- Home
- volume 12 (2008)
- numéro 3
- Studies on the antifungal activities of the novel synthesized chelating co-polymer emulsion lattices and their silver complexes
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Studies on the antifungal activities of the novel synthesized chelating co-polymer emulsion lattices and their silver complexes

Editor's Notes
Received on March 26, 2004, accepted on May 2, 2007
Résumé
Etudes de l'activité antifongique des nouveaux composés synthétiques de co-polymères de chélation en émulsion et de leurs complexes d'argent. Des nouveaux composés binaires de chélation de co-polymères d'acrylate de butyle avec les acides itaconique et maléique ont été préparés par polymérisation en émulsion. La chélation de co-polymères d'acrylate de butyle-co-acide itaconique (BuA/IA) et d'acrylate de butyle-co-acide maléique (BuA/MA) et leurs complexes d'argent ont été identifiés et caractérisés par spectroscopie infrarouge et par des mesures de calorimétrie différentielle à balayage. Les activités biologiques de ces composés ont été étudiées dans différents types de souches fongiques. Le dosage et le taux de lixiviation des ions d'argent ont été contrôlés par type de co-polymères utilisé et la solubilité dans le milieu. Les résultats confirment que les polymères contenant des biocides chimiquement liés sont utiles pour empêcher la croissance des micro-organismes. L’absorption d’argent par des souches d'organismes fongiques a été étudiée pour préciser l'action antifongique de ces composés. Les modalités d'absorption ont été examinées par microscopie électronique à transmission (TEM).
Abstract
The novel binary chelating co-polymers of butyl acrylate with itaconic and maleic acids were prepared by emulsion polymerization process. The chelating co-polymers of butyl acrylate-co-itaconic acid (BuA/IA) and butyl acrylate-co-maleic acid (BuA/MA) and their silver complexes were characterized and identified using IR spectroscopy and differential scanning calorimetry (DSC) measurements. The biological activities of these compounds were studied against various types of fungal species. The dose and the rate of leached silver ions were controlled by the type of the co-polymers used and the solubility in the medium. The results provided laboratory support for the concept that the polymers containing chemically bound biocide are useful for controlling microbial growth. The silver uptake by strains of different fungal species was studied to determine their difference in behavior to the antifungal activities of these compounds. The uptake strategy was examined by transmission electron microscopy (TEM).
Table of content
1. Introduction
1Microbial infection remains one of the most serious complications associated with the use of many biomaterials. Paints and painted surfaces provide a particularly wide range of different ecological niches that provide sites for microbial attachment. The individual components of paint often provide biodegradable substrates. In water-borne paints, the formulated product can be attacked during production in pre-scale storage and as the re-usable residues (Inoue, 1994).
2Metal toxicity towards microorganisms is of environmental concern because of possible inhibition of essential microbe-assisted processes. These metals are toxic to the environment and their application is not short lived. If these materials are attached to polymers, it would be ideal solution to overcome problems associated with their toxicity. In addition, microorganisms serve as useful models for laboratory-based metal-toxicity studies. Silver is a potentially toxic metal to most organisms that interacts with cellular nucleic acids and enzyme active sites or absorbs on the cell wall (Tobin et al., 1984; Stohs et al., 1995).
3Many research studies are dealing with the biocidal activity of synthetic polymers. Polymeric antimicrobial agents could be utilized in a variety of applications such as paints, water treatment, coating on medical devices, food packaging, medical applications and health care related materials (Kanazawa et al., 1994; Worley et al., 2000; Kenway et al., 2003). Vigo (1992) studied the application of polymeric quaternary ammonium materials as anion exchange resins in the disinfection of water supplies.
4New directions in this area involve the synthesis of environmentally acceptable materials that are insoluble but possess biocidal activities. Most of these recent studies are patents. Water based emulsions are one of these aspects.
5This study is focusing on the evaluation of the antifungal activities of the novel synthesized chelating water based emulsion lattices and their silver complexes. A brief presentation of metal-uptake by two fungal species is given to elucidate the various responses of the filamentous fungi to these compounds.
