- Startpagina tijdschrift
- volume 13 (2009)
- numéro 2
- Hen egg yolk antibodies (IgY), production and use for passive immunization against bacterial enteric infections in chicken: a review
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Hen egg yolk antibodies (IgY), production and use for passive immunization against bacterial enteric infections in chicken: a review

Nota's van de redactie
Received on Septembre 26, 2008, accepted on December 2, 2008
Résumé
Les anticorps du jaune d’œuf de poule (IgY), production et utilisation en immunisation passive contre les infections entériques bactériennes : une revue. Les infections entériques causées par Salmonella constituent un problème majeur de santé publique à travers le monde. Il est bien connu que la volaille, en particulier le poulet de chair, constitue le principal réservoir pour ce pathogène zoonotique. Par conséquent, la prévention et la surveillance de Salmonella au cours de la phase d’élevage pourrait réduire efficacement la contamination de la viande à l’abattoir et lors de la transformation. Avec l'interdiction de l’utilisation sub-thérapeutique des antibiotiques en Europe et la rigueur croissante de la législation européenne en matière d'hygiène alimentaire, l'immunisation passive par l'administration orale d’anticorps de jaune d’œuf (IgY) spécifiques du pathogène serait une alternative intéressante. Cette synthèse bibliographique donne des informations concises sur la production des IgY et leur utilisation en immunisation passive, en particulier chez la volaille.
Abstract
Enteric infections caused by Salmonella remain a major public health burden worldwide. Poultry, particularly chickens, are known to be the main reservoir for this zoonotic pathogen. Therefore, the prevention and monitoring of Salmonella infection during the live phase may greatly reduce the contamination of poultry meat during slaughter and processing. With the ban on sub-therapeutic antibiotic usage in Europe and the increasingly strictness of the European legislation on food hygiene, passive immunization by oral administration of pathogen-specific hen egg yolk antibody (IgY) may be a useful and attractive alternative. This review offers summarized information about IgY production and the use of these antibodies for passive immunization, particularly in poultry.
Inhoudstafel
1. Introduction
1Poultry meat is an important food product and the broiler chicken-related industry is an economically important component of the agro-industry in the European Union (EU). The industry however is under pressure and faces many challenges. Most importantly, there is a demand for meat of high nutritional value and free of microbiological and chemical hazards. Unfortunately poultry meat is one of the major sources of food borne bacterial infections in humans such as Salmonellosis (Mayrhofer et al., 2003).
2In recent decades, Salmonellosis becomes a considerable burden to public health. Salmonella can cause a spectrum of pathological conditions such as acute gastroenteritis and bacteraemia in humans by the mechanisms of colonization, invasion and penetration of the intestinal epithelium (Roberts et al., 1996). In Europe, although Salmonellosis records a fall in the number of cases since 2005, it remains second in the list of human zoonotic diseases across the EU with 160,649 people infected in 2006 (35 cases per 100,000). Two serovars, Salmonella Enteritidis and Salmonella Typhimurium account for most Salmonellosis associated with foods of animal origin (EFSA, 2007). In Belgium, these two serovars accounted for almost 80% of identified serovars in humans in 2006 (Collard et al., 2008).
3The contamination of poultry meat products originates primarily from chickens infected with Salmonella during processing. In this respect, S. Enteritidis and S. Typhimurium are of particular importance, since these pathogens can colonize the chicken host without causing discernible illness in the infected chickens (Barrow et al., 1987; Ziprin et al., 1989).
4Since poultry meat will, due to EU legislation, not be brought as fresh poultry meat on the market from 12/12/2010 if Salmonella is detected, the broiler industry needs to take measures to decrease the colonization of the animals and their environment. These include pre-harvest, harvest and post-harvest measures. Harvest measures are essentially hygienic measures during catching and transport, while post-harvest measures include both hygienic measures and the application of decontaminating treatments on the meat. However, all carcass disinfectants are prohibited in the EU at present, thus decontamination is not an option. Therefore, prevention and monitoring during the live phase (pre-harvest phase) are of great importance in Europe.
5There are three basic strategies that can be employed to control Salmonella in poultry production throughout the live phase. These are:
6– preventive hygienic measures,
7– vaccination,
8– nutritional strategies or feed additives.
9Preventive hygienic measures typically involve establishing effective farm-site biosecurity and poultry house sanitation protocols. Vaccination strategy cannot be applied in broilers due to the short life span of the animal. Since the ban on sub-therapeutic antibiotic usage in Europe, the use of feed additives is more and more accepted as a valuable way to combat Salmonella infection in broilers production. Among the candidate replacements for antibiotics are organic acids, prebiotics, probiotics, symbiotics, competitive exclusion products, and bacteriophages. These products are intended to reduce the intestinal colonization and subsequent faecal excretion of Salmonella. The faecal excretion reduction will lead to a decrease in the rates of the environment contamination and consequently the risk of horizontal contamination should decline.
10Passive immunization by oral administration of hen egg yolk antibody (IgY) is an emerging and promising nutritional strategy that may serve to control Salmonella in broiler chicken industry. Some studies reported that Salmonella-specific IgY can prevent fatal Salmonellosis in mice or calves by oral administration (Peralta et al., 1994; Yokoyama et al., 1998a; 1998b).
11The present review focuses on the use of IgY for passive immunization, particularly in poultry. The characteristics of these antibodies and their production are also discussed.
2. Hen egg yolk antibodies
12The almost extreme properties of antibodies to recognize small specific structures on other molecules have made them an indispensable tool in laboratory in various applications such as research, diagnostic and therapy. Antibodies presently available for these purposes are mostly mammalian monoclonal or polyclonal antibodies. The production of these antibodies requires normally the use of laboratory animals. Nowadays, most classical chosen mammals for polyclonal and monoclonal antibodies are rabbits and mice, respectively. The procedure involves two steps, each of which causes distress to the animals: the immunization itself and repeated bleeding or sacrifying for spleen removal, which is a prerequisite for antibodies preparation.
13In 1893, Klemperer first demonstrated that the immunization of a hen resulted in the transfer of specific antibodies from the serum to the egg yolk. For over a hundred years there was no scientific application for this knowledge. But when the animal welfare became a matter of serious ethical concern for the scientific community, the results of Klemperer have attracted a great attention, particularly since the 1980s.
14In the sense of animal welfare, the use of laying hens for antibody production represents a refinement and a reduction in animal use. It is a refinement in that the painful and invasive blood sampling or sacrifying are replaced by collecting eggs. It entails a reduction in the number of animals used because the antibodies productivity in laying hens is nearly 18 times greater than that in rabbits (Schade et al., 1996).
15Furthermore, because of the high yolk antibodies concentration, over 100 mg of antibodies can be obtained from one egg (Akita et al., 1992). Since a laying hen produces approximately 20 eggs per month, 2 g of antibody per month may be obtained from a single laying hen.
2.1. Avian immune system
16Like in mammals, various mechanisms have been developed in birds to protect them from invading microorganisms and foreign substances.
17The bird’s immune system mainly consists of primary lymphoid organs and secondary lymphoid organs. Primary organs are the thymus, located in the neck along the jugular vein, and the bursa of Fabricius, located adjacent to the cloaca. Secondary organs are the spleen, bone marrow, Harderian gland, caecal tonsils, and organized lymphoid tissues associated with mucosal surfaces (MALT), including bronchial-associated lymphoid tissues (BALT), gut associated lymphoid tissues (GALT), conjunctival associated lymphoid tissues (CALT) and lymphoid nodules. There is also a lymphatic circulatory system of vessels and capillaries that transport lymph fluid through the bird’s body and communicate with the blood supply (Panada et al., 2007; Davison et al., 2008).
18Functionally, the bird’s immune system can be divided into two parts: one innate, but non-specific, while the other part is acquired and specific. The acquired immune system is characterized by specificity, heterogeneity, and memory. This system is divided into cellular branch and non-cellular (humoral) branch.
