- Portada
- volume 12 (2008)
- numéro 2
- Genetic variability of fatty acids in bovine milk
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Genetic variability of fatty acids in bovine milk

Notes de la rédaction
Received on August 10, 2007, accepted on December 18, 2007
Résumé
Variabilité génétique des acides gras du lait d’origine bovine. La composition en acides gras du lait influence les propriétés technologiques du beurre et montre des effets potentiellement positifs sur la santé humaine. L’effet de la supplémentation de la ration animale sur le profil en acides gras est bien décrit dans la littérature mais peu d’information est disponible sur les effets génétiques. Sur base d’un nombre limité d’études, généralement menées pour mettre en évidence un effet alimentaire, l’effet de la race semble influencer la composition en acides gras du lait. Une variation de l'activité δ-9 désaturase, enzyme clé dans la production des acides gras monoinsaturés et des acides linoléiques conjugués (CLA), pourrait expliquer ces différences. Très peu d’études se sont focalisées sur l’estimation des paramètres génétiques des acides gras. Pourtant, les valeurs d’héritabilité modérées observées pour les principaux acides gras du lait pourraient suggérer un effet génétique potentiel.
Abstract
Fatty acids composition of bovine milk influences the technological properties of butterfat and also presents some potential benefits for human health. Impact of feeding on fat composition is well described in the literature; less information is available about the impact of genetics. Based on few studies, essentially conducted to isolate some feeding effect, the breed seemed to influence the fatty acids composition. The variation in the activity of δ-9 desaturase, key enzyme in the production of monounsaturated fatty acids and conjugated linoleic acids in milk, could explain these differences. Very few studies have been focussing on the estimation of genetic parameters of fatty acids composition. However, the moderate heritability estimates observed by these studies for the major fatty acids could suggest a potential genetic effect.
Tabla de contenidos
1. Introduction
1For a long time, the perception of consumers about fat from animal origin was linked to the increased risk of cardiovascular diseases, especially due to the large amounts of saturated fatty acids. The dairy products provide 15 to 25% of fat consumed by humans and 25 to 35% of saturated fat (Chilliard et al., 2001). This statement of cognizance, even if the potential effects of saturated fatty acids are more nuanced (Chilliard et al., 2001), has forced the dairy industry to consider new options to produce and promote healthy milk. A way of doing this, is to modify the milk fat composition to improve the nutritional quality of dairy products.
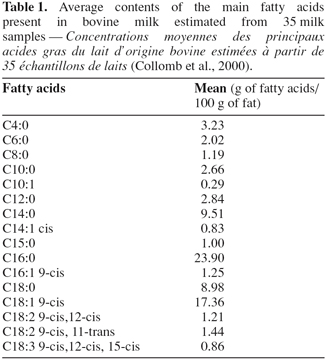
2Bovine milk fat, with a percentage varying between 3 and 5% (Jensen et al., 1990), is emulsified in the aqueous phase of milk. Milk fat globule is protected by a membrane partially derived from the plasma membrane of the lactating cell (Jensen et al., 1990; Danthine et al., 2000). The centre of this droplet is essentially composed by the lipids. The latter are complex both with the respect to lipids classes and to fatty acid components (Jensen et al., 1988). Triglycerides compose 98% of lipids present in bovine milk. The last 2% include small amounts of free fatty acids, mono- and diglycerides, phospholipids, sterols and hydrocarbons (Jenness, 1988). The analysis of fatty acids is difficult due to the volatile trend of short chain fatty acids and the large amounts of fatty acids present in milk (Jensen et al., 1988). Currently, 406 fatty acids are listed (Debry, 2001). However, few fatty acids represent the majority of fat present in bovine milk. Table 1 shows the contents of the principal fatty acids present in bovine milk based on the results obtained by Collomb et al. (2000). The 16 major fatty acids represented approximately 79% of milk fat content (Table 1). The fatty acids with the highest contents were C16:0 and C18:1 9-cis (Table 1).
