- Startpagina tijdschrift
- volume 13 (2009)
- numéro 2
- Factors influencing microbiological and chemical composition of South-Belgian raw sludge
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Factors influencing microbiological and chemical composition of South-Belgian raw sludge

Nota's van de redactie
Received on February 25, 2008, accepted on July 28, 2008
Résumé
Facteurs influençant la composition microbiologique et chimique des boues d’épuration brutes du sud de la Belgique. Les stations d’épuration produisent des boues susceptibles de contenir des microorganismes pathogènes, des éléments traces métalliques ainsi que des micropolluants organiques. L’objectif de cette étude était de mettre en évidence des facteurs qui influencent la qualité d’une boue brute, c’est-à-dire une boue fraichement produite et non traitée. La qualité des boues brutes a ainsi été caractérisée pendant un an et à chaque saison, en contrôlant certains facteurs comme le type de boue (primaire ou biologique, d’origine rurale ou urbaine) et le cycle des saisons. La qualité des boues fut estimée par la mesure de paramètres microbiologiques (Salmonella spp., Escherichia coli, entérocoques, spores de Clostridium perfringens) et chimiques (éléments traces métalliques et indice organique). L’indice organique est un nouveau paramètre basé sur la chromatographie en phase gazeuse et mis au point dans cette étude afin d’estimer la concentration en composés organiques semi-volatiles dans la boue. Les résultats ont montré des différences significatives dans la qualité des boues brutes en fonction des différents facteurs contrôlés. Ainsi, les concentrations en E. coli et en entérocoques étaient plus élevées dans les boues urbaines primaires ou biologiques comparées aux boues biologiques rurales. Les concentrations en Hg et en composés semi-volatiles étaient plus élevées dans la boue primaire urbaine comparée aux boues biologiques d’origine rurale ou urbaine. Les teneurs en As, Ni et Co étaient supérieures dans les boues brutes biologiques rurales comparées aux boues urbaines primaire ou biologique. La concentration en spores de C. perfringens dans la boue brute est plus basse en automne. L’indice organique dans les boues brutes est plus élevé en été. En conclusion, les résultats de cette étude démontrent que la qualité des boues brutes varie de manière significative en fonction du type de boue considérée et du cycle des saisons.
Abstract
Wastewater treatment plants produce sludges which are likely to contain microbial pathogens, metallic trace elements and organic micropollutants. The aim of this study was to assess factors influencing the quality of raw sludge, i.e. freshly-produced and non-treated sludge. The survey of raw sludge quality was conducted each season over a year with controlled factors such as sludge type (primary or biological; rural or urban area origin) and seasonal evolution. Quality of raw sludge was characterized by the determination of microbiological (Salmonella spp., Escherichia coli, enterococci, spores of Clostridium perfringens) and chemical parameters (metallic trace elements, organic index). The organic index is a new parameter based on a gas chromatography method and developed in this study in order to estimate global organic semi-volatile load in sludge. Results showed significant differences in raw sludge quality depending on controlled factors. Thus, E. coli, and enterococci concentrations were higher in primary and biological urban raw sludge compared to biological rural sludge. Concentrations of Hg and organic semi-volatile compounds, estimated by organic index, were higher in primary urban raw sludge than biological sludges of rural or urban origin. As, Ni and Co loads were higher in biological rural raw sludge compared to primary and biological urban sludge. Spores of C. perfringens concentration in raw sludge was lower in autumn. Organic index in raw sludge was lower in spring and in summer. In conclusion, results showed that sludge quality varies significantly, depending of sludge type and seasonal evolution.
Inhoudstafel
1. Introduction
1Generated by human activities, wastewaters are able to chemically and biologically modify the environment that collects them such as the surface waters. In order to minimize this environmental impact, wastewaters are treated in wastewater treatment plants (WWTPs). This treatment produces diverse wastes. Among these wastes, sludges are likely to contain microbial pathogens, metallic trace elements and organic micropollutants (hydrocarbons, PAHs, PCBs, etc.). According to their concentrations, these accumulated substances could be harmful for human health during their handling or after their dissemination in the environment.