2. Material and methods
2.1. Preparation of emulsion latex
6Binary co-polymeric emulsions of butyl acrylate-co-itaconic acid (BuA/IA) and butyl acrylate-co-maleic acid (BuA/MA) were prepared according to the technique of semi-batch emulsion co-polymerization processes. The following ingredients were mixed in a 500 ml three-necked flask using redox pair initiation system potassium persulfate/sodium metabisulphite KPS/NaMBS (0.54/0.832 gm) and 2 g sodium dodecyl benzene sulfonate (SDBS) as an emulsifier were dissolved in distilled water (70 ml). Finally, binary mixtures of 20 g IA with BuA or 20 g MA with BuA of 1:1 feed monomer compositions were prepared by adding the itaconic acid monomer solution in water dropwisely to the hydrophobic monomer in the reaction media. Monomers of butyl acrylate (BuA), maleic acid (MA) and itaconic acid (IA) were pure chemical grades. The polymerization reactions were carried out at 65°C for a period of 4 h. The experiments were run with mechanical stirring at 500 rpm.
7The prepared stable emulsions of the binary co-polymers of BuA/IA and BuA/MA were subjected to adsorption process by Ag+ ions from aqueous solution. The Ag complex of the binary co-polymers of the emulsions [(BuA/IA)Ag and (BuA/MA)Ag] were evaluated for controlling microbial growth compared with their free emulsions.
2.2. Microorganisms
8The fungal strains of Aspergillus flavus, Aspergillus niger, Aspergillus terreus, Botrytis allii, Diplodia oryzae, Fusarium oxysporum, Helminthosporium turcicum, Macrophomina phaseoli and Trichoderma reesei were obtained from MIRCEN Cairo, Faculty of Agriculture, Ain-Shams, University, Egypt. Mucor rouxii NRRL 1894 was obtained from the Agricultural Research Service Peoria, IL., USA.
2.3. Culture media
9The fungal strains growth medium was Czapeck's dox medium (Difco Laboratories, Detroit, Mi., USA). A stock culture of these strains is propagated on agar slants with the same constituents.
10Cells for the uptake studies were obtained by culturing the chosen fungal strain in 50 ml culture broth of Czapeck's dox medium in 250 Erlenmeyer flasks. They were incubated at 28°C on a rotary shaker (180 rpm) for 72 h.
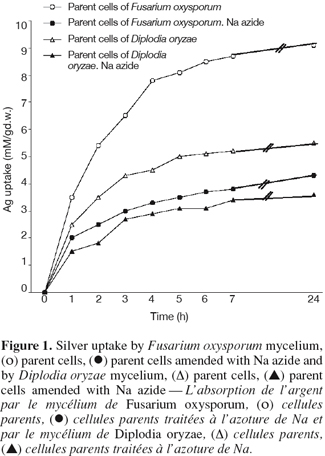
2.4. Antimicrobial activity
11The antifungal activities of the prepared chelating emulsions and their silver complexes [(BuA/IA)Ag and (BuA/MA)Ag] were evaluated by the agar diffusion technique according to the method of Penna et al. (1997). Each compound and its dilutions were tested against the various fungal strains. Each compound solution was put in a well and tested after 1, 5 and 10 days of its diffusion in uninoculated plain agar medium. After each period a film of inoculated Dox agar medium was overlayed and incubated for 48 h at 30°C. This was done to test the degree of release of Ag+ ions (Kansoh et al., 2000).
12To study whether these compounds act as fungicides and/or fungistatic agents, the prepared compounds, at their diluted concentrations as used in the applied field of paints, were sprayed on the plates inoculated with actively growing mycelia of the tested fungal strains. The plates were incubated at room temperature for 7 days. Non-sprayed plates were taken as controls. Samples from each sprayed mycelium were chosen randomly, recultured on fresh media free from the tested compounds and incubated for a week at 28°C. The growth of these mycelia was observed and recorded to test for the fungicidal or fungistatic effects of the emulsions.