3Typical milk fat from dairy cows contains 70% of saturated, 25% of monounsaturated and 5% of polyunsaturated fatty acids (Grummer, 1991). Based on the previous results on the impacts of fatty acids on human health (e.g. Noakes et al., 1996; Hu et al., 1999; Parodi, 1999; Simopoulos, 2003), a composition of milk fat with potential positive effects on human health should be composed for 60% of monounsaturated (Pascal, 1996), 30% of saturated and 10% of polyunsaturated fatty acids (Hayes et al., 1992). Even if obtaining this particular fat composition is an utopia, fatty acid profile could be improved using different sources of variation. The interest to study the variation of fat composition in bovine milk is not recent. For instance, Smith et al. in 1916 discussed the effect of cottonseed oil on fat composition. Then, many studies focussed on feed supplementation with particular feeds to modify the fatty acid profile (e.g. Chilliard et al., 2000 and 2001), especially to increase the fraction of unsaturated fatty acids in milk fat. Even if many results confirm the feeding impact, less information relate to genetic effect including breed differences and individual variability of the fatty acids. The aim of this paper is to review the impacts of genetic factors on the composition of bovine milk fat.
2. Breed differences
2.1. Fatty acids
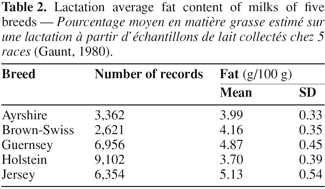
4Variation of milk fat content among dairy breeds has been known for a long time and is well described in the literature even if actual averages differ according to country and period. Table 2 gives an example of breed averages in the 1970s in the USA, representing lactation average fat content in milk produced by five dairy breeds (Gaunt, 1980). These data included over 23,000 lactation records for cows milked twice a day for 305 days in 22 states in the United States. Highest and lowest milk fat contents were observed for milk from Jersey and Holstein cows, respectively (Table 2). As the fatty acids compose the milk fat, logically variability of fatty acid profiles due to breed differences should also exist.
5Generally the breed influences on fatty acid profile were studied based on phenotypic differences observed for limited numbers of cows from various breeds fed with the same diet. Consequently although the obtained results did not represent exactly the breed differences, this type of research gives some indications. The first studies linked differences in milk fat composition to manufacturing properties. One of the oldest studies linked the technological properties of milk fat to the hardness of butterfat. Coulter et al. (1934) studied the variation of fatty acid contents among dairy breeds based on the estimation of iodine value. These authors observed that the butterfat produced by Channel Island cows was firmer than that produced by Holstein or Ayrshire cows.
6Table 3 summarizes the results of breed differences obtained by several authors based on chromatographic data. All results are expressed in % of produced fatty acid content compared to that produced by Holstein breed. Majority of the studies focussed on the differences in fat composition between Jersey and other dairy breeds, especially to study the effect of a highest fat content on fatty acid profile. In 1964, Stull et al. reported that the composition of milk fat produced by Holstein breed was significantly different compared to that produced by Jersey and Guernsey for five individual fatty acids (C10:0, C12:0, C16:0, C16:1 cis and C18:1 cis) (Table 3). Higher contents of fatty acid with short and medium chain and a lower content of C18:1 9-cis were observed by Hermansen et al. (1990) in Jersey milk fat than in fat produced by heavier breeds. Palmquist et al. (1992) studied the differences of milk fat composition between Jersey and Holstein. These authors reported that the contents of C6:0 until C14:0 were 8 to 42% higher in Jersey compared to Holstein. Jersey fat contained a higher content of C18:0 (Palmquist et al., 1992). Few years later, these same authors confirmed that Jersey milk fat contained a higher proportion of short and medium chain fatty acids and lower proportions of C18:1 9-cis than milk fat from Holstein cows (Beaulieu et al., 1995) (Table 3). They found also that the content of C16:0 was lower in Jersey than in Holstein. This observation was not in agreement with Stull et al. (1964) but was also observed by DePeters et al. (1995) (Table 3). Beaulieu et al. (1995) indicated that the amount of fat yield stayed unchanged in function of the quantity of supplement food. Consequently, the observed changes in the fatty acid profile in milk fat reflected a modification in the amount of fatty acids synthesized by the mammary gland. These authors postulated that the de novo synthesis of fatty acids in Jersey cows was more inhibited than in Holstein breed at the first amount of added fat. The differences on fatty acids composition between Holstein, Jersey and Brown-Swiss cows were studied by DePeters et al. in 1995. They reported that the content of short chain fatty acids did not change a lot between Jersey and Holstein milk fat (Table 3). Stull et al. (1964) and Beaulieu et al. (1995) reported that the proportion of medium chain fatty acids was higher and the content of C18:1 9-cis was lower in Jersey than in Holstein milk fat. Higher proportions of C6:0 until C12:0 were produced by Jersey cows (Table 3). The total unsaturated fatty acids content was lower for Jersey than other studied cows. As for the previously reported results, White et al. (2001) showed that Jersey produced significantly higher concentrations of C6:0, C8:0, C12:0 and C14:0 than Holstein. This last breed produced significantly higher contents of C16:1 and C18:1. Based on these results, Jersey cows seemed to produce more fatty acids with short and medium chain compared to Holstein. Consequently, the production of a highest content of fat in bovine milk seems to be linked to a lowest content of unsaturated fatty acids. So, this fat composition involves a production of a harder butter and milk with less interest for human health. Indeed, different studies based on the impact of fatty acids on human health (e.g. Bonanome et al., 1988; Grummer, 1991; Hu et al., 1999) showed that the fatty acids with medium carbon chain, especially C14:0, increased more the risk of cardiovascular diseases compared to other fatty acids and especially unsaturated fatty acids.
