfunction, including photosynthesis (Fridovich, 1986; Imlay et al., 1988). Molecular and biochemical studies of salt stress responses in plants demonstrated significant increases of active oxygen species (AOS), including singulet oxygen (1O2), superoxyde anion (O2-) and hydrogen peroxide (H2O2) (Jugklang et al., 2004; Tsai et al., 2004). To neutralize and repair the damage initiated by AOS, plants have developed a complex antioxidant system: superoxyde dismutase (SOD) neutralizes the O2- and produces H2O2, while H2O2 neutralization uses other enzymes such as catalase (CAT) and ascorbate peroxidase (APX). A direct connection between antioxidant defense systems and environmental stresses, including salinity was reported in many researches. Thermal stress was associated with more activation of CAT activity in adapted clones of gladiolus (Bettaieb et al., 2007) and transgenic rice (Matsumura et al., 2002) as compared to their controls. Water stress was associated with an increase of the antioxidant enzyme response and induction of new CAT isoforms in rice (Srivalli et al., 2003). Salt-tolerant cotton cultivars exhibited significantly greater in CAT, APX and SOD activities as compared to the salt-sensitive ones (Gossett et al., 1994). In a related study, in vitro selection for salt stress-resistance showed a significant increase in antioxidant enzyme activities of cotton cell lines grown under NaCl stress (Gossett et al., 1996). Modulation by NaCl of SOD, CAT and APX activities was studied in Nicotiana plumbaginifolia (Savouré et al., 1999). In rice, Benavente et al. (2004) have reported an alteration of the gene expression encoding for antioxidant enzyme under salt stress. Although results of these investigations show the role of antioxidant status in the adaptation of many plants species to salt stress conditions, little information is nowadays available on potato. This work was carried out in order to establish an eventual relationship between CAT activity and salinity tolerance in this species. Transgenic lines (modified in their CAT activity) were used for an in vitro and in vivo study of their behavior under salt stress conditions.
2. Materials and methods
2.1. Biological material
2Three groups of transgenic lines and a non transformed control (DWT) of potato cv. 'Désirée' were used in this work. The transgenic lines were generated using different strategies. Genes coding for CAT2 of Nicotiana plumbaginifolia (Cat2, antisense) and of Gossypium hirsutum (SU2, sense) were used for the partial repression of CAT activity (2AS lines and SU lines, respectively). The KatE gene coding for Escherichia coli (strain DH5α) HPII CAT was used to generate lines over-expressing CAT activity in plastids. Two constructs pCat2AS (Cat2, anti-sense) and pCatGH (SU2, sense) were kindly provided by Prof. D. Inze (Gent University, Belgium) (see Chamnongpol et al., 1996 for more details), KatE was cloned in a binary vector pBin19 (kindly provided by Prof. U. Sonnewald, Institute of Plant Genetics and Crop Plant Research, Germany) (Bajji et al., 2007). The different constructs were mobilized into Agrobacterium tumefaciens (strain 58 for pCat2AS and pCatGH and strain LBA4404 for pBin19) to transform potato internodal explants as described by Beaujean et al. (1998) and modified by M'Hamdi et al. (2003).
2.2. Salt treatments
3The behavior study under salt stress conditions of the transgenic lines modified in their CAT activity was carried out under in vitro and in vivo conditions. Four concentrations of NaCl (0, 25, 50 and 75 mM) were added in the in vitro Murashige and Skoog culture medium. In vivo, under semi-controlled greenhouses conditions, the plants were irrigated with water containing one of three NaCl concentrations (25, 50 and 75 mM) throughout the growth cycle. Concentration of 25 mM NaCl is the mean value of normal water irrigation in our conditions and it was used as a control in this study.
2.3. Biochemical characterization of transgenic lines
4The Laemmli method (1970) but without sodium dodecyl sulfate (SDS) was used for characterization of CAT activity in the transgenic lines. Soluble protein samples (50 µg per sample) were subjected to non-denaturing 10% PAGE. CAT isozymes were detected on the gel as follows: the gel was washed 3 times (15 min each) with distilled water, then incubated for 10 min in 0.88 mM H2O2 solution, rinsed again with distilled water, and finally incubated with 1% w/v of ferric chloride and potassium ferricyanide solution until bands appeared (yellow bands on a green background).
