- Portada
- Volume 30 (2026)
- Numéro 1
- Diversity of actinobacteria in eastern Algeria's hot springs: insights into their amylase, protease and lipase production
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Diversity of actinobacteria in eastern Algeria's hot springs: insights into their amylase, protease and lipase production

Documento adjunto(s)
Version PDF originaleRésumé
Diversité des actinobactéries dans les sources thermales de l'Est algérien : aperçu de leur production d'amylase, de protéase et de lipase
Description du sujet. La demande croissante en enzymes industrielles aux propriétés innovantes, telles la thermorésistance ou la résistance aux pH acides, se fait ressentir de plus en plus de nos jours. Les actinobactéries comptent parmi les groupes de microorganismes les plus recherchés en raison de leurs capacités inégalées de produire des composés bioactifs à impacts économiques, environnementaux et médicaux majeurs. L’inventaire de ces bactéries originaires des eaux thermales reste incomplet. Des investigations supplémentaires sont encore nécessaires pour exploiter leurs capacités métaboliques uniques.
Objectifs. L'objectif de cette étude est d'étudier la diversité des actinobactéries dans des sources thermales algériennes sélectionnées, d'identifier les isolats et d'examiner leur capacité à produire des thermozymes.
Méthode. Des actinobactéries ont été isolées à l'aide de milieux sélectifs à partir de quatre sources thermales de l'est de l'Algérie. Les isolats ont fait l'objet d'une caractérisation phénotypique et biochimique, suivie d'une identification moléculaire. En outre, les activités enzymatiques liées aux amylases, protéases et lipases ont été évaluées à des températures de 50 °C, 55 °C et 60 °C pour tous les isolats obtenus.
Résultats. Trente et une actinobactéries ont été isolées en utilisant des milieux sélectifs. Le milieu AIA, contenant de l'asparagine, favorise la croissance des actinobactéries par rapport aux autres microorganismes. Le milieu SCA, caractérisé par la présence d'amidon et de caséine dans sa composition, permet une croissance sélective des actinobactéries. De même, le milieu ISP5, riche en glycérol, est connu pour favoriser le développement des actinobactéries. Enfin, il a également été recouru au milieu de culture de Kenknight et Munaier largement utilisé pour l'isolement de ces bactéries. La plupart des actinobactéries isolées dans l'étude présentaient des activités hydrolytiques élevées, 87 % d'entre elles dégradant l'amidon, les protéines et certains lipides à 50 °C. Ce pourcentage de souches actives diminuait à 70 % à 50 °C. Ce pourcentage est passé à 70 % à 55 °C, puis à 60 % produisant de l'amylase thermostable et à 50 % produisant des protéases et des lipases thermostables à 60 °C. Une étude taxonomique combinant des caractéristiques morphologiques, physiologiques et moléculaires, utilisant le séquençage du gène ARNr16S, nous a permis d'assigner les actinobactéries performantes aux espèces suivantes, Streptomyces scabiei (OQ780365), S. griseochromogenes (OQ780366), S. griseochromogenes (OQ780367), S. avermitilis MA-4680 (OQ780368), S. avermitilis MA-4680 (OQ780369), S. bottropensis (OQ780370), S. thermocarboxydus (OQ733168), S. griseoviridis (OQ733169), S. albidoflavus (OP456974), S. albidoflavus (OP456975), S. griseus (OQ733170), S. griseus (OQ733171) S. thermodiastaticus strain JCM 4840 (OQ727248), S. thermodiastaticus strain JCM 4840 (OQ727246), S. griseorubens (OP456976), S. calvus strain DSM (OQ730106), S. calvus strain DSM (OQ730107), S. formicae strain 1H-GS9 (OQ730105), S. formicae strain 1H-GS9 (OQ730104), S. cellulosae strain NBC_01681 (OQ730102), S. cellulosae strain NBC_01681 (OQ730103), S. violaceoruber strain S21 (OQ733172), Thermoactinomyces daqus (OP456983), T. vulgaris (OQ733167), T. vulgaris (OP456984), T. intermedius (OP457077), Rhodococcus erythropolis (OP458556), R. ruber (OP458557), R. qingshengii (OP458558), Gordonia oryzae (OQ733166), G. polyisoprenivorans VH2 (OQ733165)).
Conclusions. Ces résultats sont très encourageants car ils révèlent l'identification de 31 espèces distinctes d'actinobactéries dans les sources thermales de l'Est algérien. En particulier, la présence d’actinobactéries dans la source thermale la plus chaude de Guelma (98 °C) est rapportée ici pour la première fois, soulignant la nature d’extrêmophiles de ces microorganismes. De plus, ces isolats présentent un potentiel de production d'enzymes industrielles thermorésistantes telles que l'amylase, la protéase et la lipase.
Abstract
Description of the subject. The growing demand for industrial enzymes with novel properties such as thermoresistance and acid pH resistance is becoming increasingly apparent. Actinobacteria are among the most sought-after groups of microorganisms because of their unrivalled ability to produce bioactive compounds with major economic, environmental and medical impacts. Today inventory of these thermal water bacteria remains incomplete. Further investigations are still needed to employ their unique metabolic abilities.
Objectives. The objective of this study is to investigate the diversity of actinobacteria in selected Algerian thermal springs, identify the isolates, and examine their capacity to produce thermozymes.
Method. Actinobacteria were isolated using selective media from four thermal springs in Eastern Algeria. The isolates underwent phenotypic and biochemical characterization, followed by molecular identification. Additionally, enzyme activities related to amylases, proteases, and lipases were assessed at temperatures of 50 °C, 55 °C, and 60 °C for all obtained isolates.
Results. Thirty-one actinobacteria were isolated using selective media. The AIA medium, which contains asparagine, promotes the growth of actinobacteria over other microorganisms. The SCA medium, characterized by the presence of starch and casein in its composition, also supports the selective growth of actinobacteria. Similarly, the ISP5 medium, rich in glycerol, is known to favor the development of actinobacteria. Finally, the Kenknight and Munaier medium, commonly used for the isolation of actinobacteria was also used. Most actinobacteria isolated in the study exhibited high hydrolytic activities, with 87% degrading starch, proteins, and certain lipids at 50 °C. This active strain percentage decreased to 70% at 55 °C, and further to 60% producing thermostable amylase and 50% producing thermostable proteases and lipases at 60 °C. A taxonomic study combining morphological, physiological and molecular characteristics, using 16S rRNA gene sequencing, enabled us to assign the high-performance actinobacteria to the species Streptomyces scabiei (OQ780365), S. griseochromogenes (OQ780366), S. griseochromogenes (OQ780367), S. avermitilis MA-4680 (OQ780368), S. avermitilis MA-4680 (OQ780369), S. bottropensis (OQ780370), S. thermocarboxydus (OQ733168), S. griseoviridis (OQ733169), S. albidoflavus (OP456974), S. albidoflavus (OP456975), S. griseus (OQ733170), S. griseus (OQ733171) S. thermodiastaticus strain JCM 4840 (OQ727248), S. thermodiastaticus strain JCM 4840 (OQ727246), S. griseorubens (OP456976), S. calvus strain DSM (OQ730106), S. calvus strain DSM (OQ730107), S. formicae strain 1H-GS9 (OQ730105), S. formicae strain 1H-GS9 (OQ730104), S. cellulosae strain NBC_01681 (OQ730102), S. cellulosae strain NBC_01681 (OQ730103), S. violaceoruber strain S21 (OQ733172), Thermoactinomyces daqus (OP456983), T. vulgaris (OQ733167), T. vulgaris (OP456984), T. intermedius (OP457077), Rhodococcus erythropolis (OP458556), R. ruber (OP458557), R. qingshengii (OP458558), Gordonia oryzae (OQ733166), G. polyisoprenivorans VH2 (OQ733165).
Conclusions. These results are highly promising, as they reveal the identification of 31 distinct actinobacterial species in the hot springs of eastern Algeria. Notably, presence of actinobacteria in Guelma’s hottest thermal spring (98 °C) is reported here for the first time, underscoring the extremophilic nature of these microorganisms. Furthermore, these isolates show potential as producers of heat-resistant industrial enzymes, such as amylase, protease and lipase.
Tabla de contenidos
Received 26 August 2024, accepted 10 October 2025, available online 3 December 2025.
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. INTRODUCTION
1Algeria is rich in hot springs, with numerous thermal sources found particularly in the East. The southern part of the country also contains abundant hot water resources and represents a significant reservoir of geothermal springs known as “Continental Intercalaire” (Saibi, 2009).
2The thermal springs in Algeria are characterized by different temperatures ranging from 22 to 98 °C. It also has the hottest terrestrial spring in the nation and the second in the world after Iceland's is Hammam Debagh, which has temperatures as high as 98 °C (Stambouli et al., 2012; Boukhenfouf & Boucenna, 2019).