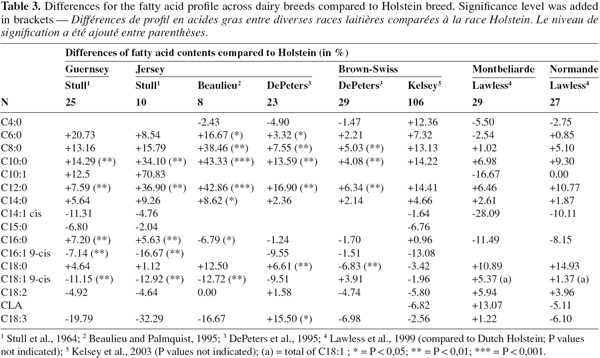
7Only one study was found to describe the differences between Brown-Swiss and Holstein. DePeters et al. (1995) showed that Brown-Swiss milk fat contained the lowest content of C18:3.
8The study of Lawless et al. (1999) focussed on the differences on fatty acid profile between Holstein, Montbeliarde and Normande. These authors showed that Dutch Holstein had higher C16:0 concentrations than the other studied breeds. Normande and Montbeliarde produced the milk fat with the highest C18:0 (Table 3).
9Thanks to its potential positive effects on human health, a specific type of fatty acids, the conjugated linoleic acids (CLA), shows a large interest. CLA is a term representing a mixture of positional and geometric isomers of octadecadienoic acid with a conjugated double bond system (Kelsey et al., 2003). Rumenic acid, C18:2 9-cis, 11-trans is the major CLA isomer found in dairy products accounting for 75 to 90% of the total CLA in milk fat (Bauman et al., 1999). This isomer is known for its anti-carcinogenic properties showed on animal model (Parodi, 1999). Another isomer, C18:2 10-trans, 12-cis seems to be also interesting for its action on the lipid status (Chilliard, 2001; Pariza et al., 2001). Moreover, other effects of CLA were shown: anti-obesity (Park et al., 1997), suppression of carcinogenesis (Ip et al., 1994; Belury, 1995), immune modulation by increasing of IgA, IgG et IgM and by decreasing IgE (Cook et al., 1993; Debry, 2001), anti-atherogen (Nicolosi et al., 1997; Debry, 2001) and effect on diabetes (Houseknecht et al., 1998). Due to these positive effects on human health, some studies are focussed on feed supplementation to increase CLA content in milk and more less studied the breed differences. However, Lawless et al. (1999) observed a variation of conjugated linoleic acid (CLA) between dairy breeds. These authors reported that breed had some influence on CLA content of milk and that a cow yielding high levels of milk fat CLA sustained this production over time. Montbeliarde milk fat contained the highest level of CLA and Normande the lowest content compared to Dutch Holstein (Lawless et al., 1999) (Table 3). Lawless et al. (1999) were not the only authors to study the variation of CLA among dairy breeds. White et al. (2001) showed that Holstein breed produced significantly higher concentrations of CLA than Jersey. Kelsey et al. (2003) studied the variation of fatty acids, especially of CLA, among Holstein and Brown-Swiss cows. Holstein milk fat contained a higher content of CLA in milk. However, these authors mentioned that the difference among studied dairy breeds were not significant. Breed accounted for less than 0.1% of the total variation in the CLA concentration in milk fat.
