- Accueil
- Volume 29 (2025)
- Numéro 1
- Changes in polyphenols content and biological activities on Tetrapleura tetraptera and Aframomum citratum fruits extracts using different extraction methods in an acid/alkaline medium
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Changes in polyphenols content and biological activities on Tetrapleura tetraptera and Aframomum citratum fruits extracts using different extraction methods in an acid/alkaline medium

Document(s) associé(s)
Version PDF originaleRésumé
Variations des teneurs en polyphénols et des activités biologiques des extraits de fruits de Tetrapleura tetraptera et Aframomum citratum obtenus suivant plusieurs méthodes d’extraction en milieux acide et alcalin
Description du sujet. Les fruits de Tetrapleura tetraptera et Aframomum citratum sont utilisés en pharmacopée traditionnelle en Afrique subsaharienne. L’extraction des principes actifs utilisent plusieurs techniques après ajout du jus de citron ou du bicarbonate de soude alimentaire.
Objectifs. Évaluer l’influence du rapport solide-liquide, du pH et des procédés d’extraction sur la teneur en polyphénols et les activités biologiques antioxydante et anti-inflammatoire de leurs extraits.
Méthode. Les fruits frais récoltés sont broyés et des extraits sont obtenus suivant trois méthodes d’extraction (infusion, 100 °C/30min ; décoction, 100 °C/30 min ; macération éthanolique, 30 min/26 °C), quatre ratios d’extraction solide/liquide (1/4, 1/6, 1/8 et 1/10 : masse de l’échantillon en g/volume du solvant en ml) et deux milieux d’extraction alcalin (pH : 7,5-10,5) et acide (4,0-6,7). Les composés phénoliques des extraits aqueux ont été identifiés et quantifiés (HPLC-DAD). La teneur en polyphénols, les activités biologiques (antioxydante et anti-inflammatoire) des extraits ont été évaluées par des méthodes spectrophotométriques. Une analyse en composantes principales fournissant une meilleure appréciation de l’information récoltée a été effectuée.
Résultats. Les extraits d’A. citratum révèlent la présence de composés tels que les acides gallique, protocatéchique, caféique, syringique et l’épicatéchine, la catéchine. Les extraits de T. tetraptera contiennent les composés majoritaires suivants : les acides protocatéchique, chlorogénique, kojique, sinapique et catéchine. Le rapport solide-liquide, le pH et la méthode d’extraction affectent significativement (p < 0,05) la teneur en polyphénols et les activités biologiques. Les meilleurs résultats sont obtenus avec T. tetraptera en utilisant la décoction en milieu alcalin à un ratio de 1/4. Pour A. citratum, la macération alcoolique en milieu alcalin au même ratio a été plus efficace.
Conclusions. Tous ces facteurs d’extraction sont importants pour optimiser l’utilisation de ces extraits de plantes et doivent être contrôlés au cours des différents process en raison de leurs effets significatifs sur les activités biologiques étudiées.
Abstract
Description. Tetrapleura tetraptera and Aframomum citratum fruits are ingredients in traditional medicines in some sub-Saharan countries. For medicinal purposes, the fruit extracts are obtained using different extraction methods and by adding lime juice or baking soda powder.
Objectives. Evaluate the influence of the solid-liquid ratio, the pH and the extraction procedures on the total polyphenols content and the biological activities (antioxidant, anti-inflammatory) of their extracts.
Method. Fresh fruits were harvested, crushed and various extracts were obtained using three extraction methods (infusion, 100 °C/30 min; decoction, 100 °C/30 min; ethanol maceration, 30 min at room temperature); four solid-liquid ratios (1/4, 1/6, 1/8, 1/10: mass of the sample in gram/volume of the solvent in ml) and two extraction mediums (alkaline and acidic). Polyphenol compounds of the aqueous extracts were identified and quantified using HPLC-DAD. The total polyphenols content, the total antioxidant capacity and the anti-inflammatory activities of both extracts were evaluated by spectrophotometric methods. Principal Component Analysis providing a transparent view of information was performed.
Results. Aframomum citratum extracts revealed the presence of major compounds like: gallic, protocatechuic, caffeic and syringic acids, epicatechin, catechin while T. tetraptera extracts revealed the presence of major compounds like protocatechuic, chlorogenic, kojik and sinapic acids, and catechin. The solid-liquid ratio, the pH and the extraction method significantly (p < 0.05) affect the total polyphenols content and the biological activities. The best results were obtained with T. tetraptera extracts using decoction in alkaline medium at a solid-liquid ratio of 1/4. Aframomum citratum best results were obtained by alcohol maceration in an alkaline medium at the same solid-liquid ratio.
Conclusions. All these extraction factors are important to optimize the use of the plant extracts. They should be controlled carefully during the various processes due to their significant effects on the biological activities.