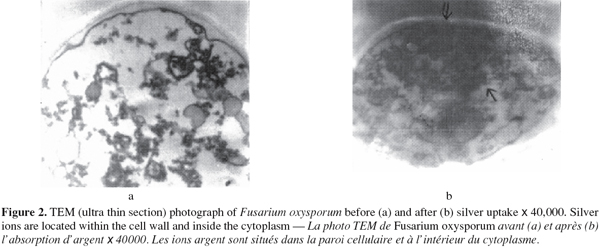
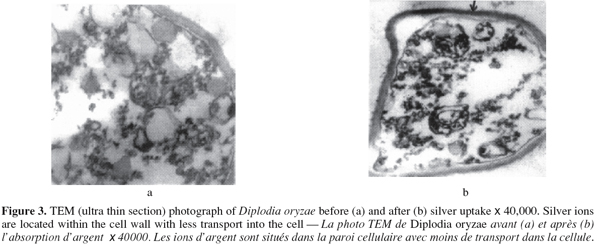
2.5. Metal uptake experiment
13The silver ion studied was used as its nitrate salt. The mycelia of the chosen fungal strains were tested as biosorbents for silver ions according to the method of Volesky et al. (1995).
14The samples were kept shaken at 28°C for 24 h, withdrawn at time intervals (0.5-2 h) and analyzed for residual silver concentrations using Atomic Absorption Spectrophotometry (Varian Spectrum AA 220). Experimental samples with no added cells were used as controls. Silver uptake was calculated as Ag mM/g.d.w. according to the method of Volesky (1990).
2.6. Electron microscopy
15The micrographs of the ultra thin sections of the fungal cells were taken by EM 10 Carl-Zeiss Transmission Electron Microscope to determine subcellular localization of the absorbed silver metal (Tsezos et al., 1982). Two samples were prepared according to the procedure of Glauert (1965). The first was used for the absorption of silver and the second was used as a blank.
3. Results and discussion
3.1. Characterization of the prepared emulsions and their silver complexes
16The prepared co-polymeric emulsions of butyl acrylate/itaconic acid and butyl acrylate/maleic acids and their silver complexes (Schemes 1 & 2) were characterized using spectrophotometric measurements, differential scanning calorimetry (DSC) and Ag ion determination.
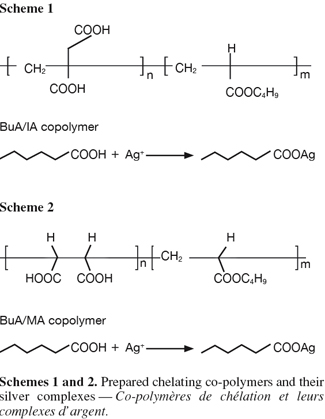
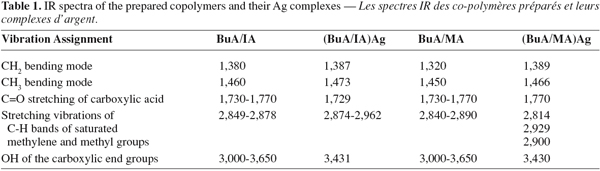
17Analysis of IR spectra for the prepared co-polymers and their Ag complexes gave good information about the structure of these materials (Table 1). The characteristic bands of C=O stretching of carboxylic groups at 1,730-1,770 cm-1 in case of BuA/IA and BuA/MA were shifted to 1,730 and 1,770 cm-1, respectively. The broad peaks of OH of the carboxyl groups at 3,000-3,650 cm-1 were also diminished due to complexation in BuA/IA and BuA/MA respectively. The reduction of the OH band of the carboxyl was greater in case of BuA/IA than BuA/MA. This clarify why the Ag content was larger in case of (BuA/IA)Ag complex (2.32%) than in (BuA/MA)Ag (0.68%).
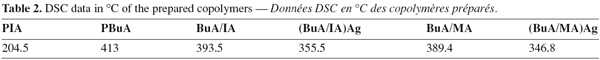
18Differential scanning calorimetry (DSC) illustrates the melting transition of the homopolymers, binary co-polymers and their Ag complexes. The results of table 2 illustrate that introduction of Ag in the trunk polymer reduces the melting transition of the prepared co-polymers.
3.2. Antimicrobial assessment of the compounds
19The biological activities of the silver complexes of the tested chelating emulsion polymers against fungal growth were evaluated by comparison to the growth observed for the emulsion co-polymers BuA/IA and BuA/MA, taken as controls (no inhibition zone). The results of table 3 show the effect of the concentrated (BuA/IA)Ag and (BuA/MA)Ag compounds against different fungal strains.
