- Home
- volume 13 (2009)
- numéro 3
- Description of two Enterococcus strains isolated from traditional Peruvian artisanal-produced cheeses with a bacteriocin-like inhibitory activity
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Description of two Enterococcus strains isolated from traditional Peruvian artisanal-produced cheeses with a bacteriocin-like inhibitory activity

Editor's Notes
Received 24 November 2008; accepted 4 March 2009
Résumé
Isolement et caractérisation de deux souches d’Enterococcus à activité bactériocinogène issues de fromages traditionnels péruviens. L’objectif de ce travail a été d’isoler et de caractériser des bactéries lactiques aux propriétés antimicrobiennes issues de 27 échantillons de fromages artisanaux d’origine péruvienne. Un total de 20 souches de bactéries Gram+ et catalase négative avec activité du type bactériocine contre Listeria monocytogenes CWBI-B2232 ont été isolées à partir d’une collection de 2 277 isolats. L’activité antimicrobienne est maintenue après neutralisation des acides organiques (acide lactique et acétique) et du peroxyde d'hydrogène. La nature protéique des composés inhibiteurs a été confirmée pour les 20 souches sélectionnées, par digestion protéolytique des surnageants de culture. Les souches référencées CWBI-B1431 et CWBI-B1430 sont celles qui présentent les activités antibactériennes les plus importantes. Elles ont fait l'objet une caractérisation polyphasique (génotypique et phénotypique). Sur cette base, CWBI-B1431 et CWBI-B1430 ont été identifiées, respectivement, comme Enterococcus mundtii et Enterococcus faecium. Ces deux souches sont sensibles à l’antibiotique vancomycine (CMI < 2 μg⋅ml-1) et ont montré l’absence d’activité hémolytique.
Abstract
The aim of this work was to isolate and to characterize strains of lactic acid bacteria (LAB) with bacteriocin-like inhibitory activity from 27 traditional cheeses artisanal-produced obtained from different Peruvian regions. Twenty Gram+ and catalase-negative strains among 2,277 isolates exhibited bacteriocin-like inhibitory activity against Listeria monocytogenes CWBI-B2232 as target strain. No change in inhibitory activity was observed after organic acid neutralization and treatment with catalase of the cell-free supernatant (CFS). The proteinic nature of the antimicrobial activity was confirmed for the twenty LAB strains by proteolytic digestion of the CFS. Two strains, CWBI-B1431 and CWBI-B1430, with the best antimicrobial activity were selected for further researches. These strains were taxonomically identified by phenotypic and genotypic analyses as Enterococcus mundtii (CWBI-B1431) and Enterococcus faecium (CWBI-B1430). The two strains were sensitive to vancomycin (MIC < 2 μg.ml-1) and showed absence of haemolysis.
1. Introduction
1From time immemorial the microorganisms have developed different strategies to find nutrients and survive in their own environments. Some of them produce the antagonistic substances to dominate their habitat, as antibiotics of wide spectrum, products of the metabolism like organic acids, chelating molecules of iron and bacteriocins (Muñoz-Rojas, 2008).
2Lactic acid bacteria (LAB) are found in many ecological niches, including certain foods, in the mouth, and in the gastrointestinal and urogenital tracts of humans and animals (Van Belkum et al., 2000). LAB have been used in food and feed preservation for centuries and their preservative effects are mainly due to the reduction of pH and the formation of organic acids, especially lactic acid (Casaus et al., 1997; O’Sullivan et al., 2002; Klaenhammer et al., 2005). This is not always sufficient; the bacteriocins can prevent bacterial spoilage or outgrowth of pathogenic bacteria. The bacteriocin or bacteriocin-producing LAB can be used as safe alternatives to chemical preservatives in foods (Casaus et al., 1997; Bennik et al., 1998). Bacteriocins are ribosomally synthesized polypeptides possessing antimicrobial activity and are rapidly digested by proteases in the human digestive tract (Cheng et al., 2003). They are cationic peptides, imparted by the presence of multiple lysine and arginine residues, and amphipathic molecules that are composed of 10 to 45 amino acids (Van Belkum et al., 2000). Genus Enterococcus are ubiquitous in nature but the role they play is often unclear (Franzetti et al., 2004) and they are found in large numbers in vegetables, plant material, and food, especially those of animal origin such as dairy products (Giraffa, 2003). Production of bacteriocins from enterococci, referred to as enterocins, can be applied as biopreservative in dairy or meat fermentation products (Franz et al., 2007). The use of the term bacteriocin-like substance is applied to antagonistic substances which are not completely defined or do not fit the typical criteria of bacteriocins (Ocaña et al., 1999) or bacterial products showing antagonist activity though not characterized (Pedron et al., 1997).