Table des matières
Received 9 May 2023, accepted 28 January 2025, available online 30 January 2025.
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1Spices are incorporated in diets around the world because of their aromatic and medicinal properties. In Cameroon markets, spices produced by harvesting non-timber forest products (Voukeng et al., 2012) are mixed with spices of conventional agriculture (thyme, cinnamon, garlic, onion, clove, ginger, turmeric, etc.). Among these spices, Tetrapleura tetraptera (Schumach. & Thonn.) Taub. and Aframomum citratum (J.Pereira) K.Schum. fruits represent a significant nutritional and therapeutic amount. Indeed, adding to their nutritional properties, they possess many traditional uses in the treatment of some diseases (cancer, cough, diabetes, arthritis, and ulcer) in Cameroon, Nigeria, Ghana and Uganda (Voukeng et al., 2012; Amadi et al., 2016; Atchan et al., 2023). The spices contain bioactive compounds that confer to them many interesting pharmacological properties (anticonvulsive, hypotensive, antioxidant, hepatoprotective, anti-malaria) according to Alade et al. (2016) and Amadi et al. (2016). It has been reported that the major components revealed on the different parts of the plant species (barks, fruits, leaves, seeds) are phenolic acids, flavonoids and alkaloids. They are also used for food preservation due to their antibacterial and insecticidal capacities (Fankam et al., 2011; Voukeng et al., 2012; Zakaria, 2012; Alade et al., 2016; Amadi et al., 2016).
2Although polyphenols exist in several plants, their quantity and type after extraction are associated to the extraction methods used, the chemical structure, the interfering compounds, the particle size of samples, and the storage conditions (Suwal & Marciniak, 2019). Also the solubility and separation properties of polyphenols are affected by their structural differences. The structure of any compound has a significant influence on its polarity level, conjugation, and interaction with the sample medium (Karishma et al., 2015). High molecular weight phenolic compounds are frequently insoluble because of their complex chemical structure. Polyphenols recovery from different sources is a challenge because of the high level of enzyme activity in plant cells. Hence, choosing the extraction process must be done carefully to avoid the chemical modification of the targeted compounds (Alara et al., 2021).
3Crude extracts are prepared using new alternative methods like solvent extraction and assisted extraction methods (use of ultrasounds, microwaves, and pressurized/supercritical fluids). Those methods are also used for phenolic compounds extraction (Alara et al., 2021). Besides, the phenolic compounds could exist in complex forms with macromolecules (proteins, carbohydrates, insoluble high molecular weight phenolic compounds) according to Alara et al. (2021). To obtain the crude form of phenolic compounds in this case, a specific extraction method should be applied to remove the unwanted compounds. To achieve this task, “Solid-phase extraction methods, fractionation and purification based on acidity are usually the methods of choice for removing these interferences” (Zwir-Ferene & Biziuk, 2006 cited by Alara et al., 2021). To optimize the extraction process, some critical factors (solvent, nature of the material, light, duration of extraction period, pH, temperature, material size, solvent/substrate ratio, liquid-liquid or solid-liquid ratio) should also be considered, according to the previous authors.
4In a home food consumption context, the conventional extraction techniques of plant extracts are maceration, decoction, percolation and infusion. Water and ethanol are used as solvents because of their safety and non-harmful effects for human consumption. Sometimes alkali or lime is added during the extraction process to improve the taste or for other purposes like deodorizing. Extraction by maceration consists of soaking a crushed sample in the appropriate solvent, followed by constant agitation (Fadjare et al., 2021). According to the same authors, decoction involves boiling the samples for a short period of time and it is mostly suitable for heat-stable and water-soluble drugs phytochemicals (Rachmat & Wulandari, 2021). After the maceration time for both extraction methods, a separation process is applied to collect the supernatant. These are easy and low-cost extraction techniques. Indeed, skilled operation is not needed compared to other modern techniques, and those techniques are economically profitable (Fadjare et al., 2021).
5Concerning A. citratum and T. tetraptera fruits, all these extraction methods have been used by many authors to obtain the plant extracts (Fankam et al., 2011; Voukeng et al., 2012; Zakaria, 2012; Moukette et al., 2015; Adesina et al., 2016; Amadi et al., 2016). However, information is not yet available about the comparative study on the effects of these traditional extraction techniques, the solid-liquid ratio and the pH medium on the recovery of polyphenols contents. Knowledge on such studies can help to optimize the extraction efficiency, especially when extracted traditionally for therapeutic purposes.
2. Materials and methods
2.1. Plant material
6Tetrapleura tetraptera and A. citratum fresh fruits were harvested respectively from Bonepoupa II in Nkam Division, Littoral region (Lat: 4.074193; Long: 10.03148), and from Boanda in Mbam and Kim Division, Centre Region (Lat: 5.4431417; Long: 11.8891720) in Cameroon. Each specimen was identified and voucher specimens (N°31611/NHC, N°37796/NHC respectively) were deposited in the National Herbarium.
