- Accueil
- volume 13 (2009)
- numéro spécial
- Control tools to detect processed animal proteins in feed and in animal by-products: specificity and challenges
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Control tools to detect processed animal proteins in feed and in animal by-products: specificity and challenges

Abstract
This paper reviews the current situation with regard to a total feed ban on the use of processed animal proteins in feed for meat producing animals within the EU. The scientific aspects surrounding the development of control tools are discussed. In particular, focus is given to methods for marking those materials prohibited in animal feeds and for the determination of species specificity in those proteins that are potentially allowed in animal feeds. The overall objective is that the advancements in science are utilized to achieve a partial relaxation of the total feed ban in the near future.
Table des matières
1. Introduction
1The feed ban instituted within the EU for animal proteins produced and used in the EU (and for animal proteins exported out of the EU), has now been in operation for over seven years. The ban was enacted to achieve one specific objective: to prevent ruminant species consuming ruminant proteins. However, while the bovine spongiform encephalopathy (BSE) epidemics in all EU countries have now been brought under control and new cases of BSE have fallen by over 40% per year over the last 10 years (OIE, 2008), the feed ban remains.
2What can be done now to partially lift the feed ban according to the EU TSE regulations (EC, 2001) –whilst at the same time continuing the eradication of transmissible spongiform encephalopathy (TSE) and maintaining a high level of prevention of any new or additional TSE epidemics in the future?
3The question normally posed regarding "what are the criteria needed to be able to lift the feed ban?" is answered by reference to the need for "control" tools. In one sense, several of the control tools indicated by the regulators are already in existence. Primarily, document controls are already used in the process chain from animal to slaughter to the production of Category 3 by-products, their processing and the dispatch of processed products. Secondly, the marking of Category 1 and 2 by-products and the products derived from them has recently been approved after a rigorous and practical series of validation tests. However, are these control tools good enough to satisfy the rigorous criteria above?
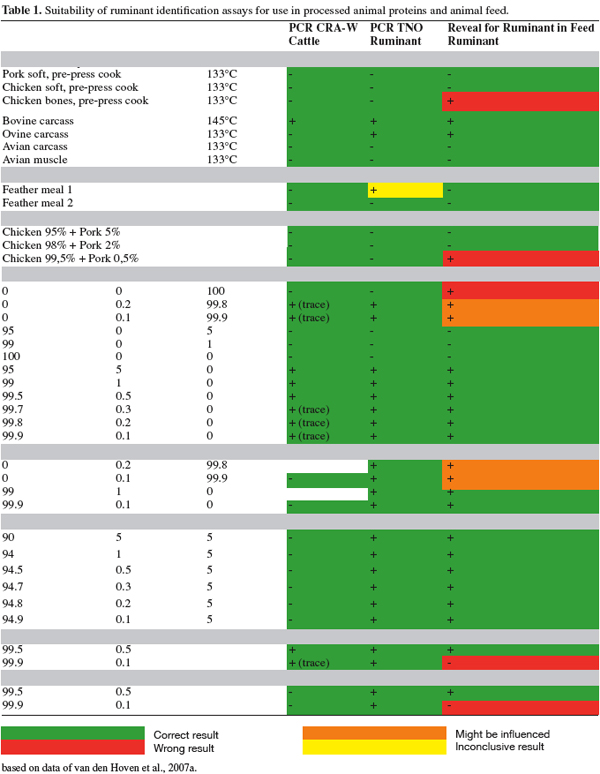
4Some preliminary work has been completed in the area of species specificity. The Joint Research Centre (JRC-IRMM) in Geel (BE) has conducted a pre-validation study for the detection of processed animal proteins (PAP) in feed by Polymerase Chain Reaction (PCR) (Prado et al., 2006). Within this study, three European institutes, CRA-W in Gembloux (BE), TNO (NL) and VLA (UK) participated in this study with their PCR methods. However is more work needed and if so, what approaches should be taken?