19The non-cellular branch includes immunoglobulins (antibodies) and the cells which produce them. Antibodies are specific (specificity) for the foreign material (antigen) to which they attach. The cells which produce antibodies are the B-lymphocytes. These cells are produced in the embryonic liver, yolk sack and bone marrow. The cells move to the bursa of Fabricius after 15 days incubation through 10 weeks of age. The bursa of Fabricius programs these cells which then move to the blood, spleen, caecal tonsils, bone-marrow, Harderian gland, and thymus. When a foreign organism enters the body, it is engulfed by a phagocytic-type cell, the macrophage. The macrophage digests partially the foreign organism and becomes an antigen presenter showing particles of foreign antigen on its surface. These particles form complexes with the glycoproteins of the MHC class II, which are self markers already present on the surface of the macrophage. Then, the macrophage transports each complex and exposes it to the T-lymphocytes (helper T cells). The latter, that presents a receptor that can bind specifically to the antigen presented, binds to the self / non-self complex of the macrophage. Subsequently, the helper T cell enters into contact with a B-lymphocyte, which also presents on its surface the foreign particles at the same time that the glycoproteins of the MHC class II. This binding activates the differentiation of B-lymphocyte to a plasmocyte, i.e. an effecter cell secreting antibodies. The B-cells respond by producing antibodies after day 5 following exposure. The lag period occurs because the B-cells must be programmed and undergo clonal expansion to increase their numbers. If the chicken is exposed a second time to the same antigen, the response is quicker and a much higher level of antibody production occurs (memory).
20Three immunoglobulin classes, which are distinguishable in concentration, structure, and immunochemical function, are found in birds: IgA, IgM, and IgY.
21The IgA and IgM are similar to mammalian IgA and IgM in molecular weight, structure and electrophoretic mobility.
22Historically, IgY, the major immunoglobulin class present in avian serum and egg yolk was called IgG, due to its function and serum concentration in comparison with the mammalian IgG. However, it has become clear that this is inappropriate, especially because of fundamental structural differences between IgG and IgY molecules, which will be discussed in more details later.
23IgY makes up about 75% of the total immunoglobulin pool. The serum concentrations of IgY, IgA, and IgM have been reported to be 5.0, 1.25, and 0.61 mg.ml-1, respectively (Leslie et al., 1973).
24The cellular branch of the bird’s immune system includes all the cells that react with specificity to antigens, except those associated with antibody production. The cells associated with this system, the T-lymphocytes, begin as the same stem cells as the B-cells. However, the T-lymphocytes are programmed in the thymus rather than the bourse of Fabricius. The T-lymphocytes include a more heterogeneous population than the B-cells. Some T-cells act by producing lymphokines; others directly destroy foreign organisms; some T-cells act to enhance the response of B-cells, macrophages, or other T-cells (helpers); and others inhibit the activity of these cells (suppressors) (Carlander, 2002; Janeway et al., 2002).
2.2. Transfer of IgY into egg yolk
25Antibodies are transferred from hen to chick via the latent stage of the egg, and play an important role in immunological function for the relatively immuno-incompetent chick to resist various infectious diseases.
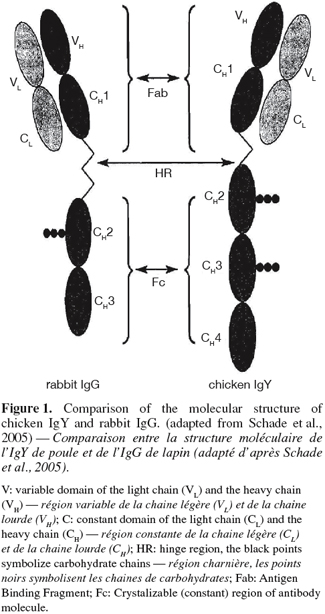
26Serum IgY is selectively transferred from the hen's circulatory system across the oolemma into the maturing oocyte in the ovarian follicle (Rose et al., 1981). This transfer occurs via a specific receptor on the surface of the yolk sac membrane which allows the selective transport of all IgY subpopulations presented by the maternal blood (Tressler et al., 1987; Mohammed et al., 1998; Morrison et al., 2001). Morrison et al. (2001) have identified several regions within the antibody molecule important for its uptake into the egg yolk. Their data demonstrate that an intact Fc (Crystalizable (constant) region of antibody molecule) and hinge region but not the Fc-associated carbohydrate (Figure 1) are required for this transport. The same findings suggest that the CH2-CH3 domain (Figure 1) is recognized by the receptor responsible for IgY transport. The transovarial passage of IgY takes approximately 3-6 days (Patterson et al., 1962; Wooley et al., 1995). The exact "lag" curve probably depends upon the number of eggs undergoing their final processing, during which IgY is transferred to the yolk (Patterson et al., 1962).
27Maternal IgA and IgM are incorporated into the egg white in the oviduct along with the egg albumen secretion. IgA and IgM in egg white are subsequently transferred to the embryonic gut via swallowed amniotic fluid (Rose et al., 1974) and IgY in egg yolk circulates in the blood of the chick via the endoderm of the yolk sac (Patterson et al., 1962). The concentrations of IgA (~0.7 mg.ml-1) and IgM (~0.15 mg.ml-1) in egg white are relatively low while that of IgY (8-25 mg.ml-1) in egg yolk is considerably high (Rose et al., 1974).
2.3. Molecular properties of IgY
28Structure of IgY. IgY has a molecular mass of ~180 kDa which is heavier than that of mammalian IgG (~150 kDa). The general structure of the IgY molecule consists of two identical heavy (H) chains and two identical light (L) chains, which are linked by disulfide bridge. The light chain of IgY consists of one variable (VL), and one constant domain (CL), like mammalian IgG. But, intra-chain disulfide linkage between the VL region and CL region of L-chain, which stabilizes the structure of the mammalian IgG L-chain is absent in the IgY L-chain and thus intra-molecular forces of IgY are weaker than those of mammalian IgG (Shimizu et al., 1993).
29The heavy chain of IgY contains one variable domain (VH), four constant domains (CH1; CH2; CH3 and CH4), unlike mammalian IgG which has three constant domains (CH1; CH2 and CH3).
30In the heavy chain of IgG, the CHl and the CH2 domains are separated by a hinge region, which gives considerable flexibility to the Fab fragment (the portion which contains the antigen-binding activity). In contrast, the heavy chain of IgY does not have a hinge region, but there are regions near the boundaries of the CHl-CH2 and CH2- CH3 domains that contain proline and glycine residues. These regions have the potential to confer limited flexibility on the molecule.
31Comparisons of C-domain sequences in IgG and IgY have shown that the CH2 and CH3 domains of IgG are the equivalents of the CH3 and CH4 domains of IgY, respectively. The equivalent of CH2 domain of IgY is absent in the heavy chain of IgG.
32The content of β-sheet structure in C domains of IgY is lower than that of mammalian IgG; therefore, the conformation of IgY domains is more disordered in comparison to mammalian IgG.
33As for IgG, the Fc part of IgY is the site of most biological effector functions. It contains two carbohydrate side-chains, in contrast to only one in IgG (Figure 1).
34Physico-chemical properties of IgY. The isoelectric point of IgY is lower than that of IgG (Polson et al., 1980). It is in the range of 5.7 to 7.6, whereas that of IgG lies between 6.1 and 8.5.
35Since the Fc fragment (the most hydrophobic moiety of the antibody molecule) of the IgY is bigger than that of the IgG, the IgY molecule is more hydrophobic than the IgG molecule (Davalos et al., 2000).
36Stability of IgY
37pH stability. The stability of IgY to acid and alkali has been studied under various conditions. It was found that the activity of IgY was decreased at pH 3.5 or lower and almost completely lost with irreversible change at pH 3 (Shimizu et al., 1988; 1992; 1993). Similar results were reported by Lösch et al. (1986), Hatta et al. (1993) and Lee et al. (2002b). Rapid decrease of the IgY activity at low pH(s) indicated conformational changes and damage in the Fab portion including the antigen-binding site.