2Sludge contains diverse microorganisms and among them pathogens from many types: parasites such as Giardia spp., viruses such as enteroviruses, helminths or bacteria (Gantzer et al., 2001; Rimhannen-Finne et al., 2004; Pourcher et al., 2005). Moreover, these pathogens might come from fecal origin (Escherichia coli, enterococci, Salmonella spp. or Campylobacter spp., etc.) or from environmental origin such as Listeria monocytogenes (Sahlström et al., 2004). In order to estimate the microbiological risk of sludge use, e.g. agriculture, many authors have studied the behaviour of these pathogens first during the sludge treatment processes such as liming or composting and then in the environment after sludge valorization in agriculture (Jepsen et al., 1997; Estrada et al., 2004; Horan et al., 2004).
3Just like pathogens, metallic trace elements and organic micropollutants have been quantified in sludge and their fate during sludge treatments has been studied by several authors (Lazzari et al., 2000; Stevens et al., 2001; Fuentes et al., 2004).
4In order to protect the environment and indirectly human health, legislation establishes the required quality of sludge before their valorization in agriculture. In the European Union, the 86/278/CEE directive defines threshold values for metallic trace elements of sludges spread on soils. This directive, elaborated in 1986, estimates quality of valorized sludges only on the basis of their metallic trace elements contents and is likely to be modified soon. Even still at the planning stage, several studies recommend to lower thresholds of metallic trace elements and to define new thresholds for microbiological (E. coli, Salmonella spp.) and for organic (HAPs, PCBs, etc.) parameters (European Commission DG Environment, 2000; Carrington, 2001).
5Except from temporal evolution of HAPs and metallic trace elements (Villar et al., 2006), little is known about factors influencing the microbiological and chemical quality of freshly-produced (or raw) sludge. The objective of this study was to test the influence of defined and controlled factors on raw sludge quality. These factors are sludge type (primary/biological; from rural/urban area) and seasonal evolution. To achieve this objective, raw sludges quality was monitored from different locations over a year by determining microbiological (faecal indicator bacteria) and chemical parameters (metallic trace elements, organic semi-volatile content). The study presented herein suggests the establishment of a new parameter to characterize raw sludge quality: the organic index, based on gas chromatography method, in order to estimate the global organic semi-volatile load of sludge.
2. Method
2.1. Sampling sites
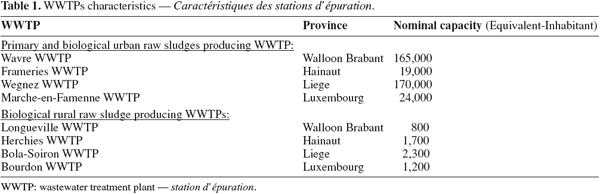
6Samples were collected in 4 of the 5 provinces of the Walloon Region, southern part of Belgium. In each province, 2 WWTPs were defined as rural or urban according to the following criteria:
7Urban WWTP:
8–nominal capacity upper than 15,000 Equivalent inhabitant,
9– treatment of domestic and industrial wastewater,
10– primary treatment by settling,
11– biological treatment by activated sludge process (secondary and occasionally tertiary treatment).
12Rural WWTP:
13– nominal capacity below 2,500 Equivalent inhabitant,
14– treatment of domestic wastewater only (no industrial wastewater),
15– no primary treatment by settling,
16– biological treatment by activated sludge process (secondary treatment).
17WWTPs selected with these criteria are the most commonly encountered in Belgium.
18These facilities produced 3 types of raw sludge:
19– primary urban raw sludge: produced by urban WWTP, during primary settling
20– biological urban raw sludge: produced by urban WWTP, during the activated sludge process
21– biological rural raw sludge: produced by rural WWTP, during the activated sludge process
22Raw sludge considered in this study was the one freshly-produced and non-treated (thickening, conditioning, dewatering, stabilization). Characteristics of WWTPs are summarized in table 1.