2.2. Fruits processing and various extraction conditions
7The fresh fruits were selected, sliced before finely crushed using IKA Universal Mill M 20. The obtained paste was used for aqueous and alcohol extraction. De-ionized water and ethanol (98%) were used as solvents. Different solid-liquid ratios were investigated for extraction during preliminary studies.
2.3. Extraction protocols description
8Preliminary studies were performed to investigate the influence of the solid-liquid ratio on the extract total polyphenols content and their total antioxidant capacity while the other extraction parameters were fixed. Five solid-liquid ratios (1/2, 1/4, 1/6, 1/8, 1/10 m/v) were randomly selected. By using 1/2 m/v for both plant species, the supernatant collected was not enough for the planned tests. Then the solid-liquid ratios used for the rest of the trials were 1/4, 1/6, 1/8, and 1/10 m/v.
9Three extraction procedures were used to obtain plant extracts: infusion, decoction and ethanol maceration. Concerning infusion, 1 g of plant material is introduced into a conical tube (Corning© centrifuge tube: 15 ml) and the quantity of distilled water heated to 100 °C is added. After homogenization, the extract is left to soak and shake for 30 min at room temperature. Using decoction, the same amount of plant material is introduced into a screw tube and the quantity of distilled water which depends on the solid-liquid ratio is added. The screwed tube is placed in a water bath preheated to 100 °C and boiled for 30 min. Ethanol maceration was done in a conical tube with the same amount of plant material. The quantity of ethanol (98%) which depends on the solid-liquid ratio was added and the mixture was left to macerate during 30 min after homogenization at room temperature.
10All the tubes were then centrifuged (4,500 rpm/10 min) and the collected supernatants were lyophilized, vacuum-packed, refrigerated and used for subsequent analyses.
2.4. Extraction using different pH
11To adjust the pH, acetic acid (C2H4O2) and baking soda (NaHCO3) were used. Stock solutions of acetic acid and baking soda of 1 g·100 ml-1 were prepared. Concerning the incorporation rates of acetic acid and baking soda, they varied from 0.05 to 1% of the total extraction volumes for both plant species. The solid-liquid ratio with the best total polyphenols content (1/4 m/v) was chosen for this analysis.
12The pH ranges of the solutions obtained after adding acetic acid and baking soda for both plant species extracts were measured (Table 1). The measurements were realized using a digital pH meter (Hildebrant, 2016) in triplicate and this assay was done for the three extraction procedures. For each extraction procedure (infusion, decoction, ethanol maceration), the influence of the pH was investigated.
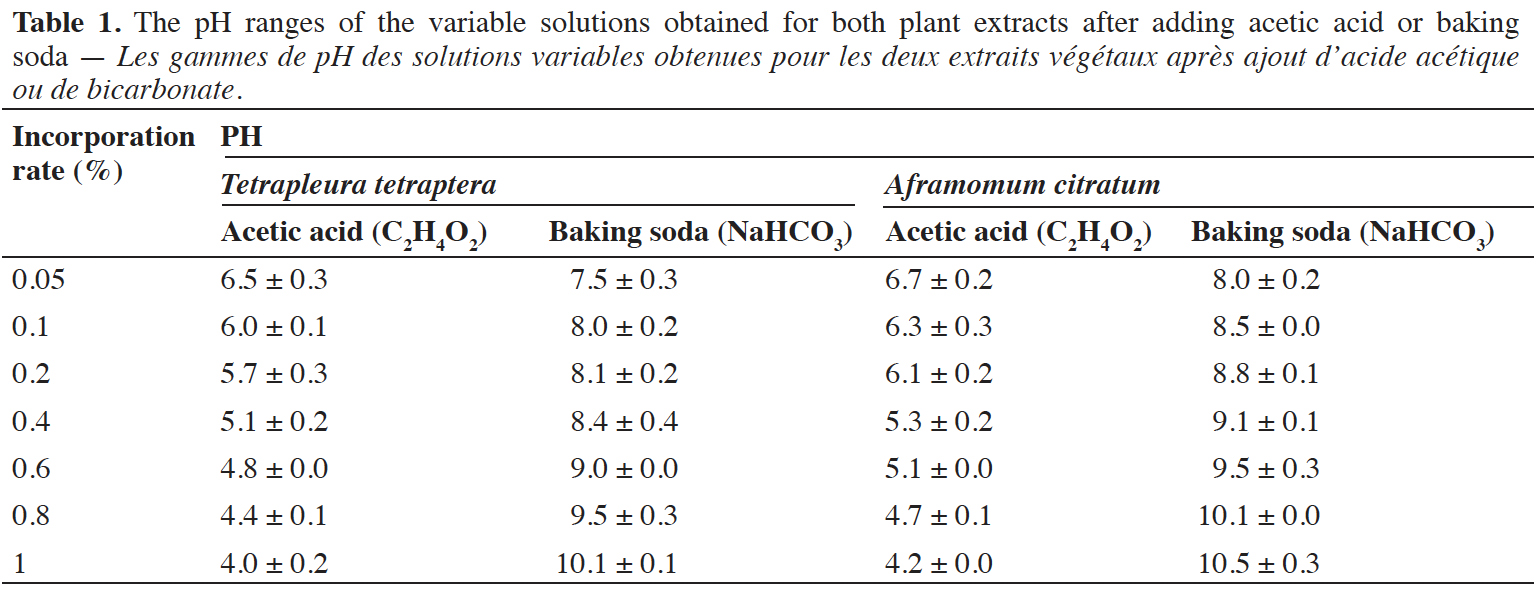
2.5. Characterization of polyphenols: HPLC-DAD analysis
13Plant extracts preparation. The solid-liquid ratio with the best total polyphenols content (1/4 m/v) was chosen for the HPLC-DAD analysis and the extracts biological activities tests. Ten grams of each spice paste were mixed with 40 ml of boiling de-ionized water (100 °C) and the mixture was let to brew for 30 min at room temperature. The mixture was then filtered and centrifuged. The filtrate was treated twice with equal volume of n-hexane (to remove non-polar compounds and reduce interference during polyphenols identification) in a separating funnel. The aqueous phase was centrifuged, collected, filtered (whatman paper N°1) freeze dried and kept into a freezer for further analysis.