5For example, should there be attempts made to validate a test to identify the species of origin of a PAP? In itself, this is an interesting question, as it does not fully explore the scope and intent of the feed ban, but rather poses further questions. The situation is complicated by the fact that the current feed ban includes all animal proteins (with some key exceptions). If there was a method of discriminating between ruminant PAP and non-ruminant PAP then perhaps this might give grounds for re-focusing the feed ban on ruminant PAP alone. If such a method was available for ruminant PAP in non-ruminant PAP, then what detection level is needed in order to result in the continuation of the BSE eradication and prevention program? In addition, if such a PAP test was validated, how would it fit into the overall feed control program, which relies heavily on the presence/absence of muscle fibres and bones in animal feed?
6Much of this discussion particularly surrounds the ability of microscopy to detect 0.1% terrestrial proteins in animal feeds. Thereafter, other detection technologies might be expected to be validated to at least this level in feeds. However, what about ruminant contamination in non-ruminant Paps? This question has not yet been resolved satisfactorily by the regulators and is now the main focus of EFPRA in their attempt to achieve the re-entry of certain Paps into mono-gastric animal feed.
2. Material and methods
7The development of the "marker" Glycerol Tri-Heptanoate (GTH) by researchers at JRC-IRMM and CCL came after a wide ranging series of tests that considered many different dyes and chemicals.
8GTH proved to be a suitable marker for processed products in rendering plants. JRC-IRMM together with CCL performed an implementation study for the use of GTH at 10 European rendering plants which represented many of the different types of approved processes in the EU.
9To assist EFPRA in determining the risk from contamination of Paps and to provide a scientific and independent platform for a risk based assessment by EU regulators, EFPRA commissioned a report from DNV in 2006 (DNV, 2006). In summary, the risk assessment considered a number of scenarios in relation to two possible areas of cross contamination. Firstly, ruminant contamination of non-ruminant PAP. Secondly, the possibility of the non-ruminant PAP being used in ruminant feed. The results indicated very low levels of risk – in terms of the chance of increased cases of BSE in the European cattle herd – and also produced a lower "risk" than seen in some EFSA opinions (EFSA, 2005; 2007). As a result, EFPRA therefore propose that a level of 1% contamination of non-ruminant PAP with ruminant PAP is acceptable as a practical threshold level, which does not increase the risk of additional TSE within ruminants.
10With regard to this proposal, EFPRA and colleagues at CCL have conducted some research in the field of species detection and identification of terrestrial proteins in animal feeds and Paps.
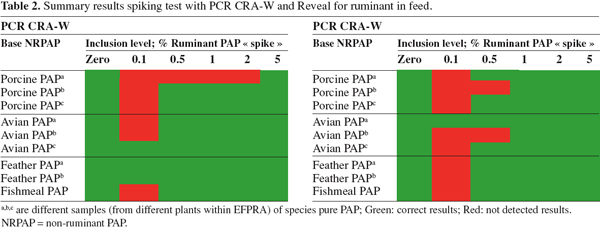
11The first step of CCL was to test the suitability of several existing techniques, other than microscopy, to identify terrestrial proteins in Paps and feed. The techniques chosen included two PCR methods; Community Reference Laboratory, Gembloux (BE) and TNO (NL) and an immunochemical (dipstick) method, Reveal kit (Neogen) for ruminant in feed.
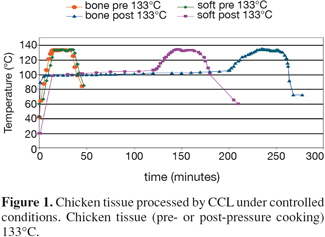
12Reference processed animal protein materials were prepared in The Netherlands and in the UK. Some reference materials (porcine soft material, porcine bone material, chicken soft material and chicken bone material) were processed by CCL in a dedicated sterilizer, under strict conditions (20 minutes at 133°C, pre- or post-pressure cooking) (Figure 1) (van den Hoven et al., 2007a). Other reference materials (bovine, ovine, porcine and avian carcasses and muscle material) were processed by PDM Ltd (Doncaster, UK) at 133°C, 137°C, 141°C and 145°C. Furthermore, some mixtures in Paps and feed were prepared with these reference materials. The main purpose of processing reference materials was to obtain materials that are definitely not contaminated by other sources. Besides the reference materials and the mixtures, based on these reference materials, "pure" commercial materials were collected from rendering plants.