3Artisanal cheeses are still made by farmers on small scales in the farmhouse using traditional techniques. Raw milk is a very suitable medium for the growth of bacteria, the indigenous LAB present in milk acidifies the raw milk for the obtaining of cheese, the quality of these cheeses depends to a great extent on the composition of their microflora (Marino et al., 2003). Cheeses made from unpasteurized milk and following traditional manufacturing procedures are an important source of strains harboring genetic diversity (Morea et al., 1999). The artisanal cheese is a dairy product widely consumed in Peru and extensive commercialized in small markets. It is made by cattleman of low economic resources who do not respect good manufacture practices. The cheeses are mainly elaborated in Andean zones. Consequently, from the 27 cheeses used in this study, 25 are from high-altitude towns (between 1,198-3,633 meters above sea level).
4In this investigation, LAB isolated from traditional raw milk cow cheeses artisanal-produced obtained from different Peruvian regions were selected by his antagonistic activity against Listeria monocytogenes CWBI-B2232. The two strains that demonstrated the best inhibitory activity were biochemical and genotypic identified. After that, the genus of the isolated strains was identified, two factors involving health risk were determined and the stability to the temperature and pH of these two strains, were evaluated.
2. Materials and methods
5LAB from 27 traditional Peruvian artisanal-produced cheeses were investigated. The cheeses were lyophilized before being analyzed. Lyophilized cheese samples (1 g) were homogenized in 9 ml of physiological sterile saline for 5 min and after were serially diluted 10-fold. Hundred μl of each dilution were plated on Man-Rogosa-Sharpe (MRS) agar and incubated at 37°C for 48 h. Colonies were picked up randomly and grown in 5 ml MRS broth at 37°C for 16 h. The antimicrobial activity of was tested by agar well-diffusion assay (Schillinger et al., 1989) against L. monocytogenes CWBI-B2232 as target strain. The antimicrobial activity was scored positive in presence of a detectable clearing zone around the well. Bacteriocin activity was defined as the reciprocal of the highest dilution showing at least a 1 mm zone definite inhibition of the indicator strains around the well and was expressed as activity units per milliliter (AU⋅ml-1). The selected isolates were examined by phase-contrast microscopy (Zeiss de type Axioskop 2 mot) to determine their cell morphologies and Gram-stained and tested for catalase activity.
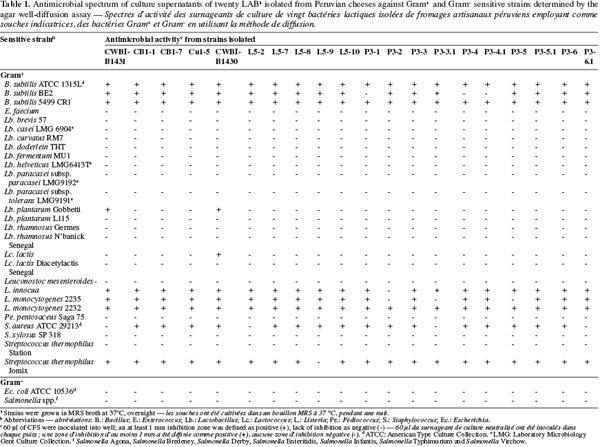
6The supernatant fluids of bacteria with antimicrobial activity were studied to determine the nature of inhibitor. In order to rule out acid inhibition and the hydrogen peroxide, the CFS was adjusted to pH 6.8 with 1N NaOH and by the addition of catalase (68 U⋅ml-1) (Fluka 60640) in another sample. The sensitivity to proteolytic enzymes of neutralized CFS was realized by the addition of proteinase K (Sigma, P-6556), protease (Sigma, P-3910), trypsin (Merck) and pepsin (Sigma, P-7000) at final concentration 1 mg⋅ml-1 (Benkerroum et al., 2000). In all the cases, a positive control sample was tested in parallel. The inhibitory activity of every sample was determined following the agar well-diffusion assay as previously described. The spectrum of activity against different indicator bacteria, listed in table 1, was determined by the well-diffusion assay as previously described.