14For phenolic compounds identification, 1 g of the dried aqueous extract was diluted into 4 ml of de-ionized water. The mixture was degassed by ultrasonic bath (Fisher scientific ultrasonic bath, Elma Germany) prior to use and filtered through 0.45 µm membrane filter (Millipore) directly on 2 ml HPLC vial.
15Identification, quantification of polyphenols compounds. Stock solutions of standards were prepared in the HPLC mobile phase (0.025-0.2 mg·ml-1) and were handled as previously described. The identification and the quantification of polyphenol compounds were determined according to the methods described in previous studies (Moukette et al., 2015; Eyenga et al., 2020a; Hafiz et al., 2020).
16Total polyphenols (TPP) contents determination. The extract total polyphenol content was estimated using the method described by Eyenga et al. (2020b) and the amounts were calculated from the gallic acid standard curve (0.05–1 mg·ml-1), and expressed as mg GAE·g-1 dw.
17Antioxidant assay: Total Antioxidant Capacity (TAC). The extracts total antioxidant capacity was determined by the Phosphomolybdenum method (Prieto et al., 1999 cited by Kumari et al., 2024) and Trolox was used as a positive control. The reducing activity was expressed as mg TE·g-1 dw based on the Trolox calibration curve (0.02–0.1 µg·ml-1).
18Anti-inflammatory assay: Lipoxygenase (5-LOX) inhibition (INH.5-LOX). The soybean 5-LOX inhibition (INH.5-LOX) activity by the extracts at various concentrations (0.05, 0.5, 5.0, 50 mg·ml-1) was estimated using the method described by Gunathilake et al. (2018) with few modifications at 234 nm, the negative and the positive controls were respectively Phosphate buffer and Diclofenac. The solid-liquid ratio with the best total polyphenols content (1/4 m/v) was chosen for this analysis.
2.6. Statistical analysis
19Results are reported as the mean of three replicates ± SD. Statistical significance between groups was analyzed by one-way ANOVA. The significance level was set at p < 0.05. The main multivariate statistical analysis approach was based on principal component analysis (PCA) to explain significant associations among quality parameters, total phenolic compounds contents, and biological activities data. Pearson’s correlations (p < 0.05) were used to identify correlations between all the variables included in the dataset. All the statistical analyses were carried out using JMP PRO 16.0.
3. Results
3.1. Phenolic compounds characterization of aqueous extracts of A. citratum and T. tetraptera fruits using HPLC-DAD
20At the various wavelengths and using the solid-liquid ration 1/4 (m/v), many compounds were identified and quantified on both aqueous extracts (Figures 1 and 2). However, at 280 nm, concerning A. citratum, the total amount of phenolic acids identified was higher than the amounts of the other phenolic compounds (Table 2). Gallic acid, protocatechuic acid, chlorogenic acid, benzoic acid, caffeic acid, p-coumaric acid and syringic acid were identified (Figures 1). Flavonoids were also identified and quantified (Table 2), among them catechin, epicatechin, cyanidin chloride, oleuropein and trans-chalcone.