38Under alkaline conditions, the activity of IgY did not change until the pH increased to 11. However, it was markedly diminished at pH 12 or higher (Shimizu et al., 1988; 1992; 1993). Similar results about pH effect were presented by Lösch et al. (1986), Hatta et al. (1993) and Lee et al. (2002b).
39Proteolysis stability. IgY is relatively resistant to trypsin or chymotrypsin digestion, but is fairly sensitive to pepsin digestion. Hatta et al. (1993) demonstrated that almost all of the IgY activity was lost following digestion with pepsin, but 39% and 41% of the activity remained even after 8 h of incubation with trypsin or chymotrypsin, respectively.
40The stability of IgY against pepsin appears to be highly dependent on pH and the enzyme/substrate ratio. At pH 5 or higher, IgY was fairly resistant to pepsin and retained its antigen-binding and cell-agglutinating activities. However, at pH 4.5 or below, both activities were lost (Shimizu et al., 1988). The results of Hatta et al. (1993) who also observed the IgY behavior with pepsin under different incubation times and pH confirmed the susceptibility of IgY to pepsin at low pH. Digestion of IgY with pepsin at pH 2 resulted in complete hydrolysis of the antibody molecule, leaving only small peptides. However, IgY digested with pepsin at pH 4 retained 91% and 63% of its activity after 1 h and 4 h incubation time, respectively.
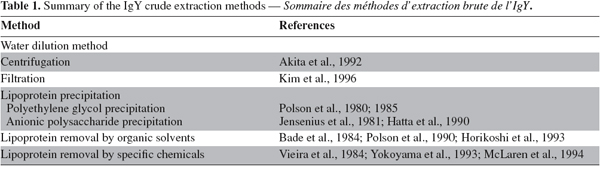
41After tryptic digestion, IgY retained its antigen-binding and cell-agglutinating activities in spite of a definite breakdown of the polypeptides. Unlike the trypsin digestion, no definite cleavage of the IgY chains was observed for chymotryptic digestion and the activities of IgY remained high for these digests (Shimizu et al., 1988; Otani et al., 1991).
42Temperature and pressure stability. IgY has been thermally treated at various temperatures for different periods of time. The binding activity of IgY with antigen decreased with increasing temperature and heating time. According to Shimizu et al. (1992) and Hatta et al. (1993), IgY is stable at temperature ranging between 60°C and 70°C. The activity of IgY decreased by heating for 15 min at 70°C or higher (Shimizu et al., 1988; 1992) and IgY denatured seriously when thermally treated at temperatures higher than 75°C (Chang et al., 1999).
43IgY is relatively stable to pressure as reported with no detectable inactivation of IgY by pressure up to 4,000 kg per cm2 (Shimizu et al., 1994).
44Freeze and spray-drying stability. Freezing and freeze-drying are low temperature processes that are usually considered to be less destructive. However, proteins may suffer from a loss of activity as a result of conformational changes, aggregation or adsorption (Skrabanja et al., 1994). There have been some reports on the stability of IgY in regard to these methods. Freezing and freeze-drying did not affect the activity of IgY unless repeated several times (Shimizu et al., 1988). However, Chansarkar (1998) showed that frozen or freeze-dried IgY resulted in some loss of antigen-binding activity and a significant drop in the solubility under the conditions of high salt and protein concentrations. The same findings were observed by Sunwoo et al. (2002). Recently, Fu et al. (2006) examined the thermal stability of IgY at various temperatures ranging between 25 and 90°C for 15 min treatment, before and after freeze-drying process. Their results indicated that freeze-dried IgY had shown a good thermal stability with no significant reduction in reactive activity except at 90°C. But this was also observed for the non freeze-dried IgY.
45Yokoyama et al. (1992) analyzed some properties of IgY powders obtained by spray-drying or freeze-drying the water-soluble fraction of egg yolks from Escherichia coli immunized hens. As compared to the freeze-dried powder, the spray-dried powder did not show a significant alteration in antibody titres and yields, even when several spray-drying temperatures were tried (140 to 170°C). However, a higher moisture content of powder was observed in the powder prepared by spray-drying than by freeze-drying.
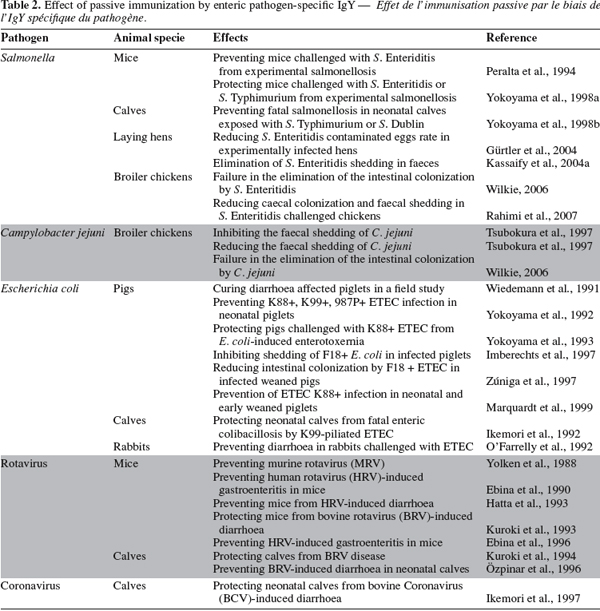
2.4. IgY production
46Immunization of the hens. Specific IgY development and production can be achieved by immunizing laying-hens with the target antigen. However, the resulting immune response of the immunized hens can not be very predictable. Mainly five factors influence this response: the antigen (dose and molecular weight), the type of adjuvant used, the route of application, the immunization frequency, and the interval between immunizations (Schade et al., 1996).
47Antigen. The immune response is triggered by contact of the organism with antigen, which is a structure that is recognized by the immune system as foreign ("non-self"). The dose of antigen influences significantly the immune response and the antibody titre that is evoked. Too much or too little antigen may induce suppression, sensitization, tolerance or other unwanted immunomodulation (Hanly et al., 1995). Behn et al. (1996) achieved better results when injecting hens with 0.1 mg of mouse IgG, instead of 1.0 mg. Schwarzkopf et al. (2000) found that the injection of antigen concentrations ranging between 10 µg and 1 mg elicited good antibodies responses, and this was also reported by other researchers (Mahn, 1998).
48Basically for each antigen, various concentrations have to be tested since the type of antigen should be also considered. Antigen can be presented to the immune system as complex multiantigens (e.g., bacteria, viruses and parasites) or as single antigens (e.g., proteins or polysaccharides) (Leenars et al., 1996). Proteins are recognized to be the most efficient immunogens because of the polymorphism of their structure and the differences existing between species and individuals (Goldsby et al., 2003). As a rule of thumb, only 10-100 µg of protein antigen should be used (Camenisch et al., 1999; Matsuda et al., 1999; Tini et al., 2002).
49Peptides (molecular weight below 10 kDa) can also be used as antigen, but they should be coupled to carriers (e.g., bovine serum albumin or keyhole limpet haemocyanin) (Schade et al., 2005). Polysaccharides antigens are efficient too. However, lipid and nucleic acids are not potent immunogens unless they are coupled to proteins or polysaccharides (Goldsby et al., 2003).
50Adjuvant. The induction of high and sustainable egg yolk antibody titre reclaims the use of adjuvant. There are more than 100 known adjuvants, which differ in their chemical characteristics, their efficacy in stimulating the immune system, and their secondary side-effects. Freund’s complete adjuvant (FCA) remains the most effective adjuvant for antibodies production in laboratory animals. In mammals, the use of this adjuvant leads systematically to severe inflammation at the injection site. In birds, the use of FCA does not seem to result in the same severe lesions as in mammals. The results of Gassmann et al. (1990) suggest that chickens show higher resistance to tissue damaging potency of FCA than rabbits. Svendsen et al. (1996) also support this finding. However, the results of Wanke et al. (1996) and Olbrich et al. (2002) contradict these data. To avoid an eventual local tissue reaction, the Freund’s incomplete adjuvant (FIA), which is the most effective substitute found to date, becomes now the most commonly used adjuvant to produce egg yolk antibody. Since FIA is less efficient than FCA, some investigators preferred the use of a combination of the two adjuvants: FCA for the first immunization and FIA for the booster immunizations (Kapoor et al., 2000; Li et al., 2006; Chalghoumi et al., 2008). In these studies good results were achieved and no adverse side-effects were reported.