2.2. Sampling procedure
23Samples were collected in every WWTP at each season (autumn, winter, spring and summer) from November 2005 to November 2006.
24Samples of raw sludge were carried out into a 10 l polypropylene bucket and in 200 ml sterile glass bottles for microbiological analysis. Right after sampling, bottles were kept at 4°C until use.
2.3. Microbiological analysis
25E. coli and enterocci numerations were conducted with the MUG/EC microplate technique according to ISO 9308-3:1998 and ISO 7899-2:2000 standardized methods respectively (AFNOR, 1999a; 1999b). For each type of microorganism, 8 dilutions were used to inoculate 12 wells per dilution.
26Spores of Clostridium perfringens were determined by the Most Probable Number (MPN) technique after incubation at 46°C according to French AFNOR V59-107 standardized method (AFNOR, 1984). After thermal treatment (15 minutes at 75°C) of the sample, 6 dilutions were used to inoculate 3 tubes of lactose-sulfite medium.
27Presence of Salmonella spp. was detected with the multi-steps method (preenrichment, enrichment, isolation, biochemical and serological confirmation) according to European norm EN 12824:1997 (AFNOR, 1998).
2.4. Metallic trace elements and arsenic
28Metallic trace elements and arsenic concentrations were determined by acid digestion and atomic absorption spectrometry according to EPA 3050 method (US Environmental Protection Agency, 1996). A PerkinElmer FIMS 400 was used for Hg and a PerkinElmer AAS 800 was used for other metallic trace elements.
2.5. GC/MS screening and Organic index
29Raw sludge sample was dried with anhydrous sodium sulfate. Ten to 50 g of sample were placed with 200 ml acetonitrile in a Soxhlet extractor and extracted for 6 hours. The raw extracts were concentrated to 1 to 5 ml. Screenings were carried out with a gas chromatograph (Interscience ThermoQuest CE Instrument TRACE GC 2000) equipped with capillary column Optima-5-MS (30 m x 0.32 mm; DF = 0.25 µm) from Macherey-Nagel (Düren, Germany). Temperature program was 90°C (for 1 min) to 320°C with a rate of 5°C per min. The mass detector was a Funnigan Trace MS Quadripole, used in full scan mode (electronic impact at 70 eV). The scanned mass ranged from 50 to 590 amu (2 scans per second). Organic index was determined by integration of the recorded chromatogram (total ion current) with substracted base line (from only solvent injection) on a hydrocarbon range from C10 to C40 (as recommended by the NBN EN 14039 norm: GLC determination of C10 to C40 hydrocarbon concentrations). It represents the total peak area and is expressed in decimal logarithmic units.
2.6. Statistical analysis
30Sampling was considered as double stratified. Variables were defined by each analyzed parameter (E. coli, Zn, etc.). Three-way analysis of variance (ANOVA, cross classification) was conducted to test the influence of factors on raw sludge quality. These factors are sludge type (fixed factor, qualitative, 3 terms) and seasonal evolution (fixed factor, qualitative, 4 terms). The geographical factor (random factor, qualitative, 4 terms) could not have been statistically tested because of the absence of repetition. ANOVA test and verification of hypotheses (normality and homogeneous variance of populations) were performed with Minitab 14 software (Minitab Inc.).
31Differences between means were determined by Newman-Keuls test and considered as being significant at P < 0.05.
3. Results and discussion
32In this study, the microbial and chemical quality of raw sludge was monitored over one year. The survey was led in 4 of 5 provinces of southern Belgium, for each season (autumn, winter, spring and summer) and in the most common types of WWTPs. The aim of these campaigns was to assess the influence of sludge type and seasonal evolution on raw sludge quality.