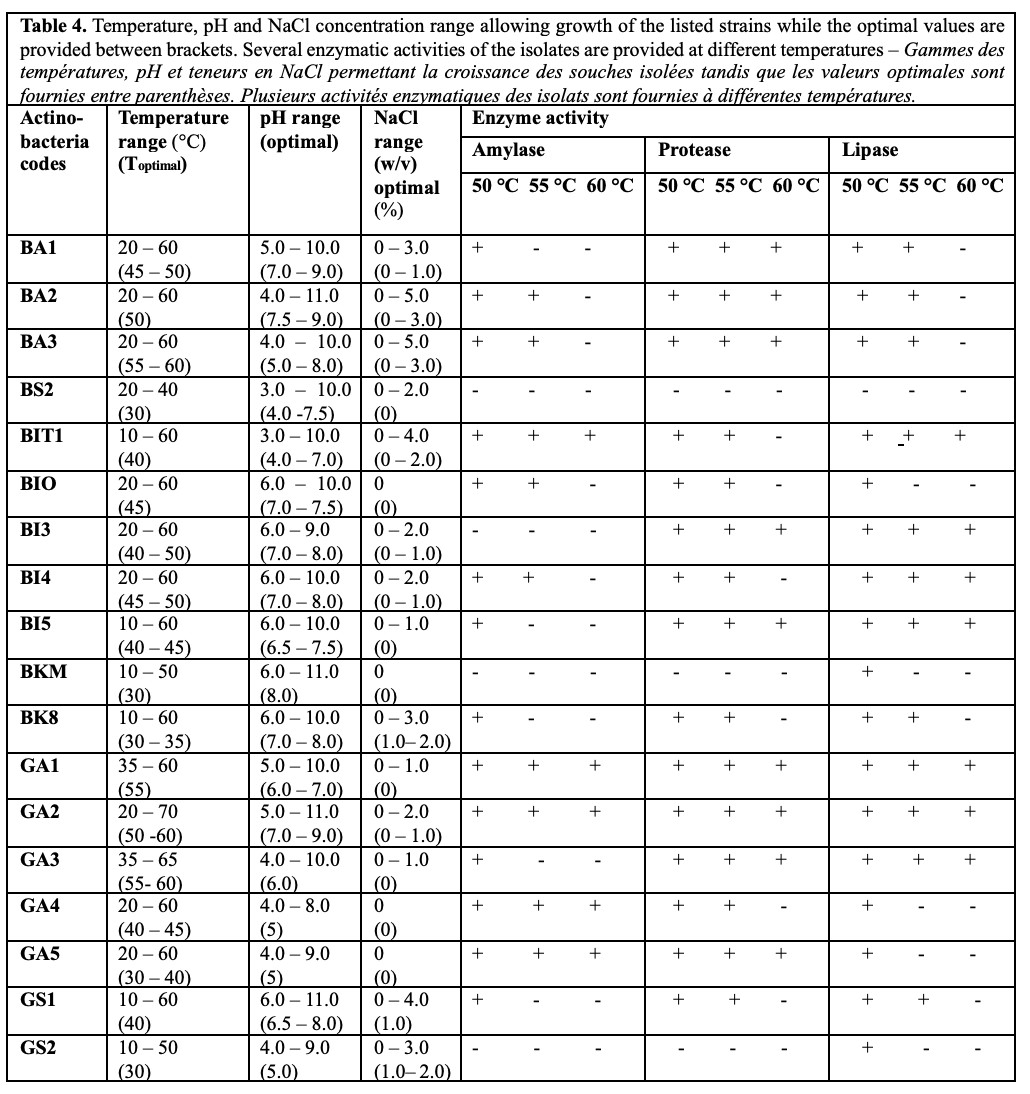
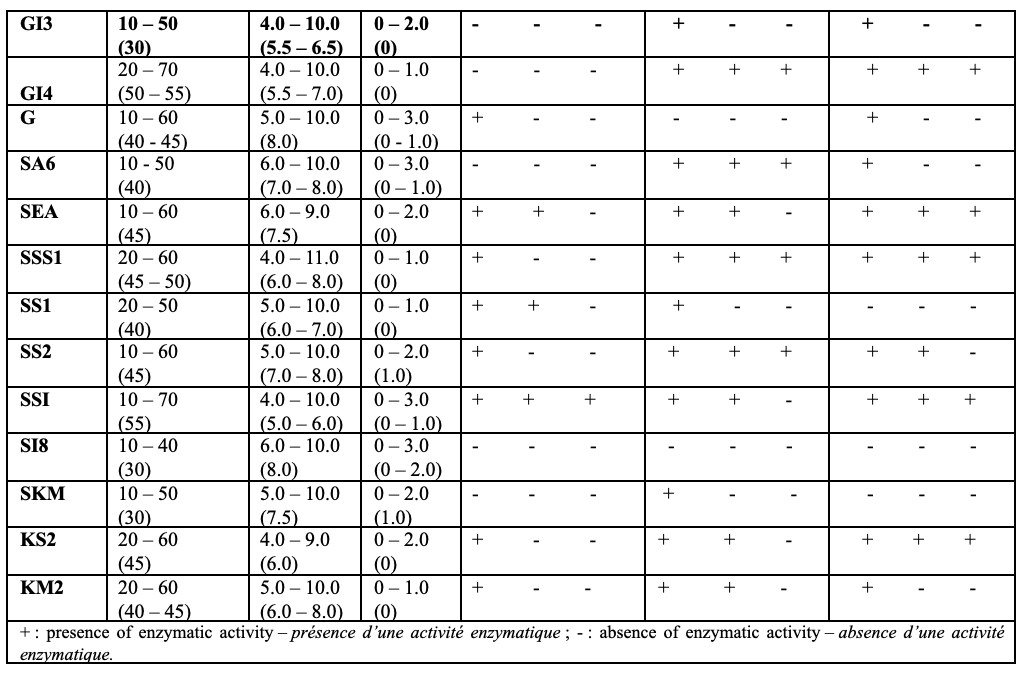
3Traditionally, Algerian hot springs have been used primarly as thermal and therapeutic resorts. However, recent research has focused on their physical, chemical and bacteriological composition (Liang et al., 2015; Ait Ouali et al., 2019). The high temperatures of these springs are the key factor influencing the diversity of thermophilic and hyperthermophilic microorganisms, with optimum growth at temperatures above 55 °C and above 80 °C, respectively (Alrumman et al., 2018; Benammar et al., 2020; Bouacem et al., 2022).
4Actinobacteria are widely distributed in nature. According to Bergey's 2007 classification, actinomycetes belong to the class Actinobacteria (Phylum: Actinobacteria, Class: Actinobacteria) with 5 subclasses, 6 orders (the main orders are: Actinomycetales, Bifidobacteriales, Corynebacteriales, Micrococcales, Propionibacteriales), 41 families, 193 genera, and 1,711 species (Bouizgarne & Ben Aouamar, 2014). These Gram-positive, predominantly aerobic bacteria posses a highly diversified primary and secondary metabolism, making them particularly interesting from a biotechnological point of view (Salwan & Sharma, 2018). In recent years, thermophilic Actinobacteria have attracted considerable attention due to their ability to produce novel bioactive molecules of commercial importance. Numerous studies have confirmed the presence of these bacteria in hot springs, with notable examples including Plinifilum yunnanense sp. Nov. LA5 (Zhang et al., 2007), Thermoactinomyces thalpophilus and T. sacchar (Uzel et al., 2011), Thermoactinomyces khenchelensis sp. Nov. (Mokrane et al., 2016), various Streptomyces species (Medjmadj et al., 2020; Cherifa et al., 2023), and members of the genera Saccharomonospora and Thermoactinomyces (Benammar et al., 2020).
5Traditionally, Actinobacteria have been recognized as prominent producers of antibiotics. However, contemporary research is increasingly focused on their capacity to produce enzymes of significant industrial and commercial value, which are extensively employed across various sectors, including food, detergents, pharmaceuticals, and chemicals (Janaki, 2017; Fernandes et al., 2022).
6To date, only 20 enzymes from microorganisms are widely utilized. Actinobacteria are particularly valuable for their ability to produce and secrete a broad range of extracellular hydrolytic enzymes that are environmentally friendly. Nevertheless, many rare genera of Actinobacteria remain largely unexplored and underexploited for their biotechnological potential (Mukhtar et al., 2017).
7For example, a thermostable keratinase (70 °C) produced by Actinomadura keratinilytica strain Cpt29 is used in the leather industries and the thermostable nitrile hydratase (50-80 °C) produced by thermophilic actinobacteria Pseudonocardia thermophila JCM 3095 is employed in acrylamide production (Habbeche et al., 2014).
8Enzymes serve diverse applications across multiple industries (Mukhtar et al., 2017). For instance, glycosidases are used to decompose plant biomass (Saini et al., 2015), while proteases find utility as detergent additives and in the tanning industry (Mukhtar et al., 2017). Isomerases have been effectively utilized to produce fructose-rich syrups (Patel & Hajoori, 2023). Additionally, enzymes derived from Actinobacteria play a significant role in the clarification of beer and wine (Edison et al., 2022) as well as serving as non-toxic food preservatives. Moreover, the enzymes produced by Actinobacteria are extensively used in the immobilization of clinical diagnostic test preparations, highlighting their importance in both food and medical applications (Abdulla et al., 2008).
9Thermophilic actinobacteria are increasingly recognized for their critical role in the enzyme production industry due to several distinctive advantages. One of the primary benefits of employing these organisms is their ability to thrive in high-temperature environments, which significantly reduces the risk of contamination from mesophilic microorganisms. This feature makes them particularly valuable in industrial fermentation processes, where maintaining a sterile environment is crucial for maximizing yields (Kumar et al., 2019). The enzymes produced by thermophilic actinobacteria, referred to as thermozymes, demonstrate remarkable stability and activity at elevated temperatures, typically retaining their optimal performance at temperatures ranging from 60 °C and up to 80 °C.