2.4. Measured parameters
5Vegetative growth and tuber yield. In the in vitro tests, our observations were made on the number of internodes three weeks after cultivation. In the in vivo tests, the final tuber yield and physiological parameters (chlorophyll fluorescence and content) were measured. In this work which was carried out for three generations both in vivo and in vitro, each observation was made on 20 plants and repeated three times.
6Chlorophyll fluorescence. A rotary system type FIM 1500 Analytical Development Limited, ADC was used to measure chlorophyll fluorescence from 90-day-old mature leaves.
7Chlorophyll content. Chlorophyll was extracted by homogenizing and boiling 1 g of fresh weight leaves in 35 ml ethanol 96%. After centrifugation for 10 min at 4,000 g, the chlorophyll content was determined spectro-photometrically from the ethanolic supernatant at 654 nm, as described by Wintermans et al. (1965).
2.5. Statistical analysis
8All data obtained were subjected to analysis of variance at 5% level using SAS program.
3. Results
3.1. Biochemical characterization of transgenic lines
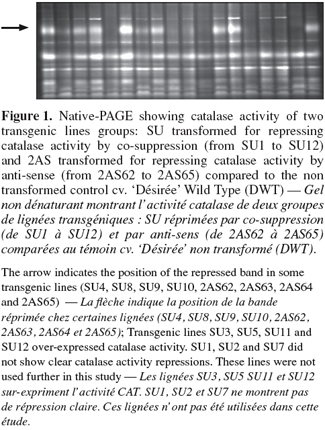
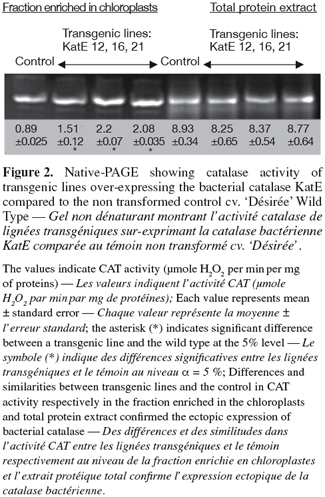
9The characterization of the transgenic lines showed a significant reduction in one of the CAT isoforms in the 2AS and SU transgenic lines groups as compared to the control (Figure 1). The arrow of figure 1 indicates the repressed band in some transgenic lines. Data derived from densitometric analysis showed a decrease of up to 66% of CAT activity in these lines. In the case of KatE lines, both spectrophotometric and native gel analyses showed that their CAT activity was significantly increased, between 70 and 147% in percoll-enriched chloroplast fraction in comparison with the wild type (Figure 2). Among all transgenic lines transformed and PCR positive, 20% showed modifications in their CAT activity.
3.2. Vegetative growth and tuber yield
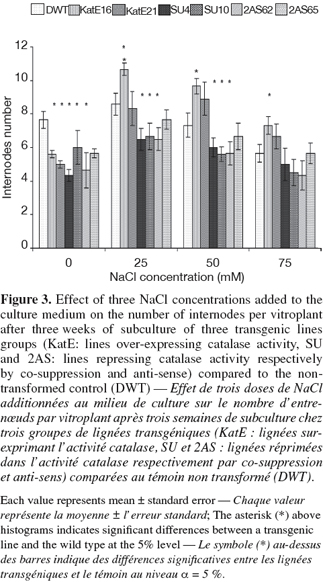
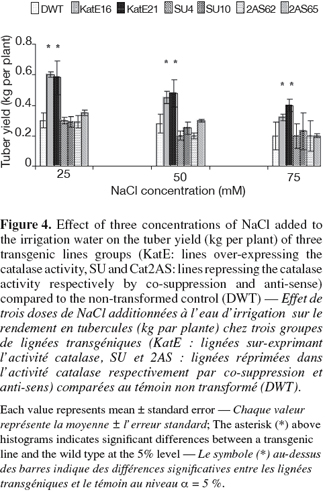
10For in vitro experiments, the vegetative growth was estimated using the number of internodes per vitroplant of three week-old culture. This parameter measuring the multiplication rate presented some variations according to the modification sense of CAT activity. A higher number of internodes per vitroplant was observed in line16 over-expressing CAT activity as compared to the control at 25, 50 and 75 mM of NaCl (Figure 3). On the contrary, the repression of the CAT activity by "anti-sense" or "co-suppression" was associated with a reduction of the multiplication rate compared to that of the control under 25 and 50 mM of NaCl (Figure 3). The highest number of internodes per vitroplant in both the transgenic and the control lines was observed at 25 mM NaCl. The tuber yield is an important agronomical parameter and was affected by CAT modification in this study. Significant differences were observed between the lines over-expressing CAT activity and the wild type. These lines gave the highest tuber yield in all used NaCl concentrations (Figure 4). In the contrary, repressed lines did not show any differences with the control under the same conditions (Figure 4).