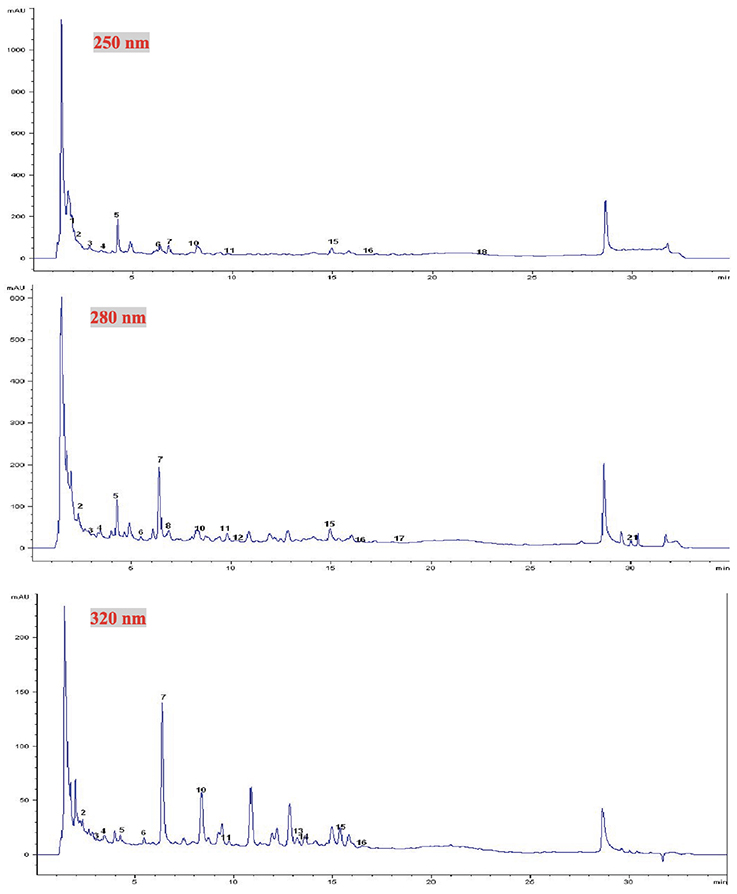
Figure 1. Chromatograms of aqueous extract of Aframomum citratum at different wavelengths (250, 280 and 320 nm) — Chromatogrammes des extraits aqueux d’Aframomum citratum à trois longueurs d’onde (250, 280 et 320 nm).
1: kojic acid — acide kojique; 2: gallic acid — acide gallique; 3: theobromine — théobromine; 4: (-) gallocatechin — (-) gallocatéchine; 5: protocatechuic acid — acide protocatéchique; 6: epigallocatechin — épigallocatéchine; 7: chlorogenic acid — acide chlorogénique; 8: (+-) catechin — (+-) catéchine;10: caffeic acid — acide cafféique; 11: epicatechin — épicatéchine; 12: epigallocatechin gallate — épigallocatéchine gallate; 13: p-coumaric acid — acide p-coumarique; 14: cyanidin chloride — cyanidine chloride; 15: syringic acid — acide syringique; 16: trans-OH-cinnamic acid — acide trans-OH-cinnamique; 17: benzoic acid — acide benzoïque; 18: oleuropein — oleuropéine; 21: trans-chalcone — trans-chalcone.
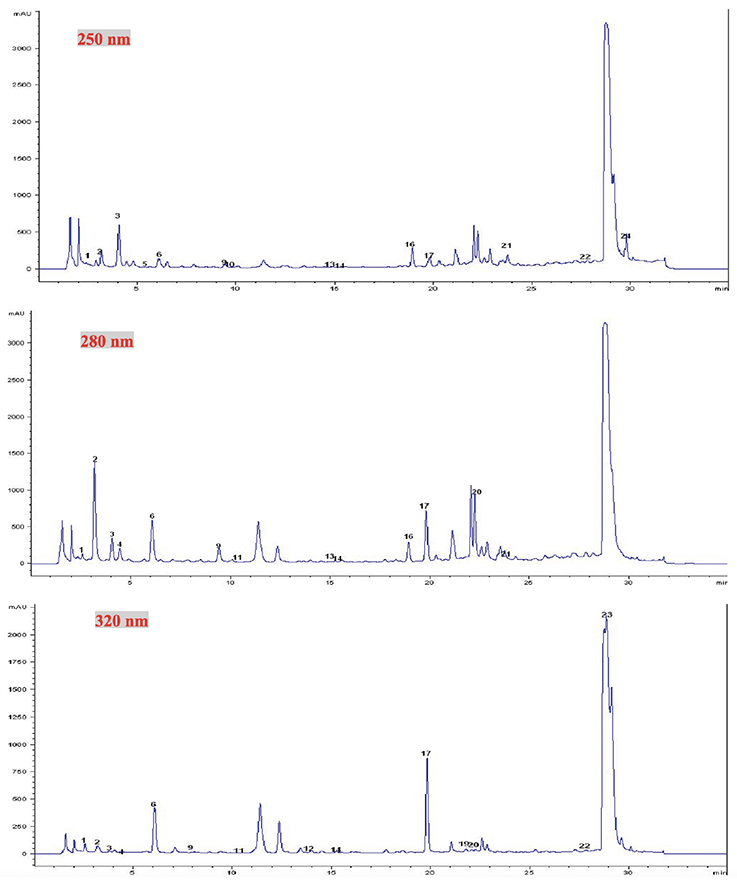
Figure 2. Chromatograms of aqueous extract of Tetrapleura tetraptera aqueous extracts at different wavelengths (250, 280 and 320 nm) — Chromatogrammes des extraits aqueux de Tetrapleura tetraptera à trois longueurs d’onde (250, 280 et 320 nm).