10Independently of the variation of fatty acids across dairy breeds, Lawless et al. (1999) showed also some significant differences, especially for C16:0, on the fatty acid profiles among Holstein cows from two separate origins, Irish and Dutch Holstein. These individual variations suggest that additional factors must affect the fat composition in bovine milk e.g. the rumen environment (pH, time of rumination, etc.), physiological differences (size of intestine, the activity of mammary gland, etc.).
2.2. -9 desaturase
11The variation of milk fat composition observed among dairy breeds could be partially explained by the metabolic process of fatty acids synthesis. Aforementioned, Beaulieu et al. (1995) have used a metabolic interpretation based on the de novo synthesis to explain the differences in the response of Jersey and Holstein cows to the addition of fat in the animal ration.
12By the introduction of a cis-double bond between carbons 9 and 10 in the carbon chain of fatty acids (Bauman et al., 1999), δ-9 desaturase is an important enzyme in the production of unsaturated fatty acids. The iron that composes the δ-9 desaturase enzyme, also named stearoyl-CoA desaturase (SCD), with the action of NADPH, cytochrome b5 reductase, cytochrome b5 and oxygen, catalyzes this desaturation (Ntambi, 1995; Yahyaoui et al., 2002). As its activity permits the conversion of C18:1 11-trans into C18:2 9-cis, 12-cis, this enzyme is responsible to the endogenous production of the major isomer of CLA in bovine milk, the rumenic acid (Kinsella, 1972; Bauman et al., 1999). The conversions of C18:0 into C18:1 9-cis, C16:0 into C16:1 9-cis, C14:0 into C14:1 9-cis and C10:0 into C10:1 9-cis in mammary cells are also regulated by this enzyme. Consequently, the variation of δ-9 desaturase activity could partially explain the fluctuation of the contents of monounsaturated fatty acids and CLA in bovine milk fat. Lock et al. (2003) estimated the ratio product/substrate (e.g. C14:1 9-cis/C14:0) to study the seasonal variation of the δ-9 desaturase. These authors suggested that a molecule contained in the grass could induce a higher δ-9 desaturase activity, and thus, explain the seasonal variation. Based on the ratio substrate/product (e.g. C14:0/C14:1 9-cis), Chouinard et al. (1999) showed that the δ-9 desaturase activity was influenced by dietary supplementation. In addition to these extrinsic factors, Peterson et al. (2002) suggested that the variation of CLA content in milk fat among individuals was related to the rumen biohydrogenation and δ-9 desaturase activity. If the variation of the δ-9 desaturase activity permitted to explain partially some phenotypic differences observed in the CLA content, the activity of this enzyme should vary genetically. In this sense, Kelsey et al. (2003) showed a δ-9 desaturase activity difference between Holstein and Brown-Swiss breeds from the estimation of the ratio substrate/(substrate + product) (e.g. C14:0/(C14:1 9-cis + C14:0). Holstein cows showed the highest δ-9 desaturase activity. The lowest contents of monounsaturated fatty acids and CLA observed by Kelsey et al. (2003) for Holstein breed could be explained by this enzymatic activity (Table 3). As the ratio C18:1/C18:0 could reflect the δ-9 desaturase activity, the lowest value of this ratio observed for Jersey could also explain the lowest contents of monounsaturated fatty acids and CLA observed by DePeters et al. (1995) for this breed.
3. Heritability
3.1. Fatty acids
13Gibson (1995) mentioned that effective genetic improvement requires genetic variation, a mechanism of selection, and an economic incentive for the improvement. Economic incentives will not be discussed as evolution of milk price is yet uncertain; however several authors reported estimation of genetic parameters for the fatty acid profile in milk. Unfortunately even if already several feeding studies concluded towards a possible genetic effect, only a restricted number focussed on the estimation of genetic parameters of fatty acid contents. Most feeding studies, as for Beaulieu et al. (1995), observed differentiated reactions of cows from different breeds to dietary changes, a clear indication that the genetic factor might be important.