7The strains showing the highest inhibitory activity were biochemically and genetically characterized by established routine methods. The ability of the selected strains to ferment various carbohydrates was determined using API 50CHL strips (BioMérieux, France) according to the manufacturer’s instructions. The genotypic identification was determined on the basis of 16S rDNA sequence according to Diop et al. (2007). The sequences obtained (400-600 bp) were then assembled using Vector NTI Software. Sequences were identified in the Ribosomal Database Project (http://rdp.cme.msu.edu). Phylogenetic analysis was realized by an alignment of sequences consensus of genes 16S rDNA collected in an international database (Genbank).
8A partial safety evaluation of these strains was investigated by testing the two main factors (hemolytic activity and vancomycin resistance) involved in a potential health risk associated with the use of enterococci (Foulquié et al., 2003).
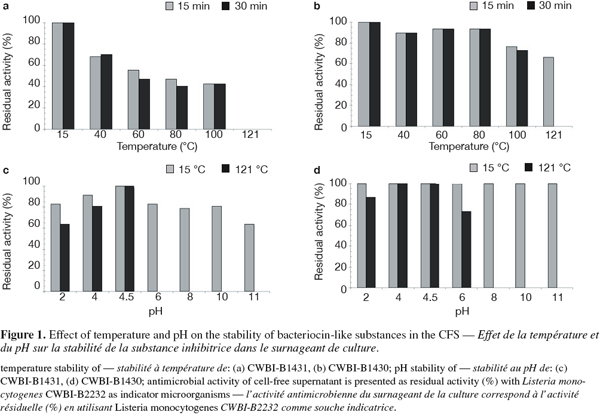
9To determine the effect of temperature and pH on the stability of bacteriocin-like substances, cultures were grown in MRS broth to initial pH 6.5, at 37°C for 16 h. To evaluate the effect of temperature on bacteriocin-like substances, the CFS corresponding to cultures were heated at 40, 60, 80 or 100°C, for 15 and 30 min, or 121°C for 15 min. To evaluate the effect of pH on bacteriocin-like substances, the pH of CFS were adjusted at 2, 4, 6, 8, 10 or 11 by adding the appropriate volumes of 4N HCl or 4N NaOH. All studies were carried out in triplicate. The samples were filtered through 0.2 μm cellulose acetate filter (Minisart® 16534, Sartotius). After 15 minutes of incubation at 15°C and treatment 121°C, the residual activity was assayed by the agar well-diffusion assay as previously described.
3. Results and discussion
10A total of 2,277 LAB were isolated from 27 traditional cheeses artisanal-produced obtained from different cities of Peru. Twenty isolates (Gram+ and catalase-negative) exhibited an inhibitory activity against L. monocytogenes CWBI-B2232. This indicates that 0.9% of the isolated strains are producers of inhibitory substances. No change in activity of CFS of the twenty strains was recorded when they were neutralized and treated with catalase, indicating that bacterial acidification of the medium and the production of hydrogen peroxide were not responsible of the inhibition. Complete inactivation was found on the neutralized-CFS of the twenty strains with proteases as proteinase K and protease P-3910, and only of the twenty strains, P3-3.1 was not sensible to trypsin and L5-8 was not sensible to pepsin. Antimicrobial activity was observed in the positive controls, consisting of untreated extracellular extracts from the respective LAB strains. These results indicate the proteinaceous nature of the substance responsible for the antimicrobial activity, suggesting that the inhibitory activity could be based on the biosynthesis and secretion of bacteriocins (Cheng et al., 2003).