10Although thermozymes exhibit mechanisms of action similar to their mesophilic counterparts, their distinct structural features confer enhanced thermal stability, enabling them to remain functional under extreme thermal conditions (Kumar et al., 2019). In addition to thermal stability, thermozymes are more resilient to fluctuations in pH, salinity, and high solute concentrations. These properties render them highly suitable for a wide range of industrial applications, including formulation of detergents, the food industry, and bioremediation. Specifically, the stability of thermozymes under fluctuating conditions offers valuable potential for processes that operate under extreme environmental constraints, thereby contributing to improved efficiency and reduced costs (Krishnasamy, 2017).
11Our main objectives in this work are to isolate thermophilic actinobacteria from hot springs, phenotypically and physiologically characterize these bacteria, and to identify the purified isolates by molecular methods. We also aim to study certain enzymatic abilities under high temperature conditions.
2. MATERIALS AND METHODS
2.1. Sampling and description of study sites
12Three samples of hot water were taken aseptically from each site of four thermal springs in Eastern Algeria: Hammam Beni Haroune (Mila) (36°33′19″N, 6°16′11″E), Hammam Dbegh (Guelma) (36°27′35″N, 7°16′10″E), Hammam Knif (35°29′11,63"N, 7°15′08,64"E) and Hammam Essalihine (Khenchela) (35°26'20,12"N, 7°05′08,46"E) as illustrated in figure 1.
13Samples were taken in 500 ml glass vials, sterilized in Pasteur oven at 180 °C/30 min (Baveja, 2019). Samples were transported to the laboratory at ambient temperature.
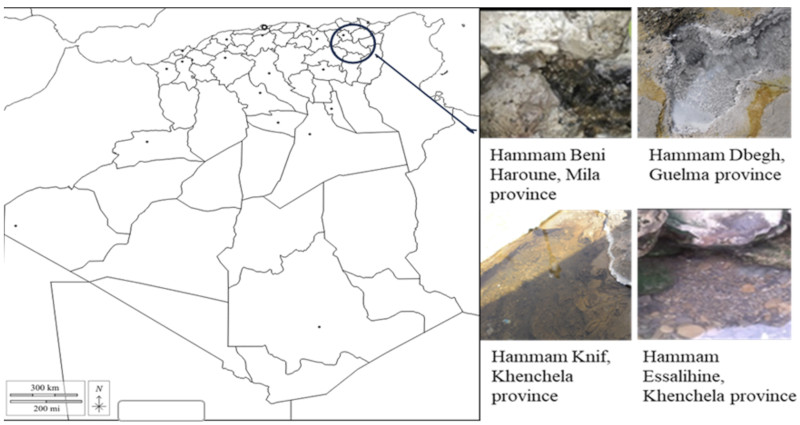
Figure 1. Map of the location of thermal waters in eastern Algeria – Carte de la localisation des eaux thermales dans l’est Algérien.
2.2. Isolation and purification of actinobacteria from hot springs
14Four selective culture media were employed for the isolation of Actinobacteria, namely AIA (Actinomycetes Isolation Agar), SCA (Starch Casein Agar) (Uzel et al., 2011), Kenknight and Munaier (Chaudhary & Prabhu, 2016), and ISP5 medium (Shirling & Gottlieb, 1966). These media were supplemented with an antifungal agent (fungizone) at a concentration of 50 μgmL-1 and an antibacterial agent, polymyxin at 10 μgmL-1, to prevent growth of fungi and bacteria (Boudemagh & Bensouici, 2014). After preparing a series of decimal dilutions in sterile physiological water up to 10-3 (Bastide, 1986), 0.1 ml of each suspension was surface-inoculated on Petri dishes (Hirsch & Christensen, 1983) and incubated at 30 and 55 °C for 20 to 50 days with humidified conditions inside the incubator to prevent the medium from drying out.
2.3. Phenotypic characterization
15Cultural characterization was performed by observing the presence and color of both aerial and substrate mycelium. Fresh and Gram-stained observations of the isolates were made using standard methods (Delarras, 2007; Duraipandiyan et al., 2010). The slide culture technique (Cross, 1989) was employed to examine the mycelia of all strains. In this technique, a previously sterilized slide (sterilized at 180 °C) was gently inserted into the ISP2 medium at a 45° angle relative to the agar surface.
16A drop of the bacterial suspension was then placed between the slide and the medium. Following an incubation period of 7 days, the coverslips were carefully removed and placed on a clean slide for observation under magnifications of X40 and then X100 using an optical microscope (Shimadzu).
17Scanning electron microscope observations (Zeiss) were carried out to examine the morphology of the mycelia and spore chains of each actinobacterium isolate. Observations were carried out at the Scientific and Technical Research Center in Physico-Chemical Analysis in Ouargla, Algeria. This microscope enables samples to be observed directly in their natural state, without requiring pretreatment by metallization. A fraction of the colony was spread out on a sterile slide, covered with another slide and sent to the analysis center.
2.4. Biochemical characterization
18The biochemical characteristics tested are: sugar utilisation (D-glucose, D-fructose, D-lactose, L-arabinose, D-mannitol and maltose), catalase and oxidase tests, gelatin liquefaction, nitrate reductase production, urease production, indole production (Tindall et al., 2007) and capacity of producing the melanoid pigments which can be detected on ISP7 medium (Margalith, 1992).
2.5. Molecular identification of isolates
19The molecular identification was carried out at Gen Life Sciences research center, Algeria. The study used the GF-1 Nucleic Acid Extraction Kit to extract bacterial genomic DNA, which was then stored at 4 °C prior to PCR amplification with 16S rRNA primers set (27F: 5’–AGAGeTTTGATCCTGGCTCAG–3’ and 1492R 5’– CCGTCAATTCCTTTGAGTTT–3’) (Edwards et al., 1989). The PCR mixture included Hot Start Taq DNA Polymerase, DNA template, primers, and distilled water. The PCR process involved denaturation, annealing, and extension steps, followed by gel electrophoresis of the PCR products on a 1.5% agarose gel. PCR products were sequenced by the Sanger method in both forward and reverse directions via separate reactions, each containing 40 µg of template DNA. To purify the sequencing products, an ethanol precipitation method was used, followed by centrifugation to remove unincorporated reagents. The purified DNA was then rehydrated in formamide (15 µl) and analyzed using a 3130 Genetic Analyzer. Two forward and two reverse sequences from each sample were aligned to create a composite sequence, which was visually assessed for quality, leading to the editing or removal of low-quality traces. Organisms were identified by comparing consensus sequences to a database using BLASTn, available on the NCBI website (http://blast.ncbi.nlm.nih.gov). The 16S rRNA gene sequences of strains were submitted to the NCBI GenBank database to obtain the accession numbers of all isolates. The phylogenetic tree was constructed using MEGA 11 software (Tamura et al., 2004; Kumar et al., 2018) The bootstrap test (1,000 replicates) was used to determine confidence in branching points (Felsenstein, 1985).
2.6. Effect of temperature, pH and NaCl on growth of actinobacteria
20Actinobacteria were tested for growth at different temperatures from 10 - 80 °C with an interval of 10 °C and for resistance to different pH from 4.0 - 11.0 with an interval of 1.0 and as well as for salt tolerance (0 - 5% w/v NaCl) were tested on ISP2 liquid medium under agitation at 180 rpm. After determining the temperature and pH ranges for each strain, another series of tests was conducted with temperatures varying by 5 °C and pH levels varying by 0.5 to accurately determine the optimum for each parameter.
21Strains were incubated at 30 °C for pH and salinity testing. For temperature and salinity tests, pH was set at 7 while for temperature and pH tests, salinity was set at 0% w/v NaCl. Each test was performed in triplicate. Strain growth was measured after three days incubation by measuring turbidity at 540 nm using a UV-visible spectrophotometer type 1800A, Shimadzu, Japan.
2.7. Enzyme production by actinobacteria
22The ability of actinobacteria to produce three extracellular hydrolytic enzymes (amylase, protease and lipase) was assessed triplicate on suitable media for each test. The thermostability of these enzymes was evaluated by incubating culture media at temperatures of 50, 55 and 60 °C for three days (Panosyan, 2019). Amylase production was tested on nutrient agar supplemented with 1% starch. After growth of the isolates, a solution of lugol was added, the positive result being reflected by the absence of black coloration around the colonies (Jeffrey, 2008). Extracellular protease production was determined by plating isolates on casein agar containing 1% skimmed milk. The appearance of a clear halo around colonies indicates casein degradation (Gordon et al., 1974). Lipase production was carried out on Sierra medium with Tween 80 added. The appearance of an opaque halo around colonies signaled the presence of lipase (Delarras, 2007).