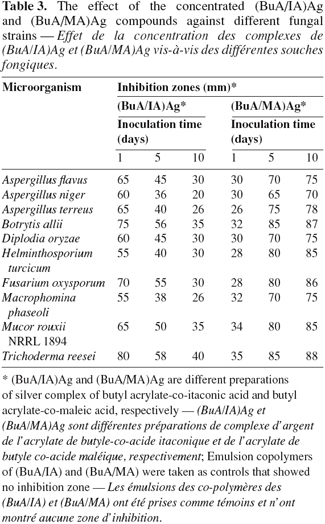
20Compound (BuA/IA)Ag showed inhibition zones that decreased by the time as the concentration of the biocide was diluted by diffusion throughout the agar medium in the plates. This can be explained on the basis that the type of the prepared emulsion and the position of the carboxylic groups in this compound formula (Scheme 1) facilitate the fast Ag+ ions release from the compound.
21On the other hand, compound (BuA/MA)Ag showed slight inhibition zones against all fungal strains at initial time that increased with time up to the 10th day. In this compound the carboxylic groups are closer to each other in its formula (Scheme 2). So, the initial release of Ag+ ions was difficult due to the difficult mobility of these ions into the medium. A low release of Ag+ ions would increase by time up to the 5th day. Then the latest Ag+ ions were released up to the 10th day.
22In general, the compounds (BuA/IA)Ag and (BuA/MA)Ag are biologically active against all the tested fungal strains, but their responses to these compounds are variable.
23The minimum inhibitory concentrations (MIC) of these compounds were studied to economize their use. As shown in table 4, the compound (BuA/IA)Ag was effective at a concentration of 0.05 mg.l-1 against Aspergillus flavus, Botrytis allii, Fusarium oxysporum and Trichoderma reesei, while concentration of 0.2 mg.l-1 was quite active against A. niger, A. terreus, D. oryzae, H. turcicum, M. phaseoli and M. rouxii NRRL 1984.
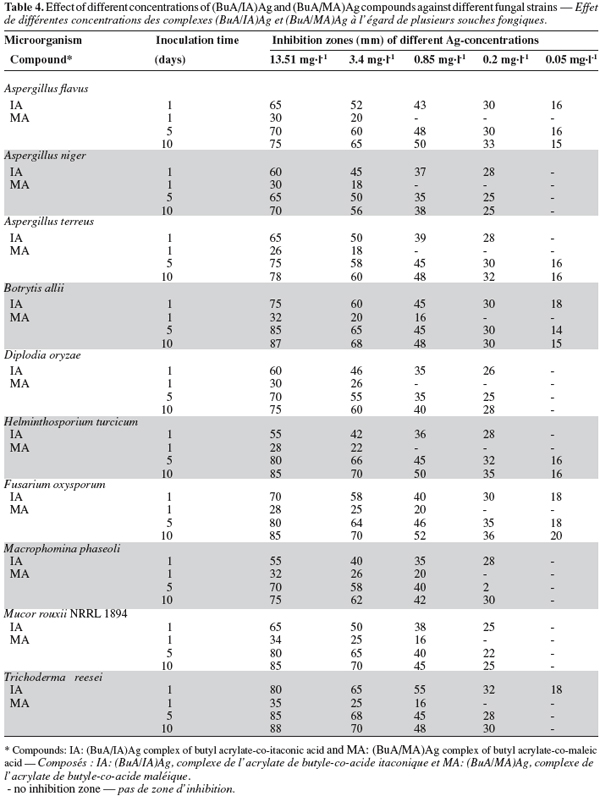
24The compound (BuA/MA)Ag was active at a concentration of 0.05 mg.l-1 against A. flavus, A. terreus, B. allii, H. turcicum and F. oxysporum, while a concentration of 0.2 mg.l-1 was effective against A. niger, D. oryzae, M. phaseoli, M. rouxii and T. reesei. Although the highly diluted solutions of the compounds have low silver concentrations, they showed a noticeable growth inhibition zones. This may be due to the complete solubility of the biocide at high dilutions with an efficient spread. These results reveal the most effective and most economical dilutions of the emulsions that can be used for application.