3. Results and discussion
3.1. GTH marker
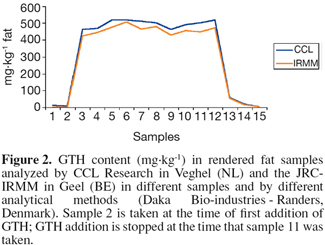
13The results of the rendering validation study are illustrated by one example from one of the rendering participants in the study. In all products (MBM and fat) from the participating rendering plants, the GTH was clearly detectable throughout the trial period (Figure 2). In practice a minimum concentration of 250 mg GTH per kg fat is efficient as marker for Category 1 and 2 materials (van den Hoven et al., 2007b).
3.2. Species identity
14The tested methods gave quite good results on these reference materials (see table 1; part of the results). The PCR methods tested were even capable of identify the material that was heated for 20 minutes at the highest temperatures.
15However, for feed analyses with PCR, permitted products such as milk, blood products and fat, will interfere with the detection of the target DNA. The Reveal test has the advantage that it is not sensitive for these permitted products, although it may have some limitations in terms of some false positive reactions to certain feed components and a lower sensitivity than PCR. For some (3 out of 28) chicken PAPs samples false positive results are obtained with the Reveal for detection of ruminant proteins. Nonetheless, this latter point needs to be taken in context and the sensitivity issue must relate to the risk assessments completed for each stage of the feed production chain.
16The Reveal kit (Neogen) for detection of ruminant proteins in feed gave more accurate results with “European PAPs” than the kit that is developed by Neogen for the detection of ruminant proteins in MBM. With ruminant in MBM kit, a high percentage of false negative results were found. This may be caused by the very high concentration of all types of animal proteins overloading the kit reagents. Consequently, Reveal kit for detection of ruminant proteins in feed was used for testing with Paps.
17From the PCR results obtained it could be concluded:
18– all tests gained a very high sensitivity, being able to detect 0.1% cattle MBM,
19– a careful setting of the cut-off level must be done in order to have enough sensitivity without loosing specificity,
20– the presence of animal fats from the rendering industry might be a source of "false positive" results in some feeds, especially pig feeds, where this fat is usually present.
21The actual state of art of the PCR makes it a promising technique to clearly identify animal species in feeds, allowing a change in European legislation towards a less restrictive use of some kind of animal materials as ingredients.
22The next step of CCL was to determine the detection level of ruminant proteins in non-ruminant Paps and feed with two of the techniques. Some pure single species non-ruminant materials (poultry meal, porcine meal, feather meal, fishmeal) in which no DNA was detectable with PCR and Reveal, were spiked with various levels of ruminant (mixture of bovine and ovine) Paps. The two techniques used (from table 1) were the PCR method of CRA-W and the Reveal kit for ruminant in feed because this method would be a promising quick screening test. Table 2 shows a summary of the spiking test with PCR CRA-W and Reveal for ruminant in feed.
23The results in table 2 show that in general levels of 1% or higher are detected with both methods. The results of the PCR test show that the detection level can be 0.1% but this is strongly dependent on the matrix or homogeneity.
4. Conclusion and recommendations
24Following the rendering validation studies, GTH is now adopted as a marker for Category 1 and 2 animal by-products (EC, 2007).
25The Reveal for ruminant in feed test appears to be a promising method for screening non-ruminant Paps for the presence of ruminant PAP at a level of 1% or higher (Vaessen et al., 2007). This method has the advantage that there is no interference with the already allowed PAPs for use in feed (e.g. milk and non-ruminant blood proteins). EFPRA consider that incorporation of the Reveal for ruminant in feed test within a control tool program, would enhance a risk based-control tool approach to allow the use of non-ruminant PAP in non-ruminant animal feeds.
26Of course, changes to the EU feed controls will be decided by risk managers in the light of all the information available. In this context, it is considered that the newly available GTH marking system and the potential use of a screening method for ruminant PAP in non-ruminant PAP will enhance the EU feed control program in eradicating and preventing TSE diseases in the future.