3. RESULTS
3.1. Isolation and enumeration of actinobacteria
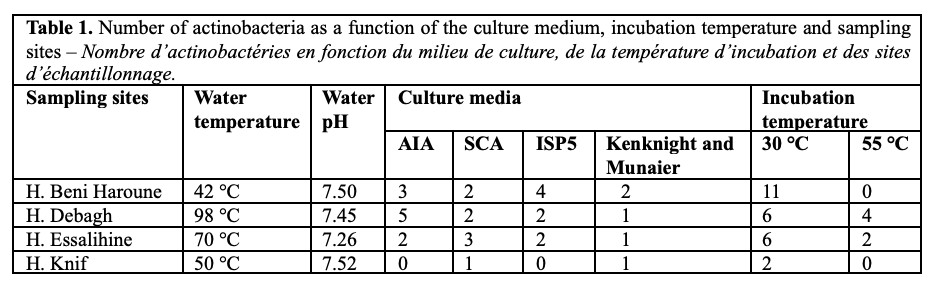
23Inoculated Petri dishes showed good growth of isolates after 40 days of incubation. The colonies obtained dysplayed differences in color, appearance and texture. Colonies exhibiting characteristics typical of actinobacteria were selected and subcultured on ISP2 medium until purification. The number of isolates per sampling site, isolation medium and incubation temperature is presented in table 1.
3.2. Morphological, cultural and biochemical characterization of isolates
24The phenotypic and cultural characteristics of the isolates revealed that the aerial mycelium color ranged from white, green, yellow, gray and to orange while substrate mycelium was generally white to transparent. Melanoid pigment production was observed in only three isolates. All isolates tested positive for catalase activity. The majority of the strains produced oxidase, gelatinase, urease, and nitrate reductase. Regarding sugar utilization, most isolates displayed a preference for glucose and fructose as their primary carbon sources over other sugars (Table 2 and Figure 2).
25Observations in the fresh state, by Gram staining, the slide technique and electron microscopy are presented in figures 2 and 3.
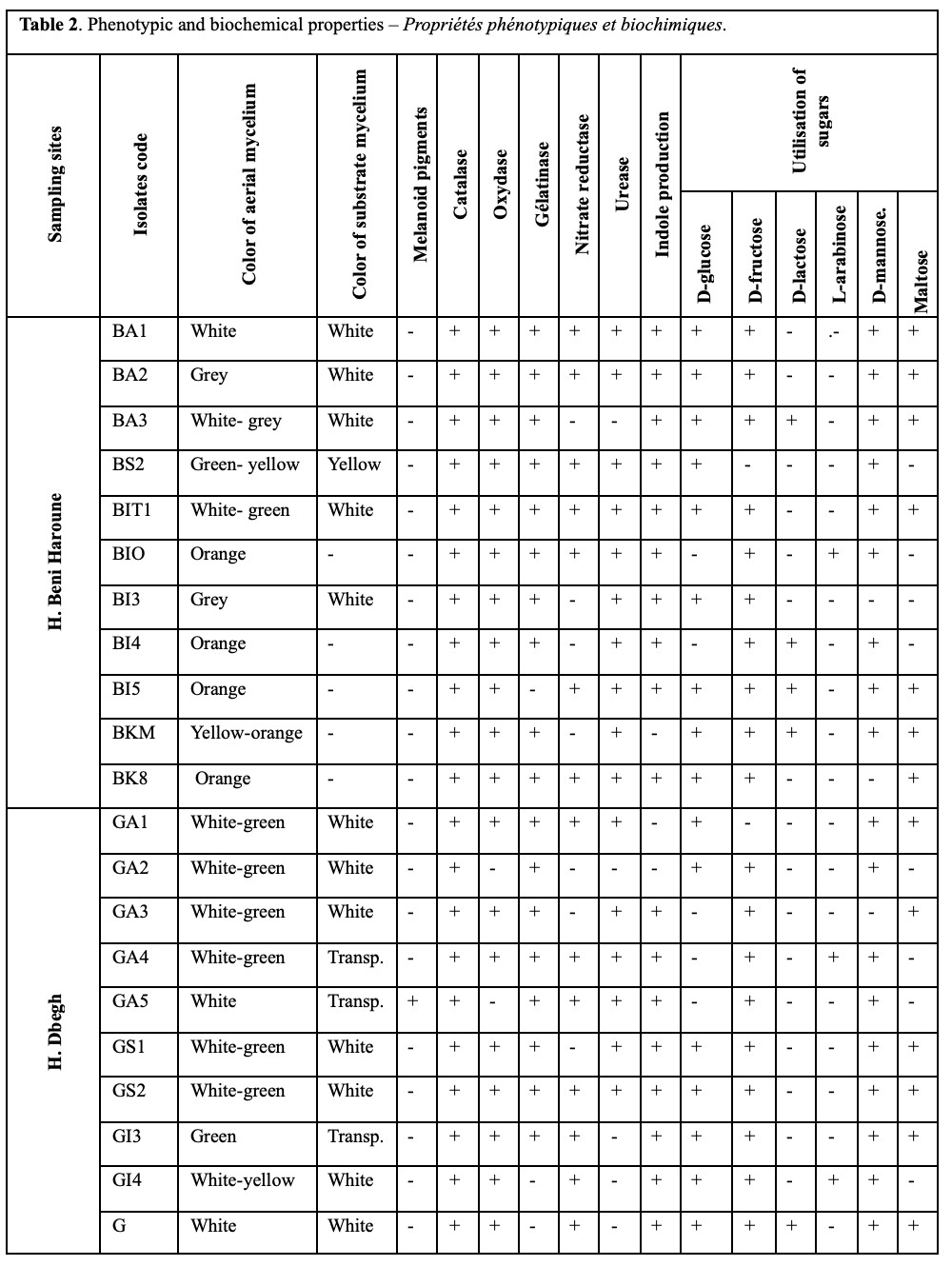
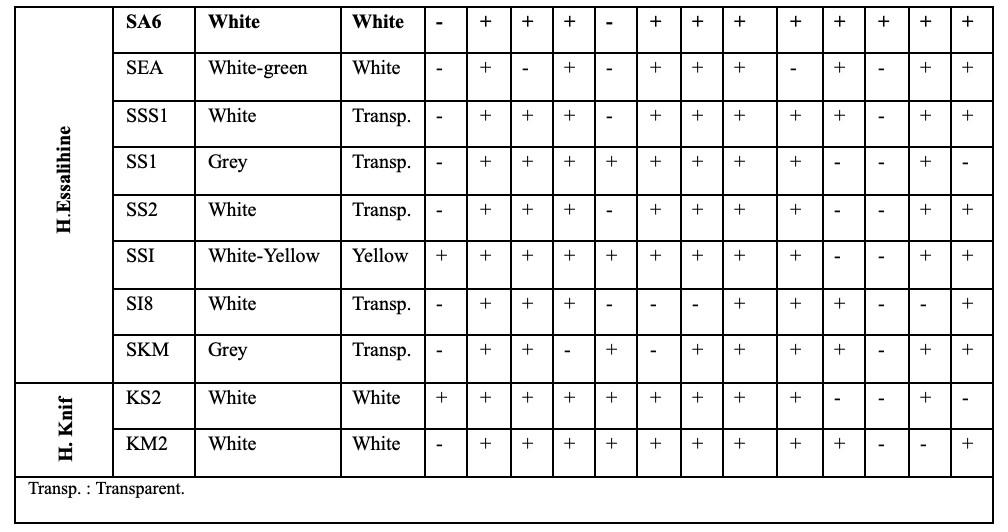
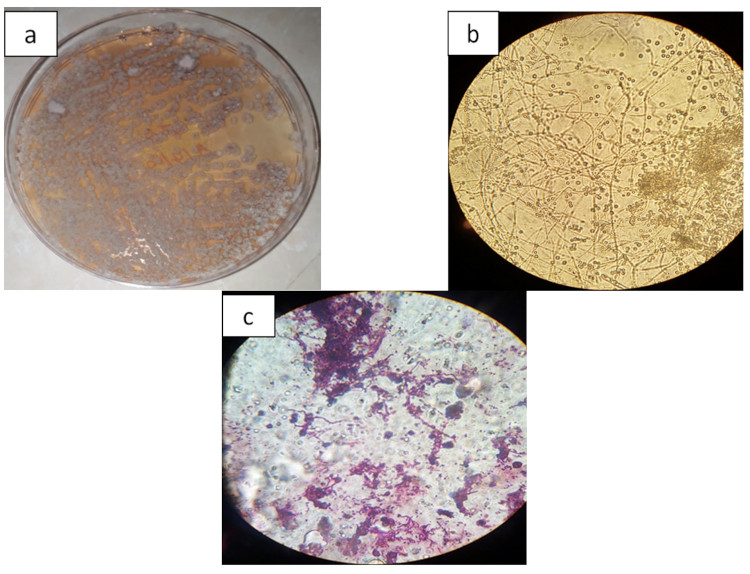
Figure 2. a. Actinobacterial culture image (GS2) on ISP2 medium – Image de la culture actinobactériale (GS2) sur le milieu de culture ISP2; b. Microscopic image of Actinobacterium GS2 without staining (magnification: X100) – Image microscopique de l’actinobactérie GS2 sans coloration (grossissement : X100); c. Microscopic image of Actinobacterium GS2 with Gram staining (magnification: X100) – Image microscopique de l’actinobactérie GS2 après coloration de Gram (grossissement : X100).