3.1. Temperature, pH
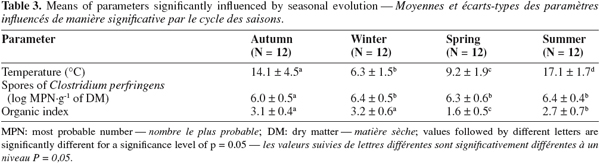
33Mean sludge temperatures during the 2005-2006 period of the survey were 12.1, 12.1 and 10.7°C for the primary urban raw sludge, biological urban raw sludge and for the biological rural raw sludge, respectively (Table 2). During this sampling campaign, temperature was significantly lower for the rural raw sludge. This fact could be explained by lower air temperature in rural areas compared to highly populated areas. Table 3 contains parameters influenced by seasonal evolution. Mean sludge temperature varied in a significant manner in raw sludge from 6.3°C in winter to 17.1°C in summer.
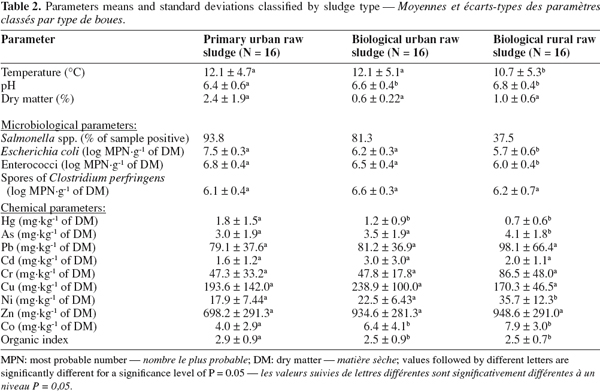
34As shown in table 2, pH was close to neutral and was significantly lower for primary urban raw sludge. Low oxygen concentration in settling basin generates an anaerobic fermentation metabolism by microorganisms in primary sludge. The acidogenic step of this fermentation could explain the significant lower pH in primary sludge. On the contrary, no such fermentation occurs in well aerated tanks where biological sludge is produced.
3.2. Microbiological parameters
35Mean levels of bacterial indicators are shown in table 2. Salmonella spp. was isolated in 93.8, 81.3 and 37.5% of primary urban raw sludge, biological urban raw sludge and biological rural raw sludge, respectively. E. coli concentrations ranged from 5.7 to 7.5 log MPN.g-1 of DM. Enterococci concentrations ranged from 6.0 to 6.8 log MPN.g-1 of DM. Spores of Clostridium perfringens concentrations ranged from 6.1 to 6.6 log MPN.g-1 of DM.
36The present results agree with others, reporting similar contaminations levels of E. coli, enterococci and the occurrence of Salmonella spp. in raw sludges (Sahlström et al., 2004; Mandilara et al., 2006).
37Globally, results showed a high contamination of raw sludge by faecal microorganisms. In order to reduce biological hazard of sludge before its valorization in agriculture, treatments with hygienization effect seem therefore mandatory. Moreover, as shown in table 2, this level of contamination was influenced by the type of sludge. Thus, biological rural raw sludges were less likely to contain Salmonella spp. Statistical analysis could not be applied to this kind of data because of their presence/absence type, so differences between means could not be qualified as significant or not. However, biological rural raw sludges were significantly less contaminated by E. coli and enterocci compared to urban raw sludges. This difference could be more than 10 times as shown for E. coli in table 2. Such a difference in rural raw sludge could be explained by less faecal contaminated wastewater at the entrance of the WWTP. It could also be explained by a high sludge age or a high retention time of wastewater in small rural WWTPs, which might increase the mortality of faecal microorganisms. In future studies, it would be interesting to verify the assumption that rural raw sludges are less contaminated by faecal pathogens, by monitoring occurrence of other microorganisms such as enteric viruses and helminth eggs.