25As a general conclusion we can suggest that the compound (BuA/IA)Ag is suitable when successive use is needed due to the fast release of Ag+ ions from the compound. The compound (BuA/MA)Ag can be used when gradual release of Ag+ ions is needed for a long time.
26Cultures of the tested fungal strains were sprayed at the minimum inhibiting concentrations of both compounds (BuA/IA)Ag and (BuA/MA)Ag for each strain and incubated for 7 days at room temperature. When mycelia from these treated plates were subcultured on biocide free media some of them failed to grow again showing the fungicidal response or began to grow weakly showing the fungistatic effect.
27The tested compounds (BuA/IA)Ag and (BuA/MA)Ag proved to be fungicidal to A. flavus, B. allii and F. oxysporum. In addition (BuA/IA)Ag compound has a fungicidal effect on T. reesei and also (BuA/MA)Ag compound acts as a fungicidal agent on A. terreus and H. turcicum. On the other hand, these compounds are fungistatic to A. niger, D. oryzae, M. phaseolli and M. rouxii.
28A comparison between the two effects (fungicidal and fungistatic) according to the process of silver uptake was studied. Nitrate salts were chosen as they are usually soluble, and the nitrate ion forms only weak complexes with the metals. To avoid the formation of metals complexes with other anions, unbuffered solutions were used (Horikoshi et al., 1981; Tobin et al., 1984).
29The plotted data of silver uptake (Figure 1) shows the relationship between the time and silver uptake of F. oxysporum and D. oryzae that showed fungicidal and fungistatic response to the tested compounds, respectively.
30The plotted data shows an initial rapid uptake during the first hour, followed by gradual uptake with equilibrium attained after 24 h. F. oxysporum cells featured higher silver uptake (9.1 mM Ag/g D.W.) than D. oryzae cells (4.4 mM Ag/g D.W.) under the same conditions.
31The extracellular binding of silver ions that adsorbed onto the fungal cell wall explains the initial rapid phase. The second, slower phase may be due to the exchange of metal ions across the cell wall and this process depends on the cell metabolism. Addition of sodium azide as a metallic inhibitor at the start of the uptake experiments reduced the silver uptake. This is due to the accumulation processes phase, which is indicative of an energy-dependent activity (Ross, 1975; Tsezos et al., 1982; Tobin et al., 1984; Brady et al., 1994; Stohs et al., 1995; Omar et al., 1996; Kansoh et al., 2000; Humphris et al., 2001).
32It is of great importance to determine the role of the metal ions in the cells to define the uptake strategy. Transmission Electron Microscope (TEM) examination of the sorbent cells before and after uptake gives the most direct evidence of this effect. The TEM photographs of F. oxysporum (Figure 2a and 2b) represent the mycelia before and after silver uptake, respectively. Figure 2b indicates that metal is absorbed onto the cell wall and a part of the metal ions has been transported into the cell, revealing electron-dense areas in the cytoplasm. Figure 3 (a and b) of D. oryzae before and after silver uptake, respectively showed strong electron dense area on the outer surface of the cells. This indicates that the cells retained the biosorped silver ions on the outer surface of the cells with less transport into the cell.
33It should be emphasized that although the primary fungistatic action of the cations is due to non-specific reaction outside the cell protoplast, ultimate fungicidal action may well be caused by secondary reactions following the formation of un-ionized complexes. The mechanisms visualized could include the breakdown of membrane permeability and the diffusion of the metal ion complex into the interior of the cells. Such observations have also been noticed by Ross (1975), Tsezos et al. (1982), Tobin et al. (1984), El-Shafei et al. (1999), Bruce et al. (2000) and Kansoh et al. (2000).
34This study provides information for the elucidation of uptake and the various responses of filamentous fungi to heavy metal ions.
Bibliographie
Brady D. & Duncan J.R., 1994. Copper resistance mechanisms in bacteria and fungi. FEMS Microbiol. Rev., 14, 121-137.
Bruce A. et al., 2000. Production of volatile organic compounds by Trichoderma in media containing different amino acids and their effect on selected wood decay fungi. Holzforschung, 54, 481-486.
El-Shafei H.A., Kansoh A.L. & Ashour E.H., 1999. Training of Candida pseudotropicalis and Saccharomyces carles to copper toxicity. J. Agric. Sci. Mansura Univ., 24(4), 1997-2008.