3.3. Physiological parameters
11The potential quantum yield (Fv/Fm), which is an indicator of the photosystemII (PSII) efficiency was affected under salt stress depending on the CAT modification (Figure 5). Q stability and a reduction of Fv/Fm values with increasing NaCl concentrations were measured respectively in the lines that over-express and repress CAT. The lowest values for all NaCl concentrations used were measured in SU4 line repressing CAT activity by "co-suppression" and which gave the lowest tubers yield, FM. The lines over-expressing CAT activity gave Fv/Fm values near to 0.8 and were comparable with those measured under non stressing conditions. In these lines, the tuber yield was higher (Figure 4). A non-transformed control had an intermediate situation between the two transgenic lines categories for the potential quantum yield.
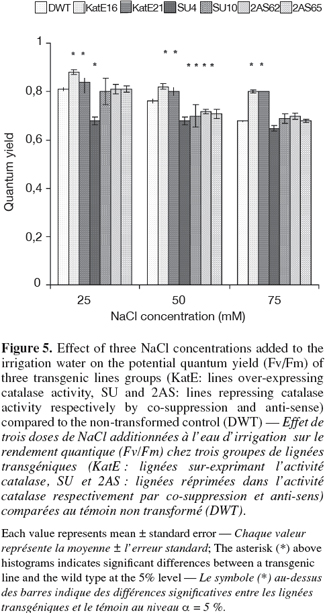
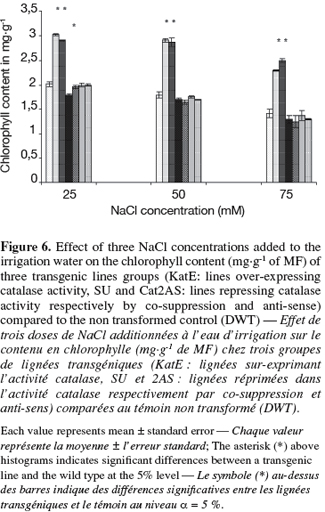
12The transgenic lines that over-express CAT activity showed superiority in chlorophyll content and gave the highest, Fv/Fm and the tuber yield. CAT repression was associated to a reduction of the pigment content only in SU4 line at 25 mM of NaCl (Figure 6).
4. Discussion
13The internodes number and the tuber yield measured were affected by CAT modification under salt stress conditions. The lines over-expressing CAT activity gave the highest values for these parameters as compared to the control at 25, 50 and 75 mM of NaCl concentrations (Figures 3 and 4). The repression of CAT activity was associated with a decrease of the internodes number at 25 and 50 mM of NaCl and any change in tuber yield (Figures 3 and 4). In fact, isoform 2 of CAT is expressed in the stems (Willekens et al., 1995) but KatE was targeted in photosynthetic tissues (chloroplasts).
14In several plant species, a relationship between AOS/antioxidant enzymes and the tolerance to abiotic stress was established: Sairam et al. (2002) observed in wheat an increase of H2O2 content, SOD, CAT and glutathione reductase (GR) activities under salt stress conditions. With barley lines repressed in 90% of CAT activity, Smith et al. (1984) measured a decrease of growth and noted necrosis development in light stressed leaves. In potato, Benavides et al. (2000) observed an increase in the reduced form of glutathion (GSH) in salinity tolerant clones compared to the sensitive ones. Glutathion is involved in the detoxication of H2O2. Willekens et al. (1997) reported that the CAT activity belongs to the normal operation of the photosynthetic apparatus in tobacco plants and is essential for the antioxidant defense in plant cells under stress conditions. They also measured a reduction of the photosynthetic activity estimated to more than 50% in the old leaves with reduced CAT activity. Matsumura et al. (2002) suggest that the improvement of low temperature tolerance in transgenic rice is associated with the increase in their capacity of H2O2 neutralization. Shikanai et al. (1998) observed an improvement of light tolerance, paraquat and drought stress in tobacco plants transformed with the same molecular construction as used in this work (KatE gene coding for HPII catalase of E. coli expressed in chloroplasts).