38Seasonal evolution influenced spores of C. perfringens concentration (Table 3). Mean concentration of spores of C. perfringens was significantly lower in autumn compared to other seasons. This observation indicated that seasonal evolution could influence microbiological quality of raw sludge.
3.3. Metallic trace elements and arsenic
39As shown in table 2, standard deviations for the trace elements were high (0.6 to 291 mg.kg-1 of DM), meaning that their concentrations in raw sludges were very variable.
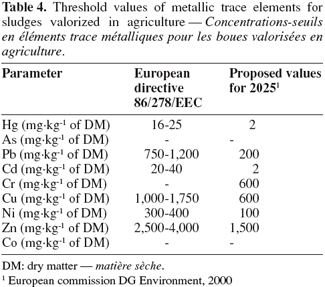
40These concentrations were below threshold values currently in application for agricultural valorized sludges in European Union and threshold values proposed for 2025 (Table 4). Concentrations of Pb, Cr, Cu, Ni, Zn in raw sludge samples were globally below threshold values. Nevertheless, if Hg and Cd concentrations in sludge do not decrease in the future, these 2 elements could limit or even prevent sludge valorization in agriculture. Indeed, at the horizon of 2025, the maximum allowed levels will be fixed at 2 mg.kg-1.
41Generally, industrial activities are considered to mainly contribute to high levels of metallic trace elements in wastewaters. When wastewaters are treated by a WWTP, metallic trace elements are likely to concentrate in sludge. This could explain significantly lower Hg concentration in rural raw sludge from low industrial activities areas (Table 2). Biological rural raw sludge area presented equivalent levels of Pb, Cd, Cr, Cu and Zn compared to urban raw sludges produced from industrial and domestic wastewaters. Moreover, levels of As, Ni and Co were significantly higher in rural raw sludges. It seems, then, that industrial activity is not the only parameter to explain metallic trace elements concentrations in sludge. Soil particles could contribute to metallic trace elements in sludge by containing relatively high contents of As and Ni. According to some authors, typical As and Ni concentrations ranged in soils from 0.1 to 40 mg.kg-1 of DM and from 0.003 to 100 mg.kg-1 of DM, respectively (Pierzynski et al., 2000; Deneux-Mustin et al., 2003). Rainwater could drag these metals to the sewer and then to the WWTP.
42According to other authors, concentrations of metallic trace elements in sludges from southern Spain follow a temporal evolution and are higher during winter (Villar et al., 2006). These authors reported no rainfall in summer, and according to them metallic trace elements would be dragged to WWTP by winter rains. In the present study, no such seasonal influence on metallic trace elements concentrations has been highlighted probably because of climate profile. In Belgium, rainfall is distributed all over the year even in summer. Indeed, according to environmental authorities, the annual distribution of rainfall is homogeneous with 160 to 200 days of precipitations up to 0.1 l.m-2 (Cellule Etat de l’Environnement Wallon, 2005).
3.4. Organic index
43In this study, we have elaborated a new parameter to estimate global semi-volatile organic load in sludge, i.e. the organic index. The organic index was based on a gas chromatography screening conducted in precise and repeatable conditions. In such conditions, semi-volatiles which represent organic compounds such as hydrocarbons (C10 to C40), PAHs, PCBs, phtalates, organochlorine pesticides are eluted. Organic index is then calculated by integration of all the peaks from the resulting chromatogram, and expressed in decimal logarithmic units.
44Results of organic index are shown in tables 2 and 3. Considering the sludge type factor, mean index were 2.9, 2.5 and 2.5 for primary urban, biological urban and biological rural raw sludges respectively. Considering the seasonal evolution factor, mean index were 3.1, 3.2, 1.6 and 2.7 for respectively autumn, winter, spring and summer.