51Route of application. The most common route for antigen injection in hens for IgY production is the intramuscular route. Injection is usually performed in the breast muscle (for review, see Schade et al., 2005). Some authors inject antigen in the leg, but the general recommendation states that intramuscular injection in the leg should be avoided, since it can lead to lame.
52Chicken can also be injected subcutaneously in the neck. With very young animals, it may be preferable to inject intramuscularly into the breast muscle, because subcutaneous injection is more difficult to perform and can therefore cause more distress (Schade et al., 1996).
53Immunization frequency and interval between immunizations. The total number of immunizations required depend on the type and dose of the antigen as well as the adjuvant employed. At least two immunizations have to be given. Yolk antibody titres should be checked 14 days after the last immunization. If antibodies titres begin to decrease, booster immunizations can be given during the laying period to maintain production of high levels of specific antibodies up to year (Schade et al., 1996).
54The success of an immunization protocol depends also on the interval between the first and second and subsequent immunizations. Often reported interval is two to four weeks (Camenisch et al., 1999; Matsuda et al., 1999; Tini et al., 2002).
55IgY extraction. Lipids and proteins are the major constituents of egg yolk. The lipid fraction, including triglycerides, phospholipids, and cholesterol, constitutes approximately one third of the yolk. Proteins consist 15 to 17% of the yolk, which can be separated by centrifugation into two main fractions, the granule (precipitate on centrifugation) and the plasma (clear fluid supernatant on centrifugation) (Stadeklman et al., 1977).
56Granules are about 22% of the total yolk proteins and composed of 70% high-density lipoproteins (HDL: α- and β-lipovitellins), 16% phosvitin (glycophospoprotein), and 12% low-density lipoproteins (LDL) (Burley et al., 1961).
57Plasma is about 78% of the total yolk proteins and composed of 86% of LDL and 14% of livetins (McCully et al., 1962). Livetins are water soluble, lipid-free globular glycoproteins, which are divided into three classes: α-, β-, and γ-livetin. According to Bernardi et al. (1960) the relative proportion of the three livetins in the yolk is 2:5:3, respectively.
58IgY is the predominant fraction of γ-livetin (Kovacs-Nolan et al., 2005). Separation of IgY requires therefore the removal of the lipoprotein and the recovery of the water-soluble fraction (WSF) followed by purification of the IgY from other livetins (Polson et al., 1980).
59The removal of lipoproteins and the recovery of the WSF can be achieved by several methods, as presented in table 1. These various IgY crude extraction methods give different results towards recovery and purity. Akita et al. (1993) compared the water dilution methods to other methods such as polyethylene glycol, dextran sulphate, and aliginates methods in terms of yield, purity and activity of IgY. The water dilution method yielded IgY in the highest level (91%), purity (31%) and with similar activity to that obtained by using other methods. Deignan et al. (2000) evaluated the dextran sulphate, the phosphotungstic acid, the polyethylene glycol and the isopropanol-aceton methods. The dextran sulphate, the phosphotungstic acid methods were both the better methods with regard to IgY recovery, followed by Polson method (Polson et al., 1985). The use of isopropanol-aceton method gave the lowest recovery.
60The choice of suitable IgY extraction method is mainly influenced by the quality of extraction (preservation of antibodies activity, purity and recovery of antibodies), scale of extraction (laboratory or industrial), cost effectiveness and technology. For example for a wide use of IgY in food application, a large-scale production of IgY with high recovery and purity is necessary. Such a separation process should be simple, economical and requiring few chemicals. In view of these requirements, the water dilution method appears the most appropriate technique.
61The recovery of the WSF can be followed by an additional step to separate IgY (γ-livetins) from the other water-soluble proteins (α- and β-livetins), and the remaining LDL. Three kinds of separation can be used to eliminate these contaminants: precipitation, chromatography and filtration. Several of these techniques could be included in one total clean-up procedure.
2.5. IgY use for passive immunization
62Passive immunity is the transfer of active humoral immunity in the form of ready-made antibodies, from one individual to another. Passive immunity can occur naturally, when maternal antibodies are transferred to the offspring. It can also be induced artificially, when high levels of antibodies specific for a pathogen or a toxin are recovered from immunized individual or from patient recovering from the infection and administered to non-immune individual. The antibodies transfer may be carried out via systemic, intravenous or oral route. This latter, is the route of choice for localized treatment of the digestive tract infections. Immunity derived from passive immunization lasts for only a short period of time as long as the antibodies remain in the organism, but it provides immediate protection.
63In both humans and animals, the administration of preformed specific antibodies is an attractive approach to establish protective immunity against viral and bacterial pathogens. It is becoming a more and more interesting alternative to control the increasing number of antibiotic-resistant organisms. Passive immunization can also be used against organisms that are non-responsive to antibiotic therapy.
64Antigen-specific IgY can be produced on a large-scale from eggs laid by chickens immunized with selected antigens (Hatta et al., 1997). Therefore, the use of IgY for passive immunization has been studied extensively, demonstrating its effectiveness in preventing or treating infectious diseases caused by various pathogens in animal models, especially those of the intestinal tract (Table 2). In this case, antibodies are usually administered in the feed in several forms: whole eggs powder, whole yolks powder, water-soluble fraction powder or purified IgY material. The utilization of preformed antigen-specific IgY for passive immunization in poultry can be schematized as presented in figure 2.
65As we can see in the table 2, although positive results have been obtained against some pathogens in mice, pigs and calves, very little research has been done to assess the ability of hen-egg antibodies in reducing enteric pathogen shedding and gastrointestinal infections in poultry.
66Tsubokura et al. (1997) used chicken immunoglobulin preparation from the eggs yolks of immunized hens for prophylactic and therapeutic treatment of chickens infected with Campylobacter jejuni. In prophylactic trial, 0.5 g of IgY preparation with a purity rate of 95% were preincubated with 106 CFU bacteria and administrated as a single dose to 14-days-old chickens. In the therapeutic experiment, C. jejuni-infected chickens were given 0.2 g of the same IgY preparation, as a single dose, 4 days after contamination. A marked prophylactic effect was noted using passive oral administration of anti-C. jejuni IgY in that it resulted in a 99% decrease in the number of bacteria in faeces as compared to controls. In the therapeutic experiment, a marked reduction of 80-95% was also observed. These observations suggest that oral passive immunization with anti-C. jejuni IgY may be a prophylactic and therapeutic approach in the elimination of this infection in an industrial or farm setting.
67Gürtler et al. (2004) investigated the influence of oral application of anti-S. Enteritidis egg yolk antibodies on the Salmonella contamination rate of eggs laid by experimentally infected hens at an infection dose of 2x108 CFU per hen. Antibodies were administrated as a whole egg powder, mixed with feed, at dose of 3 g per day per hen (~2.5% of the feed) for 23 or 26 days. In the experimental group 13.3% of the eggs were S. Enteritidis positive, which was significantly different from 29.4% S. Enteritidis-positive eggs in the control group. These results indicated that oral administration of whole egg powder containing S. Enteritidis-specific antibodies could reduce the rate of S. Enteritidis contaminated eggs and offer a potential tool for controlling S. Enteritidis infection.