14One of the first studies about the genetic variability of fatty acid profile was conducted by Edwards et al. in 1973. Very high heritability estimates ranging between 0.64 and 0.98 were obtained by these authors. In their research, the environmental variance was estimated as the sum of variances within monozygotic twins and half of the genetic variance added to the environmental variance was the sum of variances within dizygotic twins. This estimation method is today considered unreliable and these values were probably highly overestimated. Few years later, Renner et al. (1974a) reported heritability values equal to 0.26, 0.06 and 0.04 for the contents of fatty acid with short and medium chain and for the C18 family in milk fat, respectively. Estimates of 0.26, 0.25 and 0.02 for the contents of fatty acids with short and medium carbon chain and for the C18 family in milk (% in milk), respectively, were also shown by these authors. From these estimates, fatty acid contents in milk seemed to be more heritable than the contents of fatty acid in milk fat (% in milk fat). Values of heritability estimated by Karijord et al. (1982) were different from those observed by Renner et al. (1974a). They were on average 0.13, 0.14 and 0.10 for fatty acid contents with short and medium carbon chain and for the C18 family in milk fat, respectively.
3.2. -9 desaturase
15Even if no articles about the estimation of genetic parameters for the δ-9 desaturase were found, some authors who focussed on the variation of this enzymatic activity concluded towards the existence of some genetic influences. For instance, Kelsey et al. (2003) showed that the average content of CLA in the studied animal population was 4.3 mg.g-1 of fatty acids but the range among individuals was approximately threefold (2.3 to 7.2 mg.g-1 of fatty acid). Even if the stage of lactation, the milk production level, and the diet were identical among cows, a substantial variation among individuals was still observed as reported by Kelly et al. (1998). However one abstract written by Royal et al. (2005) reported the variances components and heritability of δ-9 desaturase ratios estimated on 1,520 Holstein-Friesian. These authors found heritability values equal to 0.30, 0.19 and 0.29 for C14:1/(C14:0+C14:1), C18:1 9-cis/(C18:1 9-cis+C18:0) and C18:2 9-cis,11-trans/(C18:2 9-cis,11-trans+C18:1 11-trans), respectively. Only C16:1/(C16:0+C16:1) showed a heritability equal to 0.01. These results confirmed an individual genetic variability of the δ-9 desaturase activity.
4. Genetic correlations
16Another important type of genetic parameter besides heritability, are genetic correlations. In particular, the genetic correlations among fatty acids are important to investigate the potential effects of animal selection on the fatty acid profile in bovine milk. These effects depend on the relative interdependence of fatty acids. Only two studies were found.
17The genetic correlations between traditional production traits (milk yield, fat and protein contents) and fatty acids with short and medium carbon chain and for the C18 family were estimated by Renner et al. (1974b). These authors observed that the relationships between milk yield and the studied group of fatty acids were relatively low. Only the content of fatty acids with short chain in milk seemed to be positively correlated with milk yield (0.24). Karijord et al. (1982) estimated the genetic correlations between the individual fatty acids and the traditional production traits. Their results were more diverse. Genetic correlations close to 0 were observed between C16:1, C17:0, C18:1 and milk yield. Positive genetic correlations ranged between 0.11 and 0.24 were obtained between the individual saturated fatty acids and milk yield, except for C15:0 and C16:0 (-0.58 and -0.14, respectively). Negative genetic correlations ranging between -0.11 and -0.35 were found between unsaturated fatty acids and milk yield, except for C18:2 (0.35).
18Renner et al. (1974b) indicated that the content of saturated fatty acids with medium chain became higher when the percentage of fat increased. As the percentage of fat in Jersey milk is high (Table 2), this observation could probably explain the higher contents of C12:0, C14:0 and C16:0 in Jersey fat compared to Holstein breed as observed by various authors (e.g. Beaulieu et al., 1995; DePeters et al., 1995). The genetic correlation between the content of fatty acids of the C18 family in fat and the fat percentage estimated by Renner could negative explain partially the lowest content of unsaturated fatty acids observed for Jersey breed. The same results were obtained by Karijord et al. (1982).