45According to Newman and Keuls test, organic index was significantly higher in primary urban sludge, compared to biological urban or rural ones (Table 2). Two assumptions could explain such a difference. First, WWTPs located in urban areas were much likely to receive wastewaters contaminated by hydrocarbons and PAHs from road traffic and phtalates from industrial activities. These compounds could then be concentrated in sludge during primary settling. Another assumption is based on the ability of microorganisms to degrade organic compounds. For instance, in biological processes such as sludge composting or anaerobic digestion, some PAHs and PCBs were reported to be eliminated by biodegradation (Lazzari et al., 2000; Bernal-Martinez et al., 2005). In settling tank, where the primary sludge is produced, oxygen is low causing a slow fermentative biodegradation. On the contrary in aerated basin, where biological sludge is produced, oxygen concentration is higher and a high oxidative biodegradation is expected.
46Organic index varies during seasons and is significantly lower in spring and then in summer. Temperature could partly explain this difference by influencing the microbiological metabolism. With mild temperatures, bacterial metabolism should be higher and could then contribute to organic compounds biodegradation.
47In future studies, it should be interesting to get organic index values from other authors, other sites or countries and to correlate them to PAHs and PCBs loads in sludge.
4. Conclusion
48Results showed that factors, i.e. sludge type and seasonal evolution, significantly influence microbiological and chemical quality of raw sludge:
49– E. coli, and enterococci concentrations were higher in primary and biological urban raw sludge compared to biological rural sludge,
50– concentrations of Hg and organic semi-volatile compounds, estimated by organic index, were higher in primary urban raw sludge than biological sludges of rural or urban origin,
51– As, Ni and Co loads were higher in biological rural raw sludge compared to primary and biological urban sludge,
52– spores of C. perfringens concentration in raw sludge was lower in autumn,
53– organic index in raw sludge was lower in spring and in summer.
54Acknowledgements
55This research was financially supported by the Federal Public Service of Belgium, division of Health, Food Chain Safety and Environment. T. A. Guillemet would like to thank personally Julie Guisset and Aï Sugiura for their help on this paper.
Bibliographie
AFNOR, 1984. Norme NF V59-107. Recherche des spores de Clostridium perfringens. Technique du nombre le plus probable après incubation à 46 °C. Paris : Association Française de Normalisation.
AFNOR, 1998. Norme NF EN 12824. Méthode horizontale pour la recherche des Salmonella. Paris : Association Française de Normalisation.
AFNOR, 1999a. Norme NF EN ISO 9308-3. Recherche et dénombrement des Escherichia coli et des bactéries coliformes dans les eaux de surface et résiduaires (Standard NF EN ISO 9308-3. Isolation and counting of Escherichia coli and coliforms in surface water and in wastewater). Paris : Association Française de Normalisation.
AFNOR, 1999b. Norme NF EN ISO 7899-1. Recherche et dénombrement des entérocoques intestinaux dans les eaux de surface (Standard NF EN ISO 7899-1. Isolation and counting of enterococci and coliforms in surface water and in wastewater). Paris : Association Française de Normalisation.
Bernal-Martinez A., Carrère H., Patureau D. & Delgenès J.P., 2005. Combining anaerobic digestion and ozonation to remove PAH from urban sludge. Process Biochem., 40(10), 3244-3250.
Carrington E.G., 2001. Evaluation of sludge treatments for pathogen reduction. Final Report. Report n°5026/1. Luxembourg: Office for Official Publications of the European Communities.
Cellule Etat de l’Environnement Wallon, 2005. Etat de l’environnement wallon. Namur, Belgique : Ministère de l'Agriculture, des Affaires rurales, de l'Environement et du Tourisme.
Deneux-Mustin S. et al., 2003. Mobilité et transfert racinaire des éléments en traces. Influence des microorganismes du sol. Paris : Tec & Doc-Lavoisier.
Estrada I.B. et al., 2004. The survival of Escherichia coli, faecal coliforms and enterobacteriaceae in general in soil treated with sludge from wastewater treatment plants. Bioresour. Technol., 93(2), 191-198.
European Commission DG Environment, 2000. Working document on sludge. Report ENV.E.3/LM. Brussels: European Commission.