11The sensitive of 27 Gram+ and 8 Gram- strains to the bacteriocin-like substances produced by twenty strains isolated is shown in table 1. Neutralized CFS from twenty strains isolated showed a relatively broad spectrum of activity against Bacillus subtilis, Listeria innocua, L. monocytogenes, Staphylococcus aureus and Streptococcus thermophilus. Nevertheless, they do not present activity against the seven salmonella tested and Escherichia coli.
12On the two strains with the best antimicrobial activity was continued the research. These strains noted as CWBI-B1431 and CWBI-B1430 produced an inhibitory activity 136,533 AU⋅ml-1 and 17,066 AU⋅ml-1, respectively in using L. monocytogenes CWBI-B2232 as the target strain.
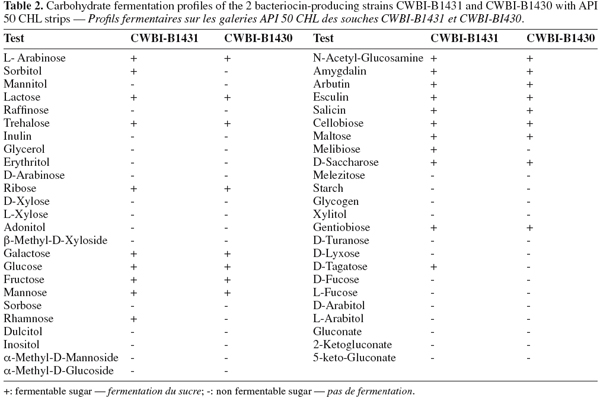
13The isolates in both cases were confirmed as Enterococcus; both strains were Gram+ coccus, catalase-negative, they grew 10, 37 and 45°C in MRS broth (pH 6.8), MRS broth (pH 9.6) at 37°C and 0.1% methylene blue milk at 37ºC and not produce gas from glucose. One strain (CWBI-B1430) grew in MRS broth containing 6.5% NaCl and reduced ammonia from arginine as was reported. CWBI-B1431 showed characteristic yellow pigmented (Kaufhold et al., 1991; Manero et al., 1999; Higashide et al., 2005) and CWBI-B1430 showed white coloration. CWBI-B1431 and CWBI-B1430 were tested for their ability to ferment sugars (Table 2). Both strains had typical fermentation reactions of arabinose, sorbitol, lactose, trehalose and inulin (Kaufhold et al., 1991; Manero et al., 1999); the conventional fermentation of mannitol and raffinose by Enterococcus mundtii and Enterococcus faecium were not observed.
14CWBI-B1431 and CWBI-B1430 strains were genotype identified using 16S rDNA gene-targeted PCR method, as previously described. The 1452-pb amplified fragment of CWBI-B1431 strain had the highest degree of sequence identity (99.99%) with the E. mundtii ATCC 43186 strain type. The CWBI-B1430 strain was identified by sequencing of 1463-pb as E. faecium exhibited 99.79% identity with E. faecium DSM 20477 strain type. The genomic 16S rDNA sequence of the strains E. mundtii CWBI-B1431 and E. faecium CWBI-B1430 is available in the GenBank database of NCBI (National Center for Biotechnology Information) under the accession numbers EF591816 and EF591817, respectively. The identification priority was based on the results of genetic identification and routine biochemical tests instead of the low percentage of similarity obtained by sugar fermentation that could be originated due to the inadequacy selection of API CH50L for well identification of Enterococcus strains as it was reported by Diop et al. (2007).
15The two strains studied displayed γ-haemolytic activity on sheep blood and horse blood, indicating absence of hemolysis activity. The vancomycin MIC value was < 2 μg⋅ml-1 for the two strains, showing sensitivity of these strains for that antibiotic; similar results for six E. faecium analyzed were reported by Foulquié et al. (2003). These are aspirant strains for safe and practical uses.