1: gallic acid — acide gallique; 2: theobromine — théobromine; 3: (-) gallocatechin — (-) gallocatéchine; 4: protocatechuic acid — acide protocatéchique; 5: epigallocatechin — epigallocatéchine; 6: chlorogenic acid — acide chlorogénique; 9: 3-oh-benzoic acid — acide 3-oh-benzoïque; 10: epicatechin — epicatéchine; 11: epigallocatechin gallate — epigallocatéchine gallate; 12: cyanidin chloride — cyanidine chloride; 13: syringic acid — acide syringique; 14: ferulic acid — acide ferulique; 16 : benzoic acid — acide benzoïque; 17: 2-oh cinnamic acid — acide 2-oh cinnamique; 19: 3-oh-tyrosol — 3-oh-tyrosol; 20: phlorhidzin — phlorhidzine; 21: resveratrol — resvératrol; 22: quercetin — quercétine; 24: flavone — flavone.
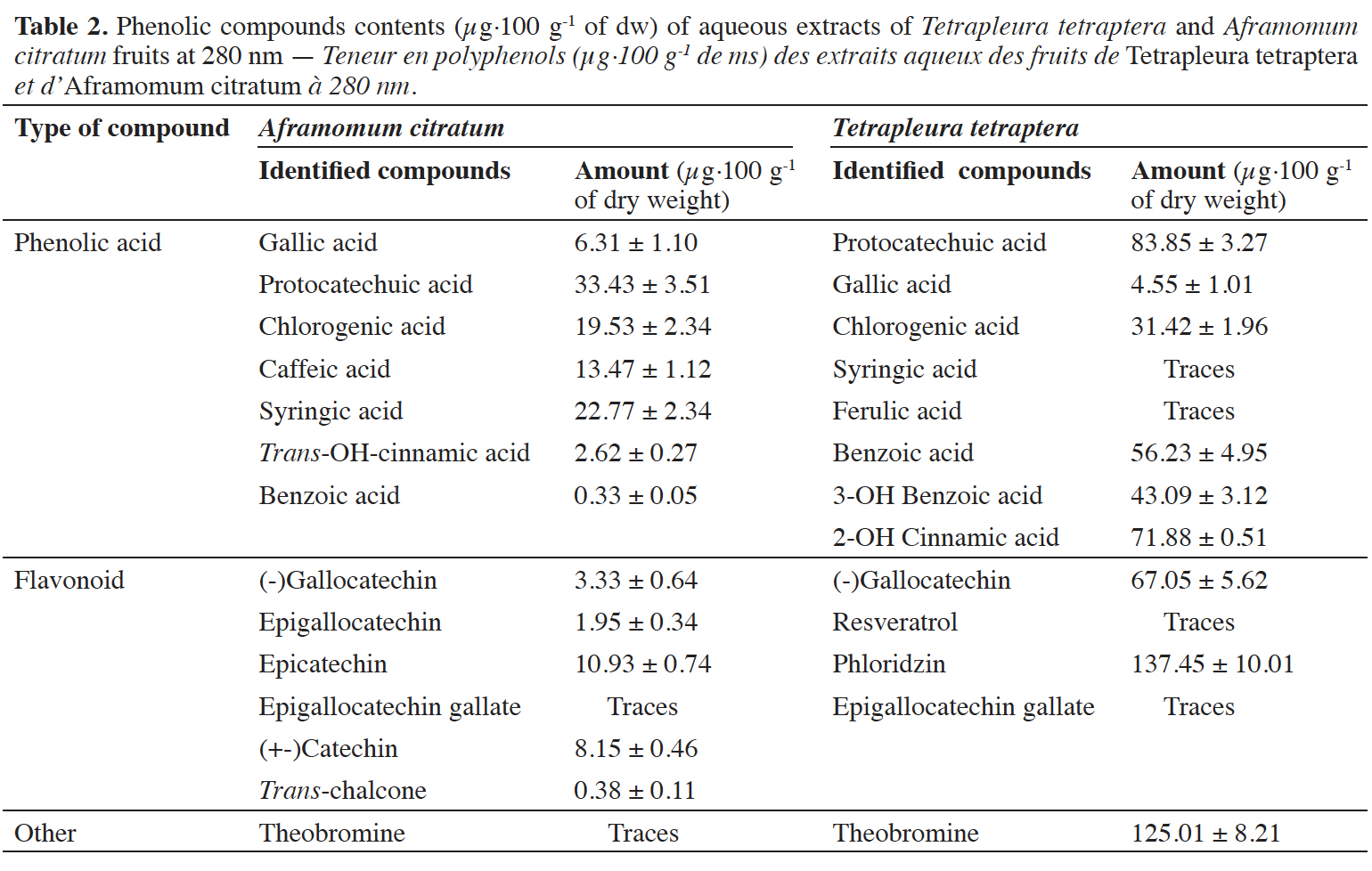
21In T. tetraptera extracts (Figure 2), phenolic acids (gallic acid, protocatechuic acid, chlorogenic acid, benzoic acid, syringic acid, ferulic acid, cinnamic acid), flavonoids (catechin, gallocatechin, epigallocatechin gallate, cyanidin chloride, myricetin, phloridzin, resveratrol, quercetin, naringenin, flavone), tyrosol, theobromine were identified and valued (Table 2).
3.2. Solid-liquid ratio influence on total phenolic (TPP) content and antioxidant activity (TAC) of plant extracts
22The solid-liquid ratio showed a significant (p < 0.05) effect on the TPP contents and the TAC of the extracts (Figure 3 for T. tetraptera extracts and Figure 4 for A. citratum extracts). Solid-liquid ratio 1/4 (m/v) exhibited the highest TAC, as well as extracted the highest TPP content in both plant extracts. In general, there was an increase of the TAC of extracts and the TPP while increasing the solid-liquid ratios in the two plant species. Trend of the TPP yields were similar with the trend obtained in TAC assays.