Figure 3. Spore chains of actinobacterium GS2 under Scanning Electron microscope (magnification: 1,500 KX) – Chaines de spores de l’actinobactérie GS2 sous le microscope électronique à balayage (grossissement : 1 500 KX).
3.3. Molecular analysis based on the partial sequence of the 16S rRNA gene
26Molecular identification of the 31 isolates showed that the majority (22) belonged to the genus Streptomyces, 4 to the genus Thermoactinomyces, 3 to the genus Rhodococcus and 2 to the genus Gordonia (Table 3). A phylogenetic tree is provided (Figure 4)
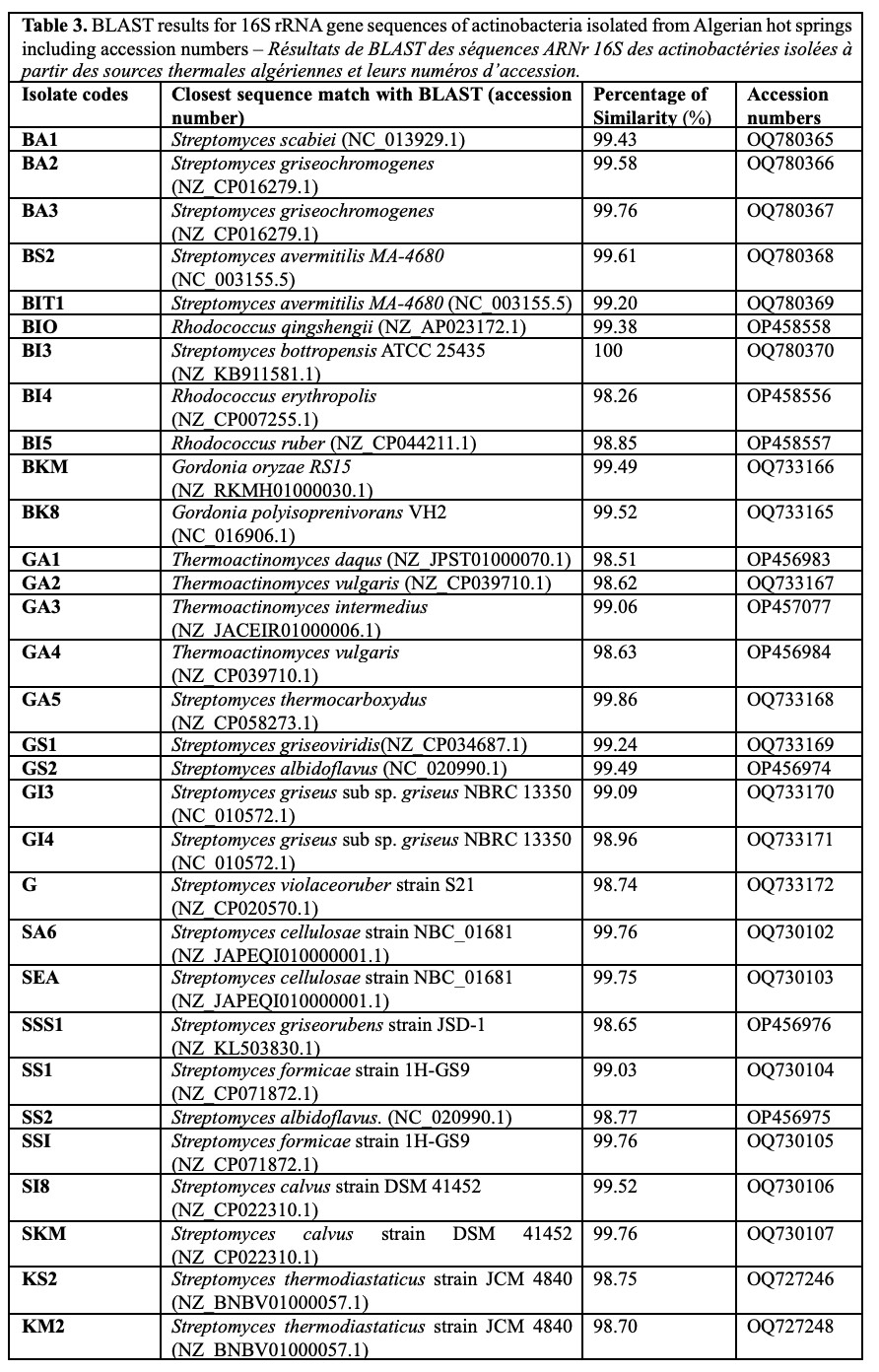
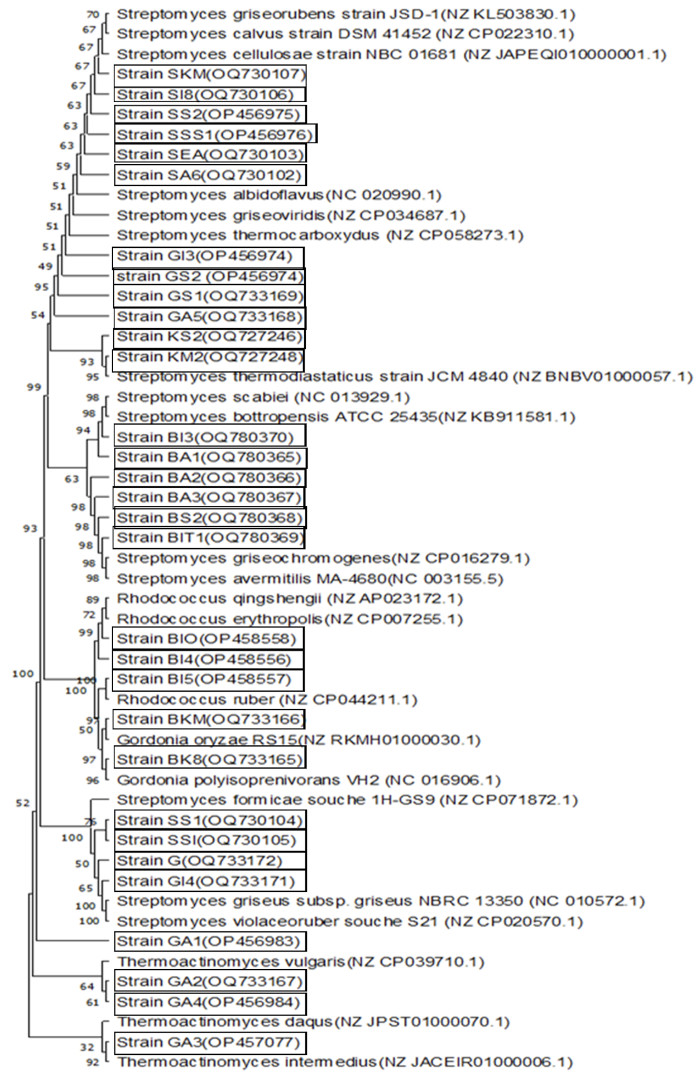
Figure 4. Neighbor-joining tree showing the phylogenetic relationships of 16S rRNA gene sequences amplified of actinobacteria isolated from Algerian hot springs to closely related sequences from the BLAST – Arbre Neighbor-joining montrant les relations phylogénétiques des séquences du gène ARNr 16S amplifiées à partir des actinobactéries isolées des sources thermales algériennes et leurs séquences étroitement apparentées issues de BLAST.
3.4. Effect of temperature, pH and NaCl on actinobacterial growth
27Table 4 indicates that 22 isolates (BA1, BA2, BA3, BT1, BI3, BI4, BIO, BI5, GA1, GA2, GA3, GI4, GS1, G, SA6, SEA, SS1, SS2, SSS1, SSI, KS2 and KM2) exhibited optimal growth between 50 and 60 °C, confirming their thermophilic nature. Among these, isolates BA1, BA2, BI3, BI4, BIO, BI5, GA2, GS1, G, SA6, SEA, SS2, SSS1 and KM2 grew within a pH range of 6-8, while isolates BA3, BIT1, GA1, GI4, GA3, SS1, SSI and KS2 displayed optimal growth in a slightly more acidic range of 5-7. The ability of these isolates to degrade the tested enzymes (amylase, protease, and lipase) was clearly demonstrated at all three temperatures (50, 55, and 60 °C) for most of the strains. In contrast, the other isolates exhibited optimal growth between temperatures of 30 and 45 °C, with a pH range of 6 to 8. The tolerance to NaCl is low for most of these isolates.
4. DISCUSSION
28The diversity of thermophiles has been studied in different hot springs around the world, however the most well-known and well-studied geothermal areas are found in North America (Yellowstone, where temperatures can reach up to 93 °C), Iceland, New Zealand, Japan, Italy and the Soviet Union. Hot springs are located throughout India, in places where the water is boiling (e.g., Manikaran, Himachal Pradesh) (Satyanarayana et al., 2005).
29In the literature, relatively few studies have investigated actinobacteria in thermal waters compared to those conducted on sediments (Zhaoqi et al., 2009; Duan et al., 2014 ; Arshia et al., 2016; Mokrane et al., 2016).
30The adaptation of actinobacteria to high temperatures is due to the presence of membrane lipids that contain more straight-chain saturated fatty acids than those of mesophiles. This composition allows thermophiles to maintain the membrane fluidity required for proper function at higher temperatures.