16The crude antimicrobial substances produced by these two strains exhibited activity against the target strains L. monocytogenes CWBI-B2232 after a treatment for 15 or 30 min at 40, 60, 80 and 100°C (Figure 1). On the other hand, an interesting antimicrobial activity was observed for the CWBI-B1430 strain at 121°C for 15 min. The treatment between pH values 2.0-11.0 at 15°C decreased the antimicrobial activity and this was not detected in pH value 6.0-11.0 after a treatment at 121ºC for 15 min for CWBI-B1431. For CWBI-B1430, low activity decreasing was obtained in the pH value 2.0-4.0 at 15°C, while that in the 6.0-11.0 pH the antimicrobial substance was stable and the antimicrobial activity was not detected in pH value 8-11 pH after a treatment at 121ºC for 15 min. These results suggested that the effect of pH was dependent on the preliminary temperature treatment. The results of the effect of the thermal treatment on antimicrobial activity suggested that the antagonistic substance produced in this study for CWBI-B1431 and CWBI-B1430 strains have on applied interest for food products; including application in food products subjected to pasteurization, cook-chilling, sterilization and other. The stability to thermal treatment of bacteriocin activity was widely reported by Campos et al. (2006) for E. faecium USC-46 and for E. mundtii USC-51. Nevertheless, these two bacteriocin-like were stable at 121°C for 15 min whereas, in our study, only CWBI-B1430 was stable at these conditions. The incorporation of enterocins as a biopreservative ingredient into model food systems has been studied. Some enterocins have been tested in food, for example, Ananou et al. (2005) evaluated of control of L. monocytogenes in sausages by enterocin AS-48 from E. faecium S-32-81. Whereas Garriga et al. (2002) demonstrated the reduction of L. monocytogenes viable counts during storage in meat model system by inoculation with enterocins A and B produced by E. faecium CTC492.
17The two strains identified display antimicrobial activity against Listeria spp., only CWBI-B1430 has an inhibitory activity against Staphylococcus aureus (Table 1). Inhibitory activity spectrum of these strains is common to the spectrum observed for some Enterococcus. Ghrairi et al. (2008) have reported that the enterocins are particularly active against pathogen bacteria such as L. monocytogenes, S. aureus and Clostridium spp. For example, enterocin A and enterocin B produced by E. faecium P21 (Herranz et al., 2001), enterocin P produced by E. faecium P13 (Cintas et al., 1997) and enterocin L50, enterocin Q and enterocin P produced by E. faecium L50 (Cintas et al., 1998; Criado et al., 2006) have shown antimicrobial activity against L. monocytogenes, S. aureus, Clostridium perfringens and Clostridium botulinum. Whereas, mundticin produced by E. mundtii ST15 showed antimicrobial activity against Lactobacillus sakei, Enterococcus faecalis, Bacillus cereus, Propionibacterium sp., Clostridium tyrobutyricum, Acinetobacter baumanii, Klebsiella pneumoniae, Pseudomonas aeruginosa, S. aureus, Streptococcus pneumonia and Streptococcus caprinus (De Kwaadsteniet et al., 2005).
18Several reports from the literature show diverse values of antimicrobial activity; however, these values are not comparable to the values obtained by the 2 strains in study because the bacteria with bacteriocin-like inhibitory activity and the target strains used are different. For example, Sarantinopoulos et al. (2002) and Aymerich et al. (2000) using L. innocua CTC 1014 as indicator strain recorded an antimicrobial activity of 800 and 4,893 AU⋅ml-1 produced by E. faecium FAIR-E 198 and E. faecium CTC492, respectively.
19This study demonstrated the presence of bacteriocin-like substance, further studies will be investigated the presence of putative bacteriocin genes in CWBI-B1431 and CWBI-B1430 strains by genetic screening PCR method. According to the results of antimicrobial activity against L. monocytogenes and L. innocua and the heat-stable of CFS, enterocins of class II will be studied.