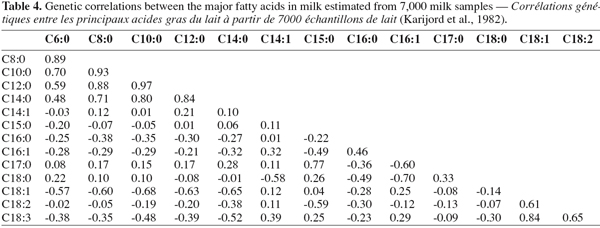
19Table 4 describes the genetic correlations estimated by Karijord et al. (1982) between the contents of fatty acids in milk fat. The values of genetic correlation estimated among the contents for major fatty acids were extremely variable and ranged from -0.68 to 0.97. Generally, positive genetic correlations were observed between the fatty acids belonging to the same class of fatty acids (saturated, monounsaturated or polyunsaturated fatty acids). On the other hand, the contents of monounsaturated or polyunsaturated fatty acids were negatively correlated with saturated fatty acids. High positive correlations between fatty acids with short and medium chain except C16:0 are shown in Table 4. The same observation can be made among the polyunsaturated fatty acids and among the monounsaturated fatty acids. C16:0 and C18:0 seemed to have a different behaviour. C16:0 was negatively correlated with all studied fatty acids except C14:1 and C16:1. C18:0 was negatively correlated with C12:0, C14:0 and unsaturated C18 family. The genetic correlations between the individual monounsaturated fatty acids and C18:2 were positive except for C16:1. This observation could be explained by the effect of the δ-9 desaturase. However, the results have to be interpreted cautiously because some doubts may exist. As a matter of fact the values of heritability estimated for milk and fat content by Karijord et al. (1982) tended to be very low (0.09 for these 2 traits), indicating potential data quantity and quality problems.
5. Conclusion
20Our literature review focussed on the genetic variability of fatty acids in bovine milk and showed a shortage of information concerning the genetic parameters of these traits. Available results showed the existence of breed effects. Especially the differences of fatty acids composition between Holstein and Jersey breeds. Jersey produced less unsaturated fatty acids in milk fat probably due in part to a lower δ-9 desaturase activity observed in this breed. Few studies reported genetic parameters showing the existence of a genetic variability of fatty acid profile in bovine milk. Globally the values of heritability were moderate. Heritabilities of saturated fatty acids were higher than those observed for unsaturated fatty acids. The variation in the δ-9 desaturase activity could partially explain the variation observed in the contents of monounsaturated fatty acids and CLA in milk. Negative genetic correlations were observed between unsaturated fatty acids and fat content. It could explain why, in the available studies, the Holstein breed with the lowest fat percentage showed the highest contents of unsaturated fatty acids. The positive genetic correlations showed strong relationships between some fatty acids in bovine milk fat. Based on an unique study reporting genetic correlations among individual fatty acids, an animal selection based on the fatty acid content could target potential interesting groups of fatty acids and not exclusively a specific fatty acid. Based on these genetic parameters and in spite of the lack of accurate estimates, the contents of monounsaturated fatty acids and CLA could increase simultaneously in bovine milk.
Bibliographie
Bauman D.E., Baumgard L.H., Corl B.A. & Griinari J.M., 1999. Biosynthesis of conjugated linoleic acid in ruminants. Proc. Am. Soc. Anim. Sci., 1-12.
Beaulieu A.D. & Palmquist D.L., 1995. Differential effects of high fat diets on fatty acid composition in milk of Jersey and Holstein cows. J. Dairy Sci., 78, 1336-1344.
Belury M.A., 1995. Conjugated dienoic linoleate: a polyunsaturated fatty acid with unique chemoprotective properties. Nutr. Rev., 53, 83-89.
Bonanome A. & Grundy S.M., 1988. Effect of dietary stearic acid on plasma cholesterol and lipoproteins. New Engl. J. Med., 318, 1244-1248.
Chilliard Y., Ferlay A., Mansbridge R.M. & Doreau M., 2000. Ruminant milk fat plasticity: nutritional control of saturated, polyunsatured, trans and conjugated fatty acids. INRA Ann. Zootech., 49, 181-205.
Chilliard Y., Ferlay A. & Doreau M., 2001. Contrôle de la qualité nutritionnelle des matières grasses du lait par l’alimentation des vaches laitières : acides gras trans, polyinsaturés, acide linoléique conjugué. INRA Prod. Anim., 14, 323-335.