31In addition, DNA-binding histone-like proteins have been identified in hyperthermophiles, which protect DNA from denaturation (Agarwal & Mathur, 2016). Other actinobacteria can adapt by producing specific enzymes, such as Streptomyces thermoautotrophicus sp. Nov. This strain produces enzymes of the autotrophic metabolic pathway, such as carbon monoxide dehydrogenase “CODH”, which facilitates growth in a warm, nutrient-deprived state by oxidizing available inorganic compounds such as carbon monoxide to CO2, which is then fixed by the RuBisCO enzyme in the microbial biomass via the Calvin-Benson cycle (King & Weber, 2007).
32In these investigations a total of 31 actinobacteria were isolated from four hot springs. Ten isolates were obtained from Hammam Debagh in Guelma, eight from Hammam Essalihine in Khenchela, two from Hammam Knif in Khenchela and eleven from Hammam Beni Haroune (Table 1). These results indicate that the thermal springs of Hammam Beni Haroune and Hammam Debagh harbor the highest abundance in actinobacteria. An intermediate number was obtained from the Hammam Essalihine thermal spring. In contrast, the number of isolates obtained from the Hammam Knif Spring was relatively low. These results are partly identical to other research (Medjemadj et al., 2020) where the authors found that Tleghma and Khenchela waters contained high levels of actinobacteria.
33The actinobacteria identified in this work belong to the genera Streptomyces, Rhodococcus, Gordonia and Thermoactinomyces. In a similar study, only three genera of actinobacteria (Rathayibacter, Streptomyces and Rhodococcus) were isolated by culture techniques from the same regions of Eastern Algeria (Medjemadj et al., 2020; Cherifa et al., 2023).
34In this work, the presence of actinobacteria in the thermal waters of Hammam Debagh, characterized by a very high temperature of 98 °C, is demonstrated for the first time. This is in contrast to the study by Medjemadj et al. (2020) who found no actinobacteria in the waters of this spring. One plausible explanation for this result are that the incubation period applied in these investigations is 50 days, in contrast to the work of Medjemadj et al. (2020), which is only 30 days. However, the absence of these bacteria in this spring was also supported by other works (Gomri et al., 2018) that used a metagenomic approach to search for endospore-forming aerobic thermophilic bacteria in the thermal springs of Hammam Ouled Ali and Hammam Debagh.
35As far as we know, the presence of actinobacteria in thermal spring waters was first demonstrated in 2007 by Zhang et al. In this work, a new strain named Planifilum yunnanense sp. Nov. was isolated from a thermal spring in Yunnan province, China. A new strain, Nocardioides pakistanensis, was isolated from a thermal spring in Pakistan (Arshia et al., 2016). Ten actinobacteria were also isolated from a thermal spring in Bora Spring in Bora (Central Sulawesi) (Sapa et al., 2023).
36The dominant genus isolated from the thermal waters studied is Streptomyces, with 22 of the 31 actinobacterial isolates identified. This result is in line with the observation made by Thakur et al. (2007) that strains growing on a wide range of culture media generally show a morphology typical of Streptomyces. In addition, the genus Thermoactinomyces is considered the most frequently isolated genus from similar habitats (Uzel et al., 2011; Mokrane et al., 2016; Sahay et al., 2017; Panosyan, 2019). The presence of the genus Rhodococcus in the thermal spring of Beni Haroune fits perfectly with Medjemadj et al. (2020).
37Among the best known thermophilic actinobacteria are Acidothermus cellulolyticus 11B isolated from the National Parc of Yellowstone (Barabote et al., 2009), Streptomyces thermoautotrophicus (Gadkari et al., 1990) and Acidithiomicrobium sp. (Norris et al., 2011) which are obligate chemoautotrophs, growing only on CO2 + H2 and sulfur, respectively, as well as Streptomyces strain G26 (Bell et al., 1988) and Amycolatopsis methanolica (Boer et al., 1990). Actinobacteria present in these environments are mainly fast-growing and spore-forming. These spores are thermoduric and are stable at higher temperatures for a longer period of time. This appears to provide an additional ecological advantage over other bacteria, making them easier to return to their vegetative forms with the advent of favorable conditions.
38The results also show that the AIA culture medium is the most favorable for isolating actinobacteria from thermal waters. According to some researchers, the presence of asparagine favors the growth of actinobacteria over other microorganisms (Suzuki, 2001). In this work, this medium enabled us to isolate 10 actinobacteria. In the work of Medjemadj et al. (2020), this medium enabled isolation of the highest number of actinobacteria from thermal waters (10 isolates). The second medium used in this research is SCA, which enabled us to selectively isolate eight actinobacteria. This medium was successfully used in the work of Suzuki (2001), where several rare genera, such as Actinomonospora, Actinopolyspara, Planomonospora and Planobispora, were isolated. Streptomyces and other genera were isolated by Medjemadj et al. (2020), using this medium.
39The use of ISP5 medium in this work, for the isolation of actinobacteria, enabled eight isolates to be obtained. The presence of glycerol is known to be favorable for actinobacteria (Jihani et al., 2012; Siddique et al., 2014). Kenknight and Munaiern culture medium was used to isolate five colonies of actinobacteria. However ,this medium is known for the isolation of actinobacteria from soil (Rao, 2005).
40This medium was used for the isolation of actinobacteria from a thermal spring located at Akoli, Vajreshwari in India by Chaudhary & Prabhu (2016).
41The production of melanoid pigments was demonstrated by isolates GA5, SSI and KS2. This characteristic is considered as a key to the classification of species in the Streptomyces genus (Shirling & Gottlieb, 1966). This production involves the presence of a tyrosinase capable of degrading tyrosine into an initially yellow compound: 5,6-dihydroxyindol acid, which forms a brownish-black melanin compound through a condensation process (Margalith, 1992).
42All isolates were tested positive for catalase production. Most strains have oxidase, gelatinase, urease and nitrate reductase. Sugar utilization shows that most isolates prefer to use glucose and fructose as their sole carbon source, rather than other sugars. These metabolic characteristics vary between genera and species of the same actinobacteria genus (Goodfellow et al., 2012) (Table 2).
43The influence of temperature on the growth of actinobacteria strains indicates that the majority of isolates are thermophiles, able to grow at 55 °C, with an optimum growth between 40 and 55 °C. These results are logical, given that the temperatures of the thermal springs tested, which range from 42 to 98 °C. Seven isolates (BS2, BKM, BK8, GS2, GI3, SI8 and SKM) are mesophiles, with optimum growth at 30 °C. Most strains show good growth in a pH range of 6 to 8. Isolates BS2, BIT1, GA4, GA5, GS2, GI3, GI4 and SSI show optimal growth in acidic pH values of 4 to 5. These isolates are acidophiles according to classification of Jiang & Xu (1993). Unlike BA1, BA2, GA2 and SEA, which are basophiles according to the same classification, they grow best at pH 9 (Table 4).
44NaCl tolerance is very low for the majority of isolates selected in this work. Only isolates BA2 and BA3 have optimal growth between 0-3% NaCl and tolerate concentrations up to 5%. Also isolates GS2, BK8 and BIT1 show optimal growth between 0-2%. According to the classification proposed by Kushner & Kamekura (1988), these isolates are light halophiles. The remaining strains are considered non-halophiles. These results are similar to those of Patel et al. (2017) who report the presence of some actinobacteria in thermal waters, which tolerate NaCl (Table 4).
45Microorganisms found in extreme environments have attracted much attention, due to the production of various natural compounds such as thermozymes and their specialized mechanisms of adaptation to extreme environments (Kiki, 2016). The major advantage of using thermozymes in industrial processes is the ability to maintain the desired enzymatic activity under thermophilic conditions, while preventing the growth of mesophilic germs responsible for contamination of the reaction medium in fermentations. Another advantage of high temperature conditions in the fermentation is the reduction of fluid viscosity (Bruins et al., 2001). High temperatures can also destroy pathogenic germs. These thermozymes, which act on sugars, lipids and many other substrates, have already been used in various fields of application such as human and animal nutrition but also in the detergent, pulp and paper industries, etc. (Kirk et al., 2005).
46Most actinobacteria isolated in this study showed very high hydrolytic activities. A total of 87% of the isolates degraded starch, proteins and certain lipids at 50 °C. This percentage decreases to 70% at 55 °C despite the optimum growth being between 40 and 55 °C. At 60 °C, 60% of isolates produce thermostable amylase and 50% thermostable proteases and lipases (Table 4). This reflects the thermal stress experienced by certain strains. Although many strains can grow at higher temperatures, their enzymatic activity and cellular function may decline near the upper limit of their tolerance. This suggests that even though these organisms can survive and grow at 55 °C, their metabolic proces may begin to decline, reduced the percentage of active strains.
47The optimum temperature for enzymatic activity often differs from the growth optimum. In the case of actinobacteria, the enzymes may exhibit peak activity at slightly lower temperatures than the maximum growth temperature, typically around 40-50 °C. This discrepancy can result in active strains showing reduced metabolic function at 55 °C, even if they are still viable.
48The production of thermostable proteases by actinobacteria has been demonstrated by numerous studies. For example, Streptomyces thermoviolaceus has the capacity to produce a thermostable proteases as Thermoactinomyces sp. AkhA-12 and T. vulgaris Tatev 35a, two actinobacterial strains capable of producing thermostable proteases at temperatures of 50, 55 and 60 °C protease (Saghatelyan et al., 2021). In addition, the Streptomyces sp. Al-Dhabi-49 strain exhibits strong proteolytic activity at 40 °C (Al-Dhabi et al., 2019).