68In multi-trials study, Kassaify et al. (2004a) examined the effects of feed supplementation with non-immunized egg yolk powder and immunized egg yolk powder (containing anti-S. Enteritidis antibodies) on the elimination of S. Enteritidis infection in laying hens. Hens were orally infected with 109 CFU S. Enteritidis. Four weeks after the bacterial challenge, hens were given daily a supplemented feed of 15% (wt/wt) of either non-immunized egg yolk powder or immunized egg yolk powder for 28 days. Oral administration of both powders resulted in a rapid decrease in the number of S. Enteritidis in faeces and an elimination of the organism after 2 weeks of feeding. In another work, Kassaify et al. (2004b) investigated the efficacy of non-immunized egg yolk powder supplement in controlling the colonization of laying hens with S. Typhimurium, C. jejuni, and E. coli. In the elimination study, four weeks after the bacterial challenge, hens were given the non-immunized egg yolk powder at concentrations ranging between 1 and 10% (wt/wt) for S. Typhimurium and C. jejuni challenge tests, and between 5 and 10% (wt/wt) for E. coli challenge test. Egg yolk powder at concentration higher than or equal to 5% (wt/wt) was capable to eliminate S. Typhimurium or to reduce significantly the bacteria shedding after 2 weeks of feeding. Egg yolk powder at concentration higher than or equal to 7.5% (wt/wt) is also capable to reduce significantly the colonization of C. jejuni and E. coli after 1 week of feeding. In the prevention trial, hens were fed supplemented feed (containing 10% of egg yolk powder) for 4 weeks and were then infected with the same pathogens. S. Typhimurium was prevented from colonization the intestine throughout the four-weeks following the challenge test, and C. jejuni and E. coli populations in the intestine were significantly suppressed. These two studies are the first and only studies to demonstrate that oral administration of non-immunized egg yolk powder can reduce the frequency of colonization of foodborne pathogens and prevent these organisms from colonizing the intestinal tract, and to suggest that the egg yolk contains other anti-infectious factors besides IgY. Subsequent in vitro work conducted by the authors (Kassaify et al., 2005) identified the protective yolk fraction against the foodborne pathogens as the granule component, HDL. However, previous studies suggested that other egg yolk components are the protective factors. Indeed, an antibacterial activity of fractionated hen egg yolk LDL from non-immunized egg yolk against two pathogenic Streptococcus strains in vitro had been reported by Brady et al. (2002). Furthermore Sugita-Konish et al. (2002) suggested that egg yolk plasma derived sialyoligosaccharides (YDS) and their derivatives are useful in preventing Salmonella infection when ingested continuously.
69Wilkie (2006) studied the effectiveness of using hen egg antibody administration on C. jejuni and S. Enteritidis colonization in the gastrointestinal tract of broiler chickens. In separate trials, day-of-hatch broiler chicks were experimentally infected with either S. Enteritidis or C. jejuni, and following challenge, anti-S. Enteritidis or anti-C. jejuni IgY were administered in form of spray-dried egg yolks powder mixed in feed at 0.05% (wt/wt) or via oral gavage with either phosphate buffered saline extracted (1/10 diluted) or ammonium-sulphate precipitated (10x concentrated) antibody. On days 4, 7, and 11 jejunum, ileum, and cecum contents were collected and plate counts obtained for the appropriate pathogen. Despite investigator observed a measurable IgY activity in vivo and in vitro, they were unable to demonstrate any significant reduction in the intestinal colonization by either S. Enteritidis or C. jejuni.
70Recently, Rahimi et al. (2007) examined the effect of S. Enteritidis-specific IgY administration on the bacterial fecal shedding, for 42 days of experiment, in 3-days old experimentally infected birds. Animals received 15 ml of yolk contained antibody mixed per 3.84 ml of drinking water on day 1 and continuing for duration of the experiment. Compared to the control animals, who did not receive antibodies, the treated animals had significantly lower faecal shedding (0% versus 14%) and lower cecal concentration of S. Enteritidis (0.27 log10 CFU versus 3.98 log10 CFU). They also had a lower isolation of S. Enteritidis from the liver, spleen and ileum. Under the condition of this study, the use of S. Enteritidis-specific IgY had a beneficial effect in reducing the colonization of Salmonella in market-aged broiler.
71The mechanism of action for orally applied IgY for pathogen reduction is still unknown. According to Peralta et al. (1994) and Tsubokura et al. (1997) antibodies are not characterized by either a bactericidal or a bacteriostatic impact. In contrast, many in vitro studies demonstrated that specific IgY have an inhibitory effect on bacterial growth of Salmonella spp. (Lee et al., 2002a; Chalghoumi et al., 2009) and E. coli (Sunwoo et al., 2002; Amaral et al., 2008; Wang et al., 2008). Agglutination, which is the interaction between antibodies and particulate antigens resulting in visible clumping, may be one mediator of growth inhibition; bacteria competing with each other in large aggregates conceivably grow more slowly than their free-swimming, single counterparts. However, agglutination may not be an important mediator of growth inhibition since the steric hindrance of two Fab arms of IgY precludes the cross-linking of bacteria (Kubo et al., 1973; Gallagher et al., 1974).
72The major mode of action is obviously the binding of antibodies to certain specific components on the bacterial surface such as outer membrane protein (OMP), lipopolysaccharide (LPS), flagella, and fimbriae (or pili). It is hypothesized that these cell surface components can easily be recognized and bound by antibodies. This binding may lead to the impairment of the biological functions of those components that play an essential role in the bacterial growth (Sim et al., 2000) and attachment to the intestinal cells (Yokoyama et al., 1998b). In this way, the antibodies protect against adhesion of bacteria at the intestinal cells (Sugita-Konishi et al., 2000; Girard et al., 2006; Chalghoumi et al., 2009) and prevent the invasion into epithelial cells (Sugita-Konishi et al., 2000).
3. Conclusion
73Salmonella is an important concern to the poultry industry mainly because of the associated contamination of poultry products for human consumption. With the EU ban on sub-therapeutic antibiotic usage in livestock industry, there is increasing interest in finding alternatives to antibiotics for poultry production. Passive immunization is one of those strategies. Egg yolk antibody prophylaxis and therapy have a natural place in poultry industry. In poultry, maternal antibodies are transmitted to the offspring via the yolk of the egg.
74Although the above success stories of prophylactic and therapeutic use of IgY in experimental models are undisputed, it would be an exaggeration, today, to consider oral IgY therapy as a common practice in livestock production, especially in poultry.
75This is undoubtedly linked to the fact that the results of experimental application of these antibodies in poultry have not always been consistent. Another interesting point is that the number of studies concerning possible beneficial effect of the use of IgY in poultry is quite low compared to studies in other animal species. Indeed, despite the fact that the European legislation does not prohibit the use of egg products in feed, since the mad cow crisis, there is still a wide spread misconception that egg yolk antibodies can not be used in chicken feed, just like animal flour in cattle feed.
76Furthermore, the production of standardized IgY, although theoretically and technically relatively simple, is labour demanding and, thus, cost intensive. Therefore, producing highly specific IgY in the most cost-effective manner possible is a key to justify commercial application of these antibodies. For example, this can be achieved by improving the immunostimulatory effect and the choice of the adjuvant used in IgY production in order to maximize the production of specific IgY (Lévesque et al., 2007). It may be possible as well to develop IgY directed against a mixture of poultry common pathogen microorganisms (Chalghoumi et al., 2008).
77Another important concern is stability of these antibodies in the gastrointestinal tract when they are fed to poultry. Orally administered antibodies, like any other protein, are susceptible to denaturation by the acidic pH of the stomach and degradation by proteases. Although, it has been shown that some immunological activity of the administrated antibodies remained detectable in the caecum, it is imperative to protect these antibodies in order to increase the fraction of immunoreactive antibodies delivered locally in the gastrointestinal tract. It has been demonstrated that microencapsulation may be an effective method for protecting IgY from gastrointestinal inactivation (Chang et al., 2002; Cho et al., 2005; Kovacs-Nolan et al., 2005), but it will induce certainly additional cost. Given that the use of IgY in the form of whole egg yolk powder might provide protection to IgY against the gastrointestinal conditions (Jaradat et al., 2000), this way of incorporation in feed seems to be the most practical and economic one, especially since it has been shown that anti-bacterial properties were associated with egg yolk components other than the IgY. Among these components are the granule HDL (Kassaify et al., 2005), the plasma LDL (Brady et al., 2002), and the YDS (Sugita-Konish et al., 2002). However, the anti-bacterial properties of these components are not yet confirmed.