15As far as physiological parameters are concerned, our results converge with those of Broetto et al. (2007) who indicate a significant reduction in the potential quantum yield in Mesembryanthemum sp. under salt and light stress conditions. On the other hand, Fedina et al. (2006) reported only a slight reduction of the chlorophyll fluorescence in barley under salt stress conditions but measured a strong increase in the H2O2 content. The reduction in chlorophyll content is a general phenomenon in salt-sensitive plants growing under salt stress conditions (Srivastava et al., 1988). In potato, Benavides et al. (2000) showed a reduction of the photosynthetic assimilation associated to a reduction of chlorophyll content and measured a 23% decrease in chlorophyll content in salt-sensitive clones. Our data for this parameter are in accordance with others: the over-expression of CAT activity was associated with high chlorophyll content and with the repression a decrease in this pigment was observed in SU4 line at 25 mM of NaCl.
16All these results show that CAT activity is involved in the salinity mechanisms tolerance in potato and its usefulness as a biochemical marker for salt stress tolerance in breeding programs is worth investigating.
17Acknowledgements
18This work was supported financially by the Research Unit of Vegetable and Floral Crops of I.N.A.T., Tunisia and the Unit of Plant Biology of Univ. Liege - Gembloux Agro-Bio Tech, Belgium.
Bibliographie
Bajji M. et al., 2007. Catalase inhibition alters suberzation and wound-healing in potato (Solanum tuberosum L.) tubers. Physiol. Plantarum, 129(3), 472-483.
Beaujean A., Sangwan R.S., Lecardonnel A. & Sangwan-Norreel B.S., 1998. Agrobacterium-mediated transformation of three economically important potato cultivars using sliced internodal explants: an efficient protocol of transformation. J. Exp. Bot., 49, 1589-1595.
Benavente M.L., Teixeira F.K., Alvim Kamei L.C. & Margis-Pinheiro M., 2004. Salt stress induces altered expression of genes encoding antioxidant enzymes in seedlings of Brazilian indica rice (Oryza sativa L.). Plant Sci., 166, 323-331.
Benavides P.M. et al., 2000. Relationship between antioxidant defence systems and salt tolerance in Solanum tuberosum. Aust. J. Plant Physiol., 27, 273-278.
Bettaieb T., M'Hamdi M., Ruiz de Galarreta & du Jardin P., 2007. Relation between the low temperature stress and catalase activity in gladiolus somaclones (Gladiolus grandiflorus Hort.). Sci. Hortic., 113, 49-51.
Broetto F., Monteiro Duarte H. & Lüttge U., 2007. Responses of chlorophyll fluorescence parameters of the facultative halophyte and C3-CAM intermediate species Mesembryanthemum crystallinum to salinity and high irradiance stress. J. Plant Physiol., 164(7), 904-912.
Chamnongpol S. et al., 1996. Transgenic tobacco with a reduced catalase activity develops nectrotic lesions and induces pathogenesis-related expression under high light. Plant J., 10, 491-503.
Fedina I., Georgieva K., Velitchkova M. & Grigorova I., 2006. Effect of pre-treatment of barley seedlings with different salts on the level of UV-B induced and UV-B absorbing compounds. Environ. Exp. Bot., 56, 225-230.
Fridovich I., 1986. Biological effects of superoxide radical. Arch. Biochem. Biophys., 247, 1-11.
Gossett D.R. et al., 1994. The effects of NaCl on antioxidant enzyme activities in callus tissue of salt-tolerant and salt-sensitive cotton cultivars (Gossypium hirsutum L.). Plant Cell Report, 13, 498-503.