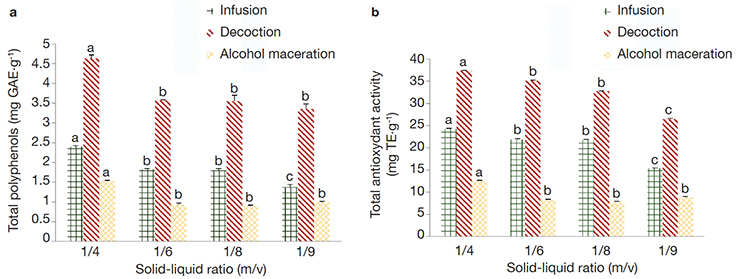
Figure 3. Total polyphenols content (a) and total antioxidant capacity (b) of Tetrapleura tetraptera extracts using different extraction ratios (1/4; 1/6; 1/8. 1/10: m/v) and extraction methods (infusion, decoction, alcohol maceration) — Teneur en polyphénols totaux (a) et capacité antioxydante totale (b) des extraits de Tetrapleura tetraptera suivant différents modes d’extraction (infusion, décoction, macération alcoolique) et ratios solide-liquide (1/4; 1/6; 1/8. 1/10 m/v).
The amount of total polyphenols and the total antioxidant capacity of the extracts followed by different letters (a, b, c) in the same colored column are significantly different at 5% level — Les moyennes suivies de lettres différentes (a, b, c) sur les cylindres de même couleur sont statistiquement différentes au seuil de 5 %; GAE: Gallic Acid Equivalent — équivalent Acide Gallique; TE: Trolox Equivalent — équivalent Trolox.
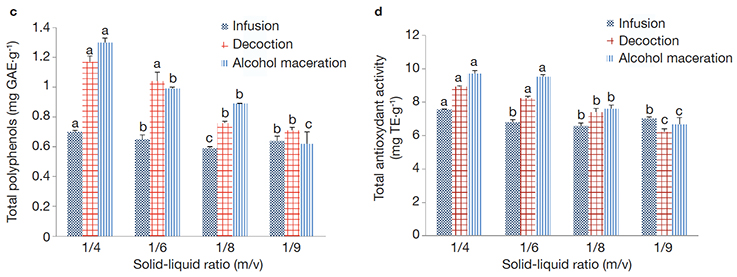
Figure 4. Total polyphenols content (c) and antioxidant activity of Aframomum citratum (d) extracts using different extraction ratios (1/4; 1/6; 1/8. 1/10: m/v) and extraction methods (infusion, decoction, alcohol maceration) — Teneur en polyphénols totaux (c) et capacité antioxydante totale (d) des extraits d’Aframomum citratum suivant différents modes d’extraction (infusion, décoction, macération alcoolique) et ratios solide-liquide (1/4; 1/6; 1/8. 1/10 m/v).
a, b, c; GAE; Te: see figure 3 — voir figure 3.