4. Conclusion
20In summary, the present investigation shows the presence of LAB producing of antagonistic substances in traditional Peruvian artisanal-produced cheeses. Two of these strains named E. mundtii CWBI-B1431 and E. faecium CWBI-B1430 were selected for further studies. These are candidate strains for safe and practical uses. They are sensitive to the glycopeptide antibiotic (vancomycin) and they did not show haemolytic activity. The remained activity after the thermal treatment of the substance produced by these strains predicts a broad potential application as biopreservatives for heat-processing treatments. Taking into account the potential of these strains, it will be interesting to optimize the fermentation conditions and medium composition to improve the production of bacteriocin-like substances and the identification of enterocins. Finally, the use of microorganism or of his bacteriocin in the food industry should be studied considering the composition and the characteristics of the food. According to what has been exposed in this work the bacteriocin-like substances produced by these strains must be identified.
21Acknowledgements
22The authors would like to thank the Belgian Technical Cooperation for financial support.
Bibliographie
Ananou S. et al., 2005. Control of Listeria monocytogenes in model sausages by enterocin AS-48. Int. J. Food Microbiol., 103, 179-190.
Aymerich T. et al., 2000. Effect of sausage ingredients and additives on the production of enterocins A and B by Enterococcus faecium CTC492. Optimization of in vitro production and anti-listerial effect in dry fermented sausages. J. Appl. Microbiol., 88, 686-694.
Benkerroum N. et al., 2000. Isolation of a bacteriocin-producing Lactococcus lactis subsp. lactis and application to control Listeria monocytogenes in Moroccan jben. J. Appl. Microbiol., 89, 960-968.
Bennik M. et al., 1998. A novel bacteriocin with a YGNGV motif from vegetable-associated Enterococcus mundtii: full characterization and interaction with target organisms. Biochim. Biophys. Acta, 1373, 47-58.
Campos C. et al., 2006. Preliminary characterization of bacteriocins from Lactococcus lactis, Enterococcus faecium and Enterococcus mundtii strains isolated from turbot (Psetta maxima). Food Res. Int., 39, 356-364.
Casaus P. et al., 1997. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology, 143, 2287-2294.
Cheng H. & Hoover D., 2003. Bacteriocins and their food applications. Comp. Rev. Food Sci. Food Saf., 2, 82-100.
Cintas L. et al., 1997. Biochemical and genetic characterization of Enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl. Environ. Microbiol., 63(11), 4321-4330.
Cintas L., Casaus P., Fernández M. & Hernández P., 1998. Comparative antimicrobial activity of enterocin L50, pediocin PA-1, nisin A and lactocin S against spoilage and foodborne pathogenic bacteria. Food Microbiol., 15, 289-298.
Criado R. et al., 2006. Immunochemical characterization of temperature-regulated production of enterocin L50 (EntL50A and EntL50B), enterocin P, and enterocin Q by Enterococcus faecium L50. Appl. Environ. Microbiol., 72(12), 7634-7643.
De Kwaadsteniet M., Todorov S., Knoetze H. & Dicks L., 2005. Characterization of a 3944 Da bacteriocin, produced by Enterococcus mundtii ST15, with activity against Gram+ and Gram- bacteria. Int. J. Food Microbiol., 105, 433-444.
Diop M. et al., 2007. Bacteriocin producers from traditional food products. Biotechnol. Agron. Soc. Environ., 11(4), 275-281.
Foulquié M. et al., 2003. Isolation and biochemical characterization of enterocins produced by enterococci from different sources. J. Appl. Microbiol., 94, 214-229.
Franz Ch. et al., 2007. Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol. Rev., 31(3), 293-310.
Franzetti L., Pompei M., Scarpellini M. & Galli A., 2004. Phenotypic and genotypic characterisation of Enterococcus spp. of different origins. Curr. Microbiol., 49, 255-260.
Garriga M. et al., 2002. Bactericidal synergism through bacteriocins and high pressure in a meat model system during storage. Food Microbiol., 19, 509-518.