Gossett D.R. et al., 1996. Antioxidant response to NaCl stress in a control and an NaCl-tolerant cotton cell line grown in the presence of paraquat, buthionine sulfoximine, and exogenous glutathione. Plant Physiol., 112, 803-809.
Imlay J.A. & Linn S., 1988. DNA damage and oxygen radical toxicity. Science, 240, 1302-1309.
Jugklang J., Sunohara Y. & Matsumoto H., 2004. Antioxidative response to NaCl stress in salt-tolerance Sesbania rostrata. Weed Biol. Manage., 4, 81-85.
Laemmli U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680-685.
M'Hamdi M., Rouvière C. & Rojas-Beltran J., 2003. Optimisation de la transformation génétique de la pomme de terre par Agrobacterium tumefaciens. Utilisation de la résistance à l'hygromycine comme marqueur sélectif. Biotechnol. Agron. Soc. Environ., 7, 183-188.
Matsumura T. et al., 2002. Wheat catalase expressed in transgenic rice can improve tolerance against low temperature stress. Physiol. Plant., 116, 317-327.
Munns R., 1993. Physiological process limiting plant growth in saline soils: some dogmas and hypotheses. Plant Cell Environ., 16, 15-24.
Sairam R.K., Veerabhadra K.R. & Srivastava G.C., 2002. Differential response of wheat genotypes to long-term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci., 163, 1037-1046.
Savouré A. et al., 1999. NaCl and CuSO4 treatments trigger distinct oxidative defence mechanisms in Nicotiana plumbaginifolia L. Plant Cell Environ., 22, 387-396.
Shikanai T. et al., 1998. Inhibition of ascorbate peroxides under oxidative stress in tobacco having bacterial catalase in chloroplasts. FEBS Lett., 428, 47-51.
Smith I.K. et al., 1984. Increased levels of glutathione in catalase-deficient mutant of barley (Hordeum vulgare L.). Plant Sci. Lett., 37, 29-33.
Srivalli B., Sharma G. & Khanna-Chopra R., 2003. Antioxidant defense system in an upland rice cultivar subjected to increasing intensity of water stress followed by recovery. Physiol. Plant., 119, 503-512.
Srivastava T.P. et al., 1988. Effect of salt stress on physiological and biochemical parameters of wheat. Ann. Arid Zone, 27, 197-204.
Tsai Y.C., Hong C.Y. & Huei Kao C., 2004. Expression of ascorbate peroxidase and glutathione reductase in roots of rice seedlings in response to NaCl and H2O2. J. Plant Physiol., 162, 291-299.
Willekens H., Inzé D., Van Montagu M. & Van Camp W., 1995. Catalases in plants. Mol. Breed., 1, 207-228.
Willekens H. et al., 1997. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J., 16, 4806-4816.
Wintermans J.F. & De Mots A., 1965. Spectrophotometric characteristics of chlorophylls a and b and their photosynthesis in ethanol. Biochem. Biophys. Acta, 109, 448-453.
Om dit artikel te citeren:
Over : Mahmoud M’Hamdi
Ecole supérieure d’Agriculture du Kef. Complexe universitaire de Boulifa. Route de Dahmani Km 7. Boulifa. TU-7119 Kef (Tunisia). E-mail: mhamdimahmoud@yahoo.fr – Institut national agronomique de Tunisie. Unité des cultures maraichères et florales. 43, Avenue Charles Nicole. TU-1082 Cité Mahrajène, Tunis (Tunisie).
Over : Taoufik Bettaieb
Institut national agronomique de Tunisie. Unité des cultures maraichères et florales. 43, Avenue Charles Nicole. TU-1082 Cité Mahrajène, Tunis (Tunisie).
Over : Youssef Harbaoui
Institut national agronomique de Tunisie. Unité des cultures maraichères et florales. 43, Avenue Charles Nicole. TU-1082 Cité Mahrajène, Tunis (Tunisie).
Over : Abdel Aziz Mougou
Institut national agronomique de Tunisie. Unité des cultures maraichères et florales. 43, Avenue Charles Nicole. TU-1082 Cité Mahrajène, Tunis (Tunisie).
Over : Patrick du Jardin
Univ. Liege - Gembloux Agro-Bio Tech. Unit of Plant Biology. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