78Evidence suggest that with the increasing resistance of microorganisms to antibiotics, research on all aspects related to the development of specific IgY against pathogenic microorganisms will have to be intensified.
79Abbreviations
80BALT: Bronchial Associated Lymphoid Tissues
81CALT: Conjunctival Associated Lymphoid Tissues
82FCA: Freund’s Complete Adjuvant
83FIA: Freund’s Incomplete Adjuvant
84GALT: Gut Associated Lymphoid Tissues
85HDL: High-Density Lipoproteins
86LDL: Low-Density Lipoproteins
87IgA: Immunoglobulin A
88IgG: Immunoglobulin G
89IgM: Immunoglobulin M
90IgY: Immunoglobulin Y
91MALT: Mucosal Associated Lymphoid Tissues
92MHC II: Major Histocompatibility Complex
93YDS: Sialyoligosaccharides
94WSF: Water-Soluble Fraction
95Acknowledgements
96The authors gratefully acknowledge the Funds for the Research in Industry and Agronomy (FRIA, Brussels, Belgium) for the financial support to the author.
Bibliographie
Akita E.M. & Nakai S., 1992. Immunoglobulins from egg yolks: isolation and purification. J. Food Sci., 57, 629-634.
Akita E.M. & Nakai S., 1993. Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic Escherichia coli strain. J. Immunol. Methods, 160(2), 207-214.
Amaral J.A., De Franco M.T., Zapata-Quintanilla L. & Carbonare S.B., 2008. In vitro reactivity and growth inhibition of EPEC serotype O111 and STEC serotypes O111 and O157 by homologous and heterologous chicken egg yolk antibody. Vet. Res. Commun., 32, 281-290.
Bade H. & Stegemann H., 1984. Rapid method of extraction of antibodies from hen egg yolk. J. Immunol. Methods, 72(2), 421-426.
Barrow P.A., Huggins M.B., Lovell M.A. & Simpson J.M., 1987. Observations on the pathogenesis of experimental Salmonella Typhimurium infection in chickens. Res. Vet. Sci., 42,194-199.
Behn I., Hommel U., Oertel M. & Hauschildt S., 1996. Kinetics of IgY formation after immunization of hens with different protein antigens. ALTEX, 13(5), 18-21.
Bernardi G. & Cook W.H., 1960. An electrophoretic and ultracentrifugal study on the proteins of the high density fraction of egg yolk. Biochim. Biophys. Acta, 44, 86-96.
Brady D. et al., 2002. A lipoprotein-derived anti-microbial factor from hen-egg yolk is active against Streptococcus species. J. Food Sci., 67, 3096-3103.
Burley R.W. & Cook W.H., 1961. Isolation and composition of avian egg yolk granules and their constituent alpha- and beta-lipovitellins. Can. J. Biochem. Physiol., 39, 1295-1307.
Camenisch G. et al., 1999. General applicability of chicken egg yolk antibodies: the performance of IgY immunoglobulins raised against the hypoxia-induced factor 1α. FASEB J., 13, 81-88.
Carlander D., 2002. Avian IgY antibody. In vitro and in vivo. PhD Thesis: University of Uppsala, Faculty of Medecine (Sweden).
Chalghoumi R., Théwis A., Portetelle D. & Beckers Y., 2008. Production of hen egg yolk immunoglobulins simultaneously directed against Salmonella Enteritidis and Salmonella Typhimurium in the same egg yolk. Poult. Sci., 87(1), 32-40
Chalghoumi R. et al., 2009. Adhesion and growth inhibitory effect of chicken egg yolk antibody (IgY) on Salmonella enterica serovars Enteritidis and Typhimurium in vitro. Foodborne Pathog. Dis., accepted.
Chang H.M., Ou-Yang R.F., Chen Y.T. & Chen C.C., 1999. Productivity and some properties of immunoglobulin specific against Streptococcus mutans serotype C in chicken egg yolk (IgY). J. Agric. Food Chem., 47, 61-66.
Chang H.M., Lee Y.C., Chen C.C. & Tu Y.Y., 2002. Microencapsulation protects immunoglobulin in yolk (IgY) specific against Helicobacter pylori urease. J. Food Sci., 67(1), 15-20.
Chansarkar N.L., 1998. Studies on structural stability of hen’s egg yolk immunoglobulin (IgY). PhD Thesis: University of British Columbia (Canada).
Cho Y.H. et al., 2005. Protective effect of microencapsulation consisting of multiple emulsification and heat gelation processes on immunoglobulin in yolk. J. Food Sci., 70(2), 149-151.
Collard J.M. et al., 2008. Drastic decrease of Salmonella Enteritidis isolated in humans in Belgium in 2005, shift in phage types and influence on foodborne outbreaks. Epidemiol. Infect., 136, 771-781.
Davalos L., Ortega-Vinuesa J.L., Bastos-Gonzalez D. & Hidalgo-Alvarez R., 2000. A comparative study between the adsorption of IgY and IgG on latex particles. J. Biomater. Sci. Polym. Ed., 11(6), 657-673.
Davison F., Kaspers B. & Schat K.A., 2008. Avian immunology. Amsterdam: Elsevier.
Deignan T., Kelly J., Alwan A. & O’Farrelly C., 2000. Comparative analysis of methods of purification of egg yolk immunoglobulin. Food Agric. Immunol., 12, 77-85.
Ebina T. et al., 1990. Gastroenteritis in suckling mice caused by human rotavirus can be prevented with egg yolk immunoglobulin (IgY) and treated with a protein bound polysaccharide preparation (PSK). Microbiol. Immunol., 34, 617-629.
Ebina T., 1996. Prophylaxis of rotavirus gastroenteritis using immunoglobulin. Arch. Virol., 12(1), 217-223.
EFSA (European Food Safety Authority), 2007. Report of the task force on zoonoses data collection on the analysis of the baseline survey on the prevalence of Salmonella in broiler flocks of Gallus gallus, in the EU, 2005-2006 [1] - Part A: Salmonella prevalence estimates, http://www.efsa.europa.eu/EFSA/Report/zoon_report_ej98_finbroilers_en,0.pdf, (10/05/07).
Fu C.Y. et al., 2006. Preparation and evaluation of anti-SARS coronavirus IgY from yolks of immunized SPF chickens. J. Virol. Methods, 133, 112-115.
Gallagher J.S. & Voss E.W., 1974. Conformational state of chicken 7S immunoglobulin. Immunochemistry, 11, 461-465.
Gassmann M., Thömmes P., Weiser T. & Hübscher U., 1990. Efficient production of chicken egg antibodies against a conserved mammalian protein. FASEB J., 4, 2528-2532.
Girard F. et al., 2006. Use of virulence factor-specific egg yolk-derived immunoglobulins as a promising alternative to antibiotics for prevention of attaching and effacing Escherichia coli infections. FEMS Immunol. Med. Microbiol., 46, 340-350.
Goldsby R.A., Kindt T.J., Osborne B.A. & Kuby J., 2003. Immunology. 5th ed. New York, USA: Freeman W.H. & Company.
Gürtler M., Methner U., Kobilke H. & Fehlhaber K., 2004. Effect of orally administered egg yolk antibodies on Salmonella Enteritidis contamination of hen’s eggs. J. Vet. Med. B, 51, 129-134.
Hanly W.C., Artwohl J.E. & Bennett B.T., 1995. Review of polyclonal antibody production procedures in mammals and poultry. ILAR News, 37, 93-118.
Hatta H., Kim M. & Yamamoto T., 1990. A novel isolation method for hen egg yolk antibody, "IgY". Agric. Biol. Chem., 54, 2531-2535.