Chouinard P.Y. et al., 1999. Conjugated linoleic acids alter milk fatty acid composition and inhibit milk fat secretion in dairy cows. J. Nutr., 129, 1579-1584.
Collomb M. & Bühler T., 2000. Analyse de la composition en acides gras de la graisse de lait. 1. Optimisation et validation d’une méthode générale à haute résolution. Mitt. Lebensm. Hyg., 91, 306-332.
Cook M.E., Miller C.C., Park Y. & Pariza M., 1993. Immune modulation by altered nutrient metabolism: nutritional control of immune-induced growth depression. Poult. Sci., 72, 1301-1305.
Coulter S.T. & Hill O.J., 1934. The relation between the hardness of butter and butterfat and the iodine number of the butterfat. J. Dairy Sci., 17, 543-550.
Danthine S. et al., 2000. Evolution des connaissances sur la membrane du globule gras du lait : synthèse bibliographique. Lait, 80, 209-222.
Debry G., 2001. Lait, nutrition et santé. Cachan, France : Editions Tec & Doc/Lavoisier.
DePeters E.J., Medrano J.F. & Reed B.A., 1995. Fatty acid composition of milk fat from three breeds of dairy cattle. Can. J. Anim. Sci., 75, 267-269.
Edwards R.A., King J.W.B. & Yousef I.M., 1973. A note on the genetic variation in the fatty acid composition of cow milk. Anim. Prod., 16, 307-310.
Gaunt S.N., 1980. Genetic variation in the yields and contents of milk constituents. Bull. Int. Dairy Fed., 125, 73-82.
Gibson J.P., 1995. The potential for genetic change in milk fat composition. J. Dairy Sci., 74, 3258-3266.
Grummer R.R., 1991. Effect of feed on the composition of milk fat. J. Dairy Sci., 74, 3244-3257.
Hayes K.C. & Khosla D.R., 1992. Dietary fatty acid thresholds and cholesterolemia. FASEB J., 6, 2600-2607.
Hermansen J.E. & Lund P., 1990. Fatty acid composition and milk quality related to feeding Ca-saponified palm acid oil to different breeds of dairy cows. J. Dairy Sci., 57, 23-31.
Houseknecht K.L. et al., 1998. Dietary conjugated linoleic acid normalizes impaired glucose tolerance in the Zucker diabetic fatty fa/fa rat. Biochem. Biophys. Res. Commun., 244, 678-682.
Hu F.B. et al., 1999. Dietary saturated fat and their food sources in relation to the risk of coronary heart disease in women. Am. J. Clin. Nutr., 70, 1001-1008.
Ip C., Simeca J.A. & Thompson H.J., 1994. Conjugated linoleic acid: a powerful anticarcinogen from animal fat sources. Cancer, 74, 1050-1054.
Jenness R., 1988. Composition of milk. In: Wong N.P., Keeney M. & Marth E.H., eds. Fundamentals of dairy chemistry, 3rd ed. New York, USA: Van Nostrand Reinhold Company.
Jensen R.G. & Clark R.W., 1988. Lipid composition and properties. In: Wong N.P., Keeney M. & Marth E.H., eds. Fundamentals of dairy chemistry, 3rd ed. New York, USA: Van Nostrand Reinhold Company.
Jensen R.G., Ferris A.M., Lammi-Keefe C.J. & Henderson R.A., 1990. Lipids of bovine and human milks: a comparison. J. Dairy Sci., 73, 223-240.
Karijord Ø., Standal N. & Syrstad O., 1982. Sources of variation in composition of milk fat. Z. Tierzüchtg. Züchtgsbiol., 99, 81-93.
Kelly M.L. et al., 1998. Dietary fatty acid sources affect conjugated linoleic acid concentrations in milk from lactating dairy cows. J. Nutr., 128, 881-885.
Kelsey J.A., Corl B.A., Collier R.J. & Bauman D.E., 2003. The effect of breed, parity, and stage of lactation on conjugated linoleic acid (CLA) in milk fat from dairy cows. J. Dairy Sci., 86, 2588-2597.
Kinsella J.E., 1972. Stearoyl CoA as a precursor of oleic acid and glycerolipids in mammary microsomes from lactating bovine: possible regulatory step in milk triglyceride synthesis. Lipids, 7, 349-355.