49Our results agree with research of Gousterova et al. (2014) who found that 81% of actinobacteria isolated from hot ecosystems in Livingston Island, Antarctica, exhibit lipolytic activity at a temperature of 45 °C. Two strains, Thermoactinomyces sp. AkhA-12 and T. vulgaris Tatev 35a, are capable of producing thermostable lipases at temperatures of 50, 55 and 60 °C (Saghatelyan et al., 2021).
50The production of thermostable amylase by thermophilic actinobacteria isolated from thermal springs has been demonstrated in a strain named Streptomyces sp. Al-Dhabi, which produces a thermostable α-amylase at 40 °C (Al-Dhabi et al., 2020). Another recent study reported amylolytic activity at temperatures up to 70 °C in two strains of actinobacteria called Streptomyces rhizosphaericola and Streptomyces cavourensis isolated from a thermal spring in eastern Algeria (Cherifa et al., 2023).
5. CONCLUSIONS
51Culture based-techniques enabled the isolation of 31 actinobacteria isolates from the four hot springs studied. Morphological characterization revealed that these isolates are Gram-positive bacteria, most of which exhibit branched filaments that occasionally fragment into coccobacillus elements as well as diffusible pigments and melanoid production. Polyphasic identification combining morphological, physiological, biochemical and molecular analyses allowed the assignment of 31 actinobacteria to the genera Streptomyces, Thermoactinomyces, Rhodococcus and Gordonia. These actinobacteria tolerate high temperatures (60 °C) and are therefore classified as thermotolerant and thermophilic. They also display the capacity to grow across a wide pH range (4-9) and some strains tolerate high concentrations of sodium chloride (3% of NaCl).
52These bacteria can produce thermostable amylases, proteases and lipases at temperatures of 50, 55 and 60 °C. Their ability to synthesize thermostable enzymes — highly valued in biotechnology — combined with their robustness under high-temperature conditions, makes them excellent candidates for industrial fermentation processes requiring elevated temperatures.
Bibliographie
Abdulla H., May E., Bahgat M. & Dewedar A., 2008. Characterisation of actinomycetes isolated from ancient stone and their potential for deterioration. Pol. J. Microbiol., 57(3), 213-220.
Agarwal A. & Mathur N., 2016. Thermophilic actinomycetes are potential source of novel bioactive compounds: A review. Eur. J. Pharm. Med. Res., 3(2), 130-138.
Ait Ouali A. et al., 2019. Geothermal potential in the Ouarsenis-Biban-Kabylie (North Central Algeria): hot spring catalogue. Arab. J. Geosci., 12, 741, doi.org/10.1007/s12517-019-4945-4
Al-Dhabi N.A., Esmail G.A., Ghilan A.M. & Arasu M.V., 2019. Isolation and screening of Streptomyces sp. Al-Dhabi-49 from the environment of Saudi Arabia with concomitant production of lipase and protease in submerged fermentation. Saudi J. Biol. Sci., 27(2020), 474-479, doi.org/10.1016/j.sjbs.2019.11.011
Al-Dhabi N.A. et al., 2020. Isolation and purification of starch hydrolysing amylase from Streptomyces sp. Al-Dhabi-46 obtained from the Jazan region of Saudi Arabia with industrial applications. J. King Saud Univ. Sci., 32(2020), 1226-1232, doi.org/10.1016/j.jksus.2019.11.018
Alrumman S., Mostafa Y.S.M., Al-Qahtani S. & Taha T.H.H., 2018. Hydrolytic enzyme production by thermophilic bacteria isolated from Saudi hot springs. Open Life Sci., 13, 470-480, doi.org/10.1515/biol-2018-0056
Arshia A. et al., 2016. Nocardioides pakistanensis sp. Nov., isolated from a hot water spring of Tatta Paniin Pakistan. Antonie van Leeuwenhoek, 109, 1101-1109, doi.org/10.1007/s10482-016-0711-8
Barabote R.D. et al., 2009. Complete genome of the cellulolytic thermophile Acidothermus cellulolyticus 11B provides insights into its ecophysiological and evolutionary adaptations. Genome Res., 19, 1033-1043, doi.org/10.1101/gr.084848.108
Bastide A. et al., 1986. Isolement et sélection de souches d'actinomycète productrices de substances antifongiques de structure non-polyéniques. Mircen J., 2, 453-466.
Baveja C.P., 2019. Textbook of microbiology. 7th ed. New Delhi : Arya Publication.
Bell J.M., Colby J. & Williams E., 1988. CO oxidoreductase from Streptomyces strain G26 is a molybdenum hydroxylase. Biochem. J., 250(2), 605-612, doi.org/10.1042/bj2500605
Benammar L. et al., 2020. Diversity and enzymatic potential of thermophilic bacteria associated with terrestrial hot springs in Algeria. Braz. J. Microbiol., 51,1987-2007, doi.org/10.1007/s42770-020-00376-0
Boer L.D. et al., 1990. Amycolatopsis methanolica sp. Nov., a facultatively methylotrophic actinomycete. Int. J. Syst. Bacteriol., 40, 194-204, doi.org/10.1099/00207713-40-2-194
Bouacem K. et al., 2022. Isolation and characterization of moderately thermophilic aerobic cultivable bacteria from Hammam Righa hot spring (Algeria): description of their hydrolytic capacities. Alg. J. Environ. Sci. Technol., 8, 3.
Boudemagh A. & Bensouici K., 2014. The effect of thermic pretreatment and antibiotics on the selective isolation of the culturable actinomycetes from Algerian desert soil. Sci. Technol., 9, 25-32.
Bouizgarne B. & Ben Aouamar A.A., 2014. Diversity of plant associated actinobacteria. In : Maheshwari D.K., ed. Bacterial diversity in sustainable agriculture. Cham, Switzerland: Springer International Publishing, 41-99, doi.org/10.1007/978-3-319-05936-5_3
Boukhenfouf W. & Boucenna A., 2019. Comparison of the radioisotopic composition between hot spring water of Hammam Debagh and its associated deposits. Radiat. Prot. Dosim., 187(1), 369-377, doi.org/10.1093/rpd/ncz177
Bruins M.E., Janssen A.E.M. & Boom R.M., 2001. Thermozymes and their applications. Appl. Biochem. Biotechnol., 90, 155-186, doi.org/10.1385/ABAB:90:2:155
Chaudhary N. & Prabhu S., 2016. Thermophilic actinomycetes from hot water spring capable of producing enzymes of industrial importance. Int. J. Res. Stud. Biosci., 4(6), 29-35, doi.org/10.20431/2349-0365.0406005
Cherifa L., Meissa M., Uzel A. & Allaoueddine B., 2023. Thermophilic actinobacteria isolated from Tleghma hot spring: a potential source of thermostable α-amylase. Carpathian J. Food Sci. Technol., 15(4), 168-182, doi.org/10.34302/crpjfst/2023.15.4.13
Cross T., 1989. Growth and examination of actinomycetes some guidelines. In: Bergey’s manual of systematic bacteriology. 4th ed. Baltimore, MD, USA: Williams and Wilkins Company, 2340-2343, doi.org/10.1016/j.mycmed.2009.11.002
Delarras C., 2007. Microbiologie pratique pour le laboratoire d’analyse ou de contrôle sanitaire. Paris : Lavoisier, 126-173.
Duan YY. et al., 2014. Streptomyces calidiresistens sp. Nov., isolated from a hot spring sediment. Antonie van Leeuwenhoek, 106, 189-196, doi.org/10.1007/s10482-014-0180-x
Duraipandiyan V. et al., 2010. Antimicrobial properties of actinomycetes from the soil of Himalaya. J. Mycol. Med., 20, 15-20.
Edison L.K., Anu S. & Pradeep N.S., 2022. Exploitation of actinobacteria for beta-glucanolytic enzymes – screening and characterization. In: Pradeep N. & Edison L.K., eds. Microbial beta glucanases. Singapore: Springer, doi.org/10.1007/978-981-19-6466-4_8
Edwards U. et al., 1989. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res., 17(19), 7843-7853, doi.org/10.1093/nar/17.19.7843
Felsenstein J., 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39(4), 783-791, doi.org/10.1111/j.1558-5646.1985.tb00420.x
Fernandes de Souza H. et al., 2022. Recent advances in the application of xylanases in the food industry and production by actinobacteria: a review. Food Res. Int., 162(Pt B), 112103, doi.org/10.1016/j.foodres.2022.112103
Gadkari D. et al., 1990. Streptomyces thermoautotrophicus sp. Nov., a thermophilic CO- and H(2)-oxidizing obligate chemolithoautotroph. Appl. Environ. Microbiol., 56(12), 3727-3734, doi.org/10.1128/aem.56.12.3727-3734.1990
Gomri M.A., El Moulouk Khaldi T. & Kharroub K., 2018. Analysis of the diversity of aerobic, thermophilic endospore-forming bacteria in two Algerian hot springs using cultural and non-cultural methods. Ann. Microbiol., 68, 915-929, doi.org/10.1007/s13213-018-1401-8
Goodfellow M. et al., 2012. Bergey's manual of systematic bacteriology. Vol. 5: The actinobacteria. 2nd ed. Heidelberg, Germany: Springer.