Hatta H. et al., 1993. Productivity and some properties of egg yolk antibody (IgY) against human rotavirus compared with rabbit IgG. Biosci. Biotechnol. Biochem., 57(3), 450-454.
Hatta H. et al., 1997. Passive immunization against dental plaque formation in humans: effect of mouth rinse containing egg yolk antibodies (IgY) specific to Streptococcus mutans. Caries Res., 31, 268-274.
Horikoshi T., Hiraoka J., Saito M. & Hamada S., 1993. IgG antibody from hen egg yolks: purification by ethanol fractionation. J. Food Sci., 58, 739-742.
Ikemori Y. et al., 1992. Protection of neonatal calves against fatal enteric colibacillosis by administration of egg yolk powder from hens immunized with K99-piliated enterotoxigenic Escherichia coli. Am. J. Vet. Res., 53(11), 2005-2008.
Ikemori Y. et al., 1997. Passive protection of neonatal calves against bovine coronavirus-induced diarrhea by administration of egg yolk or colostrum antibody powder. Vet. Microbiol., 58, 105-111.
Imberechts H., Deprez P., Van Driessche E. & Pohl P., 1997. Chicken egg yolk antibodies against F18ab fimbriae of Escherichia coli inhibit shedding of F18 positive Escherichia coli by experimentally infected pigs. Vet. Microbiol., 54, 329-341.
Janeway C.A., Travers P., Walport M. & Shlomchick M., 2002. Immunologie. 5th ed. Berlin; Heidelberg, Deutschland: Spektrum Akademischer Verlag.
Jaradat Z.W. & Marquardt R.R., 2000. Studies on the stability of chicken IgY in different sugars, complex carbohydrates and food materials. Food Agric. Immunol., 12, 263-272.
Jensenius J.C. et al., 1981. Eggs: conveniently packaged antibodies. Methods for purification of yolk IgG. J. Immunol. Methods, 46, 63-68.
Kapoor P., Compton M.M. & Howarth B., 2000. Immunization of chickens with quail and turkey perivitelline membrane proteins: production of antibodies and their effects on fertility. Poult. Sci., 79, 245-256.
Kassaify Z.G. & Mine Y., 2004a. Effect of food protein supplements on Salmonella Enteritidis infection and prevention in laying hens. Poult. Sci., 83, 753-760.
Kassaify Z.G. & Mine Y., 2004b. Non-immunized egg yolk powder can suppress the colonization of Salmonella Typhimurium, Escherichia coli O157:H7, and Campylobacter jejuni in laying hens. Poult. Sci., 83, 1497-1506.
Kassaify Z.G., Li E.W.Y. & Mine Y., 2005. Identification of antiadhesive fraction(s) in non-immunized egg yolk powder: in vitro study. J. Agric. Food Chem., 53, 4607-4614.
Kim H. & Nakai S., 1996. Immunoglobulin separation from egg yolk: a serial filtration system. J. Food Sci., 61(3), 510-512.
Klemperer F., 1893. Ueber natürliche Immunität und ihre Verwertung für die Immunisierungstherapie. Arch. Exp. Pathol. Pharmakol., 31, 356-382.
Kovacs-Nolan J. & Mine Y., 2005. Microencapsulation for the gastric passage and controlled intestinal release of immunoglobulin Y. J. Immunol. Methods, 296, 199-209.
Kubo R.T., Zimmerman B. & Grey H.M., 1973. Phylogeny of immunoglobulins. In: Sela M., ed. The antigens. San Diego, CA, USA: Academic Press, 117-177.
Kuroki M. et al., 1993. Passive protection against bovine rotavirus-induced diarrhea in murine model by specific immunoglobulins from chicken egg yolk. Vet. Microbiol., 37, 135-146.
Kuroki M. et al., 1994. Passive protection against bovine rotavirus in calves by specific immunoglobulins from chicken egg yolk. Arch. Virol., 138, 143-148.
Lee E.N., Sunwoo H.H., Menninen K. & Sim J.S., 2002a. In vitro studies of chicken egg yolk antibody (IgY) against Salmonella Enteritidis and Salmonella Typhimurium. Poult. Sci., 81, 632-641.
Lee K.A. et al., 2002b. Acid stability of anti-Helicobacter pyroli IgY in aqueous polyol solution. J. Biochem. Mol. Biol., 35, 488-493.
Leenaars M., Claassen E. & Boersma W.J.A., 1996. Modulation of the humoral immune response: antigens and antigen presentation. In: Lefkovits I., ed. Immunology methods manual. London: Academic Press Ltd, 989-1013.
Leslie G.A. & Martin L.N., 1973. Studies on the secretory immunologic system of fowl. 3. Serum and secretory IgA of the chicken. J. Immunol., 110(1), 1-9.
Lévesque S., Martinez G. & Fairbrother J.M., 2007. Improvement of adjuvant systems to obtain a cost-effective production of high levels of specific IgY. Poult. Sci., 86, 630-635.
Li X., Shuai J. & Fang W., 2006. Protection of Carassius auratus Gibelio against infection by Aeromonas hydrophila using specific immunoglobulins from hen egg yolk. J. Zheijiang Univ. Sci. B, 7, 922-928.
Lösch U., Schranner I., Wanke I. & Jügens L., 1986. The chicken egg, an antibody source. J. Vet. Med. B, 33, 609-619.
Mahn K., 1998. Etablierung eines Immunisierungsschemas für Legehennen. PhD Thesis: University of Munich (Germany).
Marquardt R.R. et al., 1999. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early weaned piglets. FEMS Immunol. Med. Microbiol., 23, 283-288.
Matsuda H. et al., 1999. A chicken monoclonal antibody with specificity for the N-terminal of human prion protein. FEMS Immunol. Med. Microbiol., 23, 89-194.
Mayrhofer S., Paulsen P., Smulders F.J.M. & Hilbert F., 2003. Antimicrobial resistance profile of five major food-borne pathogens isolated from beef, pork and poultry. Int. J. Food Microbiol., 97(1), 23-29.
McCully K.A., Mok C.C. & Common R.H., 1962. Paper electrophoretic characterization of proteins and lipoproteins of hen’s egg yolk. Can. J. Biochem. Physiol., 40, 937-952.
McLaren R.D., Prosser C.G., Grieve R.C.J. & Borrisenko M., 1994. The use of caprylic acid for the extraction of the immunoglobulin fraction from egg yolk of chickens immunized with ovine α-lactalbumin. J. Immunol. Methods, 177, 175-184.
Mohammed S.M. et al., 1998. Deposition of genetically engineered human antibodies into the egg yolk of hens. Immunotechnology, 4, 115-125.
Morrison S.L. et al., 2001. Sequences in antibody molecules important for receptor-mediated transport into the chicken egg yolk. Mol. Immunol., 38, 619-625.
O’Farrelly C., Branton D. & Wanke C.A., 1992. Oral ingestion of egg yolk immunoglobulin from hens immunized with an enterotoxigenic Escherichia coli strain prevents diarrhea in rabbits challenged with the same strain. Infect. Immun., 60, 2593-2597.
Olbrich C. et al., 2002. Stable biocompatible adjuvants: a new type of adjuvant based on solid lipid nanoparticles: a study on cytotoxicity, compatibility and efficacy in chicken. ATLA, 30, 443-458.
Otani H., Matsumoto K., Saeki A. & Hosono A., 1991. Comparative studies on properties of hen egg yolk IgY and rabbit serum IgG antibodies. Lebensm. Wiss. Technol., 24, 152-158.
Özpinar H. et al., 1996. Dose-dependent effects of specific egg-yolk antibodies on diarrhea of newborn calves. Prev. Vet. Med., 27, 67-73.
Panada A.K. & Reddy M.R., 2007. Boosting the chick’s immune system through early nutrition. Poult. Int., http://www.poultryinternationaldigital.com/poultryinternational/200707/?pg=23, (10/06/08).
Patterson R., Youngner J.S., Weigle W.O. & Dixon F.J., 1962. Antibody production and transfer to egg yolk in chickens. J. Immunol., 89, 272-278.