Lawless F. et al., 1999. Influence of breed on bovine milk cis-9, trans-11-conjugated linoleic acid content. Livest. Prod. Sci., 62, 43-49.
Lock A.L. & Garnsworthy P.C., 2003. Seasonal variation in milk conjugated linoleic acid and Δ9-desaturase activity in dairy cows. Livest. Prod. Sci., 79, 47-59.
Nicolosi R.J. et al., 1997. Dietary conjugated linoleic acid reduces plasma lipoproteins and early aortic atherogenesis in hypercholesterolemic hamsters. Artery, 22, 266-277.
Noakes M., Nestel P.J. & Clifton P.M., 1996. Modifying the fatty acid profile of dairy product through feedlot technology lowers plasma cholesterol of human consuming the products. Am. J. Clin. Nutr., 63, 42-46.
Ntambi J.M., 1995. The regulation of stearoyl-CoA desaturase (SCD). Prog. Lipid Res., 34(2), 139-150.
Palmquist D.L. & Beaulieu A.D., 1992. Differences between Jersey and Holstein cows in milk fat composition. J. Dairy Sci., 75(Suppl. 1), 292 (Abstr.).
Pariza M.W., Park Y. & Cook M.E., 2001. The biologically active isomers of conjugated linoleic acid. Prog. Lipid Res., 40, 283-298.
Park Y. et al., 1997. Effect of conjugated linoleic acid on body composition in mice. Lipids, 32, 853-858.
Parodi P.W., 1999. Symposium: a bold new look at milk fat, conjugated linoleic acid and other anticarcinogenic agents of bovine milk fat. J. Dairy Sci., 82, 1339-1349.
Pascal G., 1996. Les apports quotidiens recommandés en lipides et en acides gras. OCL, 3, 205-210.
Peterson D.G., Kelsey J.A. & Bauman D.E., 2002. Analysis of variation in cis-9, trans-11 conjugated linoleic acid (CLA) in milk fat of dairy cows. J. Dairy Sci., 85, 2164-2172.
Renner E. & Kosmack U., 1974a. Genetische Aspekte zur Fettsäurenzusammensetzung des Milchfettes. 2. Fettsäurenmuster der Milch von Nachkommenpopulationen. Zuechtungskunde, 46, 217-226.
Renner E. & Kosmack U., 1974b. Genetische Aspekte zur Fettsäurenzusammensetzung des Milchfettes. 2. Genetische Korrelationen zum Fettgehalt und zur Fettleistung. Zuechtungskunde, 46, 257-264.
Royal M.D. & Garnsworthy P.C., 2005. Estimation of genetic variation in Δ9-desaturase enzyme activity in dairy cows. In: Proceedings of the British Society of Animal Science, York, April 4-6, 2005. York, UK: British Society of Animal Science, 52.
Simopoulos A.P., 2003. Importance of ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev. Nutr. Diet., 92, 1-22.
Smith F.H., Wells C.A. & Ewing P.V., 1916. The changes in composition of butter fat produced by feeding cottonseed oil. Georgia Exp. Stn Bull., 122. Experiment.
Stull J.W. & Brown W.H., 1964. Fatty acid composition of milk. II. Some differences in common dairy breeds. J. Dairy Sci., 47, 1412 (Technical Notes).
White S.L. et al., 2001. Comparison of fatty acid content of milk from Jersey and Holstein cows consuming pasture or a total mixed ration. J. Dairy Sci., 84, 2295-2301.
Yahyaoui M.H., Sanchez A. & Folch J.M., 2002. Rapid communication: partial nucleotide sequence of the goat stearoyl coenzyme A desaturase cDNA and gene structure. J. Anim. Sci., 80, 866-867.
Para citar este artículo
Acerca de: Hélène Soyeurt
Gembloux Agricultural University – FUSAGx. Animal Science Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: soyeurt.h@fsagx.ac.be – Fonds de Recherche dans l’Industrie et l’Agriculture. Rue d’Egmont, 5. B-1000 Brussels (Belgium).
Acerca de: Nicolas Gengler
Gembloux Agricultural University – FUSAGx. Animal Science Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium) – National Fund for Scientific Research. Rue d’Egmont, 5. B-1000 Brussels (Belgium).