Glauert A.M., 1965. The fixation and embedding of biological specimen. In: Rey D.H., ed. Techniques for electron microscopy. Philadelphia, USA: Davis Co., 166-212.
Horikoshi T., Nakajima A. & Sakaguchi T., 1981. Studies of the accumulation of heavy metal elements in biological systems. XIX. Accumulation of uranium by microorganisms. Eur. J. Appl. Microbiol. Biotechnol., 12, 90-96.
Humphris S.N., Wheatley R. & Bruce A., 2001. The effects of specific volatile organic compounds produced by Trichoderma spp. on the growth of wood decay basidiomycetes. Holzforschung, 55, 233-237.
Inoue M., 1994. Fungal contamination of paint film and plastic wall covering. In: Garg R.L., Garg N. & Mukerji R.G., eds. Recent advances in biodeterioration and biodegradation. Vol. 2. Biodeterioration and biodegradation of natural and synthetic products. Calcutta, India: Naya PROKASH, 71-80.
Kanazawa A., Ikeda I. & Endo I., 1994. Polymeric phosphonium salts as a novel class of cationic biocides. VIII. Synergistic effect on antimicrobial activity of polymeric phosphonium and ammonium salts. J. Appl. Polym. Sci., 53, 1245.
Kansoh A.L., Abu-Ayana Y.M. & Abdel-Mohsen F.F., 2000. Preparation an evaluation of encapsulated copper salts or copper complexes by various types of vinyl polymers as fungicides. Pakistan J. Biol. Sci., 3(3), 415-422.
Kenway E.R., Abdel-Hay F.I., Abouel-Magd A. & Mahmoud Y., 2003. Biologically active polymers. VII. Synthesis and antimicrobial activity of some crosslinked co-polymers with quaternary ammonium and phosphonium groups. 3th. Arab Conference on Materials Science (ACMS-III), Hurghada, Egypt.
Noren G.K., Clifton M.F. & Migdal A.H., 1986. Investigation of microencapsulated fungicides for use exterior trade sales paints. J. Coatings Technol., 58, 31-39.
Omar N.B., Merroun M.L., Gonzalez-Munoz M.T. & Arias J.M., 1996. Yeast as a biosorbent for uranium. J. Appl. Bacteriol., 81, 283-287.
Penna C.A. et al., 1997. Antimicrobial activity of Eupatorium species growing in Argentina. J. Herbs Spices Med. Plants., 5, 21-28.
Ross I.S., 1975. Some effects of heavy metals on fungal cells. Trans. Mycol. Soc., 64(2), 175-193.
Stohs S.J. & Bagchi D., 1995. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biol. Med., 18, 321-326.
Tobin J.M., Copper D.G. & Neufeld R.J., 1984. Uptake of metal ions by Rhizopus arrhizus biomass. Appl. Environ. Microbiol., 47(4), 821-824.
Tsezos M. & Volesky B., 1982. The mechanism of thorium biosorption by Rhizopus arrhizus. Biotechnol. Bioeng., 24, 385-401.
Vigo T.L., 1992. Antimicrobial fibers and polymers. In: Seymour R.B. & Porter R.S., eds. Manmade fibers: their origin and development. New York, USA: Elsevier Applied Science, 214-226.
Volesky B., 1990. Biosorption of heavy metal. Boston, USA: CRC Press.
Volesky B. & Phillips H.A., 1995. Biosorption of heavy metals by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol., 42, 797-806.
Worley S.D., Sun G., Sun W. & Chen T., 2000. Monomeric and polymeric cyclicamine and N-Halamine compounds. US Patent no6020491.
To cite this article
About: Amany L. Kansoh
National Research Centre. Microbial Chemistry Department and Polymers & Pigments Department. Dokki, ET-Cairo (Egypt). E-mail: amany_kansoh@yahoo.com
About: E.A.M. Youssef
National Research Centre. Microbial Chemistry Department and Polymers & Pigments Department. Dokki, ET-Cairo (Egypt).
About: M.A. Abd-El-Ghaffar
National Research Centre. Microbial Chemistry Department and Polymers & Pigments Department. Dokki, ET-Cairo (Egypt).