Peralta R.C. et al., 1994. Passive immunisation against experimental Salmonellosis in mice by orally administered hen egg-yolk antibodies specific for 14-kDa fimbriae of Salmonella Enteritidis. J. Med. Microbiol., 41, 29-35.
Polson A., Von Wechmar M.B. & Regenmortel M.H.V.V., 1980. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol. Commun., 9, 475-493.
Polson A. et al., 1985. Improvements in the isolation of IgY from the yolks of eggs laid by immunized hens. Immunol. Invest., 14(4), 323-327.
Polson A., 1990. Isolation of IgY from the yolks of eggs by a chloroform polyethylene glycol procedure. Immunol. Invest., 19(3), 253-258.
Rahimi S. et al., 2007. Prevention of Salmonella infection in poultry by specific egg-derived antibody. Int. J. Poult. Sci., 6, 230-235.
Roberts T.A., Baird-Parker A.C. & Tompkin R.B., 1996. Salmonellae. In: Microorganisms in foods: characteristics of microbial pathogens. 5th ed. London: Blackie Academic & Professional.
Rose M.E., Orlans E. & Buttress N., 1974. Immunoglobulin classes in the hen’s egg: their segregation in yolk and white. Eur. J. Immunol., 4, 521-523.
Rose M.E. & Orlans E., 1981. Immunoglobulins in the egg, embryo, and young chick. Dev. Comp. Immunol., 5, 15-20.
Schade R. & Hlinak A., 1996. Egg yolk antibodies, state of the art and future prospects. ALTEX, 13(1), 5-9.
Schade R. et al., 2005. Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine. ATLA, 33, 129-154.
Schwarzkopf C., Staak C., Behn I. & Erhard M., 2000. Immunization. In: Schade R. et al., eds. Chicken egg yolk antibodies, production and application: IgY Technology. Berlin; Heidelberg, Deutschland; New York, USA: Springer Lab Manuals, 25-64.
Shimizu M., Nakai S. & Fitzsimmons R.C., 1988. An-E. coli immunoglobulin Y isolated from egg yolk of immunized chickens as a potential food ingredient. J. Food Sci., 5, 1360–1366.
Shimizu M. et al., 1992. Molecular stability of chicken and rabbit immunoglobulins G. Biosci. Biotechnol. Biochem., 56, 270-274.
Shimizu M., Nagashima H., Sano K. & Hashimoto K., 1993. Comparative studies on molecular stability of immunoglobulin G from different species. Comp. Biochem. Physiol., 106(2), 255-261.
Shimizu M., Nagashima H., Hashimoto K. & Suzuki T., 1994. Egg yolk antibody (IgY) stability in aqueous solution with high sugar concentrations. J. Food Sci., 59(4), 763-772.
Sim J.S., Sunwoo H.H. & Lee E.N., 2000. Ovoglobulin Y. In: Naidu A.S., ed. Natural food antimicrobial systems. New York, USA: CRC Press, 227-252.
Skrabanja A.T, De Meere A.L, De Ruiter R.A. & Van Den Oetelaar P.J., 1994. Lyophilization of biotechnology products. PDA J. Pharm. Sci. Technol., 48(6), 311-317.
Stadeklman W.J. & Cotterill O.J., 1977. Egg science and technology. 2nd ed. Westport, CT, USA: AVI Pub. Co.
Sugita-Konishi Y. et al., 2000. Blockade of Salmonella Enteritidis passage across the basolateral barriers of human intestinal epithelial cells by specific antibody. Microbiol. Immunol., 44, 473-479.
Sugita-Konishi Y. et al., 2002. Inhibition of bacterial adhesion and Salmonella infection in BALB/c mice by sialyoligosaccharides and their derivatives from chicken egg yolk. J. Agric. Food Chem., 50, 3607-3613.
Sunwoo H.H. et al., 2002. Growth inhibitory effect of chicken egg yolk antibody (IgY) on Escherichia coli O157:H7. J. Food Sci., 75, 342-345.
Svendsen L., Crowley A., Stodulski G. & Hau J., 1996. Antibody production in rabbits and chickens immunized with human IgG. A comparison of titre and avidity development in rabbit serum, chicken serum and egg yolk using three different adjuvants. J. Immunol. Methods, 191, 113-120.
Tini M. et al., 2002. Generation and application of chicken egg-yolk antibodies. Com. Biochem. Physiol. A, 131, 569-574.
Tressler R.L. & Roth T.F., 1987. IgG receptors on the embryonic chick yolk sac. J. Biol. Chem., 262, 15406-15412.
Tsubokura K. et al., 1997. Oral administration of antibodies as prophylaxis and therapy in Campylobacter jejuni-infected chickens. Clin. Exp. Immunol., 108, 451-455.
Vieira J.G.H. et al., 1984. Egg yolk as a source of antibodies for human parathyroid hormone (hPTH) radioimmunoassay. J. Immunoassay, 5, 121-129.
Wang X.Z. et al., 2008. In vitro inhibition of oral Candida albicans by chicken egg yolk antibody (IgY). Mycopathologia, 165, 381-387.
Wanke R. et al., 1996. Freund's complete adjuvant in the chicken: efficient immunostimulation with severe local inflammatory reaction. Zent. Veterinarmed A, 43(4), 243-253.
Wiedemann V. et al., 1991. Chicken egg antibodies for prophylaxis and therapy of infectious intestinal diseases. J. Vet. Med., 38, 283-291.
Wilkie D.C., 2006. Non-antibiotic approaches to control pathogens in the gastrointestinal tract of the broiler chicken. PhD Thesis: University of Saskatchewan (Canada).
Woolley J.A. & Landon J., 1995. Comparison of antibody production to human interleukin-6 (IL-6) by sheep and chickens. J. Immunol. Methods, 178, 253-265.
Yokoyama H. et al., 1992. Passive protective effect of chicken egg yolk immunoglobulins against experimental enterotoxigenic Escherichia coli infection in neonatal piglets. Infect. Immun., 60, 998-1007.
Yokoyama H. et al., 1993. Detection of passage and absorption of chicken egg yolk immunoglobulins in the gastrointestinal tract of pigs by use of enzyme-linked immunosorbent assay and fluorescent antibody testing. Am. J. Vet. Res., 54(6), 867-872.
Yokoyama H. et al., 1998a. Prevention of fatal Salmonellosis in neonatal calves, using orally administered chicken egg yolk Salmonella: specific antibodies. Am. J. Vet. Res., 59(4), 416-420.
Yokoyama H. et al., 1998b. Oral passive immunization against experimental Salmonellosis in mice using chicken egg yolk antibodies specific for Salmonella Enteritidis and S. Typhimurium. Vaccine, 16(4), 388-393.
Yolken R.H. et al., 1988. Antibodies to rotaviruses in chicken’s eggs: a potential source of antiviral immunoglobulins suitable for human consumption. Pediatrics, 81, 291-295.
Ziprin R.L., Corrier D.E. & Elissalde M.H., 1989. Maturation of resistance to Salmonellosis in newly hatched chicks: inhibition by cyclosporine. Poult. Sci., 68, 1637-1642.
Zúňiga A. et al., 1997. Reduced intestinal colonization with F18-positive enterotoxigenic Escherichia coli in weaned pigs fed chicken egg antibody against the fimbriae. FEMS Immunol. Med. Microbiol., 18, 153-161.
Om dit artikel te citeren:
Over : Raja Chalghoumi
Gembloux Agricultural University – FUSAGx. Animal Science Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: chalghoumi.r@fsagx.ac.be – Funds for the Research in Industry and Agronomy. Rue d’Egmont, 5. B-1000 Brussels (Belgium).
Over : Yves Beckers
Gembloux Agricultural University – FUSAGx. Animal Science Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : Daniel Portetelle
Gembloux Agricultural University – FUSAGx. Animal and Microbial Biology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : André Théwis
Gembloux Agricultural University – FUSAGx. Animal Science Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