Ghrairi T., Frere J., Berjeaud J. & Manai M., 2008. Purification and characterisation of bacteriocins produced by Enterococcus faecium from Tunisian rigouta cheese. Food Control, 19, 162-169.
Giraffa G., 2003. Functionality of enterococci in dairy products. Int. J. Food Microbiol., 88, 215-222.
Herranz C. et al., 2001. Enterococcus faecium P21: a strain occurring naturally in dry-fermented sausages producing the class II bacteriocins enterocin A and enterocin B. Food Microbiol., 18, 115-131.
Higashide T. et al., 2005. Endophthalmitis caused by Enterococcus mundtii. J. Clinical Microbiol., 43(3), 1475-1476.
Kaufhold A. & Ferrieri P., 1991. Isolation of Enterococcus mundtii from normally sterile body sites in two patients. J. Clinical Microbiol., 29(5), 1075-1077.
Klaenhammer T. et al., 2005. Genomic features of lactic acid bacteria effecting bioprocessing and health. FEMS Microbiol. Rev., 29, 393-409.
Manero A. & Blanch A., 1999. Identification of Enterococcus spp. with a biochemical key. Appl. Environ. Microbiol., 65(10), 4425-4430.
Marino M., Maifreni M. & Rondinini G., 2003. Microbiological characterization of artisanal Montasio cheese: analysis of its indigenous lactic acid bacteria. FEMS Microbiol. Lett., 229, 133-140.
Morea M., Baruzzi F. & Cocconcelli P., 1999. Molecular and physiological characterization of dominant bacterial populations in traditional Mozzarella cheese processing. J. Appl. Microbiol., 87, 574-582.
Muñoz-Rojas J., 2008. Bacteriocinas: una estrategia de competencia microbiana propuesta como alternativa de antibióticos dirigidos para el futuro humano. In: Martínez E. & Martínez J., eds. Microbio en línea, http: www.microbiología.org-mx/microbiosenlínea, (28/07/08).
O’Sullivan L., Ross R. & Hill C., 2002. Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality. Biochimie, 84, 593-604.
Ocaña V., Pesce A. & Nader-Macías N., 1999. Characterization of a bacteriocin-like substance produced by a vaginal Lactobacillus salivarius strain. Appl. Environ. Microbiol., 65, 5631-5635.
Pedron E., Niederauer R., Salete R. & Paiva M., 1997. Bacteriocin-like substance of Aeromonas hydrophila. Mem. Inst. Oswaldo Cruz, 92(1), 115-116.
Sarantinopoulos P. et al., 2002. Bacteriocin production by Enterococcus faecium FAIR-E 198 in view of its application as adjunct starter in Greek Feta cheese making. Int. J. Food Microbiol., 72, 125-136.
Schillinger U. & Lucke F., 1989. Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol., 55(8), 1901-1906.
Van Belkum M. & Stiles M., 2000. Nonlantibiotic antibacterial peptides from lactic acid bacteria. Nat. Prod. Rep., 17, 323-365.
To cite this article
About: Ana Aguilar Galvez
Universidad Nacional Agraria La Molina (UNALM). Instituto de Biotecnología (Área de Biotecnología Industrial). Av. La Molina s/n. PE-Lima 12 La Molina (Perú) – Univ. Liege - Gembloux Agro-Bio Tech. Unit of Bio-industry. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: bioindus@fsagx.ac.be.
About: Robin Dubois-Dauphin
Univ. Liege - Gembloux Agro-Bio Tech. Unit of Bio-industry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
About: Hakim Ghalfi
Univ. Liege - Gembloux Agro-Bio Tech. Unit of Bio-industry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
About: David Campos
Universidad Nacional Agraria La Molina (UNALM). Instituto de Biotecnología (Área de Biotecnología Industrial). Av. La Molina s/n. PE-Lima 12 La Molina (Perú).
About: Philippe Thonart
Univ. Liege - Gembloux Agro-Bio Tech. Unit of Bio-industry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