27There remains one area of concern, i.e. the ban on intra-species (porcine/poultry) recycling. PCR methods for identification of porcine or chicken DNA in Paps are available now, but have to be further validated.
28However and most importantly, this problem may be temporarily overcome by an EFPRA (EFPRA, 2007) proposal for terrestrial non-ruminant PAP to be approved for use in aquatic species feeds (Aqua-Feeds). This proposal considers all of the above mentioned and available control tools incorporated into a holistic risk based approach through dedicated channels. EFPRA recognizes that this approach may represent the first step in the re-authorization for all Paps into all non-ruminant (e.g. pig and poultry) feeds.
29How do these ideas and proposals fit into the SAFEED-PAP project? It is important to recognize of course that there are no real conflicts, as controls for both feed and PAP are necessary. However, from a practical industry perspective it would be helpful to consider the aspects of PAP cross contamination with a higher level of priority than it currently receives. For the rendering process industry, PAP is the real means of utilization that requires control. What is more, release of certain non-ruminant Paps from the feed ban will in principle allow export of these Paps to third countries. EFPRA therefore consider that PAP specific control tools are essential for harmonious international trade. Furthermore EFPRA propose that significant effort is expended to ensure international validation of any successfully validated EU PAP species methods.
Bibliographie
DNV, 2006. DNV report for EFPRA 22514037 final (04-08-2006). Brussels: EFPRA.
EC, 2001. Regulation (EC) n° 999/2001 of the European Parliament and of the Council of 22 May 2004 laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. Off. J. Eur. Communities, L147, 31/05/2001, 1.
EC, 2007. Regulation (EC) n° 1432/2007. As regards the marking and transport of animal by-products. Off. J. Eur. Communities, L 320, 06/12/2007, 13.
EFPRA, 2007. EF-07-085 Proposal for the approval non-ruminant processed animal proteins in feeds for aquatic species (Aqua-feeds). Brussels: EFPRA.
EFSA, 2005. Opinion of the scientific panel on biological hazards (BIOHAZ) on the quantitative risk assessment of the animal BSE risk posed by meat and bone meal with respect to the residual BSE risk (EFSA-Q-2003-099). EFSA Journal, 257, 1-30.
EFSA, 2007. Certain aspects related to the feeding of animal proteins to farm animals: scientific opinion of the panel on biological hazards (EFSA-Q-2007-084). EFSA Journal, 576, 1-41.
OIE (World Organisation for Animal Health), 2008. Http://www.oie.int/eng/info/en_esbmonde.htm, (21.04.08).
Prado M., Boix A. & Von Holst C., 2006. Prevalidation study for the detection of processed animal proteins (PAPs) in feed by Polymerase Chain Reaction (PCR). GE/R/FSQ/02/2006. Geel, Belgium: JRC/IRMM.
Vaessen J., van den Hoven S. & Margry R., 2007. CCL report RAP-1001353, 17th October 2007. Veghel, The Netherlands: CCL-Nutricontrol.
van den Hoven S. et al., 2007a. Suitability of ruminant identification assays for use in processed animal proteins and animal feed. Poster at Feed safety international conference, 27th and 28th November 2007, Namur, Belgium, Book of abstracts, 76-77.
van den Hoven S. et al., 2007b. Development of glyceroltriheptanoate (GTH) as a chemical marker for animal by-products. Poster at Feed safety international conference, 27th and 28th November 2007, Namur, Belgium. Book of abstracts, 78-79.
Pour citer cet article
A propos de : Stephen Lewis Woodgate
European Fat Processors and Renderers Association (EFPRA). Boulevard Baudouin, 18/4. B-1000 Brussels (Belgium). E-mail: stephen@beaconresearch.co.uk
A propos de : Suzanne van den Hoven
CCL-Nutricontrol. PO Box 107. NL-5460 AC Veghel (The Netherlands).
A propos de : Judith Vaessen
CCL-Nutricontrol. PO Box 107. NL-5460 AC Veghel (The Netherlands).
A propos de : Rob Margry
CCL-Nutricontrol. PO Box 107. NL-5460 AC Veghel (The Netherlands).