Gordon R.E., Barnett D.A., Handarhan J.E. & Hor-Nay-Pang C., 1974. Nocardia coeliaca, Nocardia autotrophica and the nocardin strains. Int. J. Syst. Bacteriol., 24(1), 54-63.
Gousterova A., Paskaleva D. & Vasileva-Tonkova E., 2014. Characterization of culturable thermophilic actinobacteria from Livingston Island, Antarctica. Int. Res. J. Biol. Sci., 3(30), 30-36.
Habbeche A. et al., 2014. Purification and biochemical characterization of a detergent-stable keratinase from a newly thermophilic actinomycete Actinomadura keratinilytica strain Cpt29 isolated from poultry compost. J. Biosci. Bioeng., 117(4), 413-421, doi.org/10.1016/j.jbiosc.2013.09.006
Hirsch C.F. & Christensen D.L., 1983. Novel method for selective isolation of actinomycetes. Appl. Environ. Microbiol., 46, 925-929.
Janaki T., 2017. Enzymes from actinomycetes – Review. Int. J. ChemTech Res., 10(2),176-182.
Jeffrey L.S.H., 2008. Isolation, characterization and identification of actinomycetes from agriculture soils at Semongok. Afr. J. Biotechnol., 7(20), 3697-3702.
Jiang C. & Xu L., 1993. Actinomycete diversity in unusual habitats. Actinomycetes, 4, 47-57.
Jihani S. et al., 2012. Isolation and molecular identification of antibiotic-producing actinomycetes from an old house in the medina of Fez, Morocco. Afr. J. Microbiol. Res., 6(47), 7370-7376, doi.org/10.5897/AJMR12.1711
Kiki M.J., 2016. A new medium for the isolation and enrichment of halophilic actinobacteria. Life Sci. J., 13(1), 65-71, doi.org/10.7537/marslsj13011610.
King G.M. & Weber C.F., 2007. Distribution, diversity and ecology of aerobic CO-oxidizing bacteria. Nat. Rev. Microbiol., 5(2), 107-108, doi.org/10.1038/nrmicro1595
Kirk O., Borchert T.V. & Fuglsang C.C., 2005. Industrial enzyme applications. Curr. Opin. Biotechnol., 13(4), 345-351, doi.org/10.1016/S0958-1669(02)00328-2
Krishnasamy N. et al., 2017. Purification, characterization, and statistical optimization of a thermostable α-amylase from desert actinobacterium Streptomyces fragilis DA7-7. 3 Biotech, 7(5), 350, doi.org/10.1007/s13205-017-0981-5
Kumar S. et al., 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol., 35, 1547-1549, doi.org/10.1093/molbev/msy096
Kumar S. et al., 2019. Thermozymes: adaptive strategies and tools for their biotechnological applications. Bioresour. Technol., 278, 372-382, doi.org/10.1016/j.biortech.2019.01.088
Kushner D.J. & Kamekura M., 1988. Physiology of halophilic eubacteria. In: Rodriguez-Valera F., ed. Halophilic bacteria. Boca Raton, FL, USA: CRC Press,109-140.
Liang J. et al., 2015. Carbonate ion-enriched hot spring water promotes skin wound healing in nude rats. PLoS One, 10, e0117106, doi.org/10.1371/journal.pone.0117106
Margalith P.Z., 1992. Pigment microbiology. London: Shapman and Hall, 5-114.
Medjemadj M., Juan-José E.R., Boudemagh A. & González-Siso M.I., 2020. Actinobacteria isolated from Algerian hot spring waters: a potential source of important enzymes. Ecol. Environ. Conserv., 26(3), 1145-1157.
Mokrane S. et al., 2016. Thermoactinomyces khenchelensis sp. Nov., a filamentous bacterium isolated from soil sediment of a terrestrial hot spring. Antonie Van Leeuwenhoek, 109(2), 311-317, doi.org/10.1007/s10482-015-0634-9
Mukhtar S. et al., 2017. Actinomycetes: a source of industrially important enzymes. J. Proteomics Bioinf., 10, 316-319, doi.org/10.4172/jpb.1000456
Norris P.R., Davis-Belmar C.S., Brown C.F. & Calvo-Bado L.A., 2011. Autotrophic, sulfur-oxidizing actinobacteria in acidic environments. Extrémophiles, 15, 155-163.
Panosyan H., 2019. Thermoactinomycetes isolated from geothermal springs in Armenia capable of producing extracellular hydrolases. Environ. Sustainability, 2, 219-226, doi.org/10.1007/s42398-019-00066-0
Patel K.S., Naik J.H., Chaudhari S. & Amaresan N., 2017. Characterization of culturable bacteria isolated from hot springs for plant growth promoting traits and effect on tomato (Lycopersicon esculentum) seedling. C.R. Biol., 340(4), 244-249, doi.org/10.1016/j.crvi.2017.02.005
Patel H. & Hajoori M., 2023. Glucose isomerase: an enzyme of industrial significance. Int. J. Pharm. Res. Appl., 8(3), 2312-2322, doi.org/10.35629/7781-080323122322
Rao N.S., 2005. Soil microorganisms and plant growth. 4th ed. Oxford and IBH Publishing Co.
Saghatelyan A., Margaryan A., Panosyan H. & Birkeland N.K., 2021. Microbial diversity of terrestrial geothermal springs in Armenia and Nagorno-Karabakh: a review. Microorganisms, 9(7), 1473, doi.org/10.3390/microorganisms9071473
Sahay H. et al., 2017. Hot springs of Indian Himalayas: potential sources of microbial diversity and thermostable hydrolytic enzymes. 3 Biotech, 7, 118.
Saibi H., 2009. Geothermal resources in Algeria. Renew. Sustainable Energy Reviews, 13, 2544-2552, doi.org/10.1016/j.rser.2009.06.019
Saini A., Aggarwal N.K., Sharma A. & Yadav A., 2015. Actinomycetes: a source of lignocellulolytic enzymes. Enzyme Res., 2015, ID 279381, doi.org/10.1155/2015/279381
Salwan R. & Sharma V., 2018. The role of actinobacteria in the production of industrial enzymes. New and future developments in microbial biotechnology and bioengineering. Elsevier, 165-177, doi.org/10.1016/B978-0-444-63994-3.00011-4
Sapa A.R., Lestari A., Ananda M. & Nengah Suwastika I., 2023. Potential of actinomycetes from Bora hot springs Central Sulawesi to produce antibacterial compounds. In : Proceedings of the 4th International seminar on science and technology (ISST 2022). Atlantis Press, 221-227.
Satyanarayana T., Raghukumar C. & Shivaji S., 2005. Extremophilic microbes: diversity and perspectives. Curr. Sci., 89(1), 112-119.
Shirling E.B. & Gottlieb D., 1966. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol., 16, 313-340.
Siddique S., Syed Q., Adnan A. & Ashraf Qureshi F., 2014. Isolation, characterization and selection of avermectin-producing Streptomyces avermitilis strains from soil samples. Jundishapur J. Microbiol., 7(6), e10366, doi.org/10.5812/jjm.10366
Stambouli A.B., Khiat Z., Flazi S. & Kitamura Y., 2012. A review on the renewable energy development in Algeria: current perspective, energy scenario and sustainability issues. Renew. Sustainable Energy Reviews, 16, 4445–4460, doi.org/10.1016/j.rser.2012.04.031 21
Suzuki S.I., 2001. Etablishement and use of gellan gum media for selective isolation and survey of specific rare actinomycetes. Actinomycetologica, 15(2), 55-60.
Tamura K., Nei M. & Kumar S., 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. PNAS, 101(30), 11030-11035, doi.org/10.1073/pnas.0404206101
Thakur D., Yadav A., Gogoi B.K. & Bora T.C., 2007. Isolation and screening of Streptomyces in soil of protected forest areas from the states of Assam and Tripura, India, for antimicrobial metabolites. J. Mycologie Médicale, 17, 242-249, doi.org/10.1016/j.mycmed.2007.08.001
Tindall B.J., Sikorski J., Smibert R.A. & Krieg N.R., 2007. Phenotypic characterization and the principles of comparative systematics. In: Methods for general and molecular microbiology. Washington, DC: ASM Press, 330-393.
Uzel A., Esin H. & Kocabaş E., 2011. Prevalence of Thermoactinomyces thalpophilus and T. sacchari strains with biotechnological potential at hot springs and soils from West Anatolia in Turkey. Turk. J. Biol., 35, 195-202, doi.org/10.3906/ biy-0907-72
Zhang Y.X., Dong C. & Biao S., 2007. Planifilum yunnanense sp. Nov., a thermophilic thermoactinomycete isolated from a hot spring. Int. J. Syst. Evol. Microbiol., 57(8),1851-1854, doi.org/10.1099/ijs.0.64646-0
Zhaoqi S. et al., 2009. Actinobacterial diversity in hot spring in Tengchong (China), Kamchatka (Russia), and Nevada (USA). Geomicrobiol. J., 26(4), 256-263, doi.org/10.1080/01490450902892373