Fuentes A. et al., 2004. Simple and sequential extractions of heavy metals from different sewage sludges. Chemosphere, 54(8), 1039-1047.
Gantzer C. et al., 2001. Monitoring of bacterial and parasitological contamination during various treatment of sludge. Water Res., 35(16), 3763-3770.
Horan N.J. et al., 2004. Die-off of enteric bacterial pathogens during mesophilic anaerobic digestion. Water Res., 38(5), 1113-1120.
Jepsen S.E., Krause M. & Grüttner H., 1997. Reduction of fecal streptococcus and Salmonella by selected treatment methods for sludge and organic waste. Water Sci. Technol., 36(11), 203-210.
Lazzari L., Sperni L., Bertin P. & Pavoni B., 2000. Correlation between inorganic (heavy metals) and organic (PCBs and PAHs) micropollutant concentrations during sewage sludge composting processes. Chemosphere, 41(3), 427-435.
Mandilara G.D. et al., 2006. Correlation between bacterial indicators and bacteriophages in sewage and sludge. FEMS Microbiol. Lett., 263(1), 119-126.
Pierzynski G.M., Sims J.T. & Vance G.F., 2000. Soils and environmental quality. 2nd ed. Washington, DC: CRC Press.
Pourcher A.M. et al., 2005. Decrease of enteric microorganisms from rural sewage sludge during their composting in straw mixture. J. Appl. Microbiol., 99(3), 528-539.
Rimhannen-Finne R. et al., 2004. Comparative analysis of Cryptosporidium, Giardia and indicator bacteria during sewage sludge hygienization in various composting processes. Lett. Appl. Microbiol., 38(4), 301-305.
Sahlström L. et al., 2004. Bacterial pathogen incidences in sludge from Swedish sewage treatment plants. Water Res., 38(8), 1989-1994.
Stevens J., Green N.J. & Jones K.C., 2001. Survey of PCDD/Fs and non-ortho PCBs in UK sewage sludges. Chemosphere, 44(6), 1455-1462.
US Environmental Protection Agency, 1996. Method 3050B. Acid digestion of sediments, sludges and soils. Washington, DC: US Environmental Protection Agency.
Villar P. et al., 2006. Temporal evolution of polycyclic aromatic hydrocarbons (PAHs) in sludge from wastewater treatment plants: comparison between PAHs and heavy metals. Chemosphere, 64(4), 535-541.
Om dit artikel te citeren:
Over : Thibault A. Guillemet
Gembloux Agricultural University – FUSAGx. Laboratoire d'Ecologie microbienne et d’Epuration des Eaux. Bâtiment 52. Avenue Maréchal Juin, 27. B-5030 Gembloux (Belgium). E-mail: guillemet_t@yahoo.fr
Over : Philippe Maesen
Gembloux Agricultural University – FUSAGx. Bureau Environnement Analyses de Gembloux (BEAGx). Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : Émile Delcarte
Gembloux Agricultural University – FUSAGx. Bureau Environnement Analyses de Gembloux (BEAGx). Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : Georges C. Lognay
Gembloux Agricultural University – FUSAGx. Unité de Chimie analytique. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : Adeline Gillet
Gembloux Agricultural University – FUSAGx. Unité de Statistique, Informatique et Mathématique appliquées. Avenue de la Faculté, 8. B-5030 Gembloux (Belgium).
Over : Jean-Jacques Claustriaux
Gembloux Agricultural University – FUSAGx. Unité de Statistique, Informatique et Mathématique appliquées. Avenue de la Faculté, 8. B-5030 Gembloux (Belgium).
Over : Marc Culot
Gembloux Agricultural University – FUSAGx. Laboratoire d'Ecologie microbienne et d’Epuration des Eaux. Bâtiment 52. Avenue Maréchal Juin, 27. B-5030 Gembloux (Belgium).