3.3. Extraction methods effects on total phenolic content (TPP) and antioxidant activities (TAC) of plant extracts
23The various results are presented in Tables 3 and 4 and in Figures 3 and 4. The decoction extraction mode significantly (p < 0.05) presents the highest extraction yields as well as the highest biological activities (antioxidant and anti-inflammatory) in T. tetraptera aqueous extracts, while alcohol maceration was the most effective regarding the parameters studied concerning A. citratum extracts.
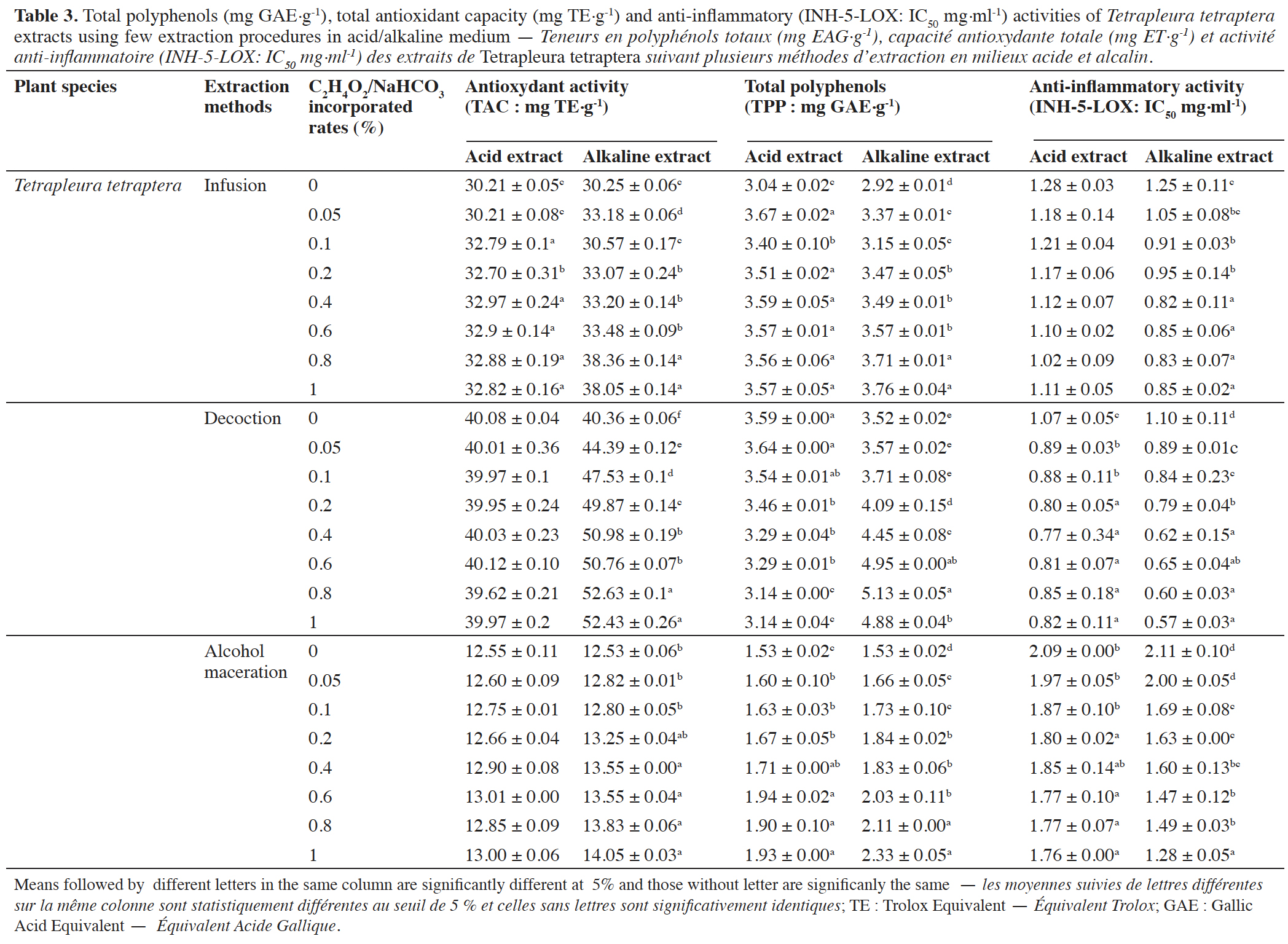
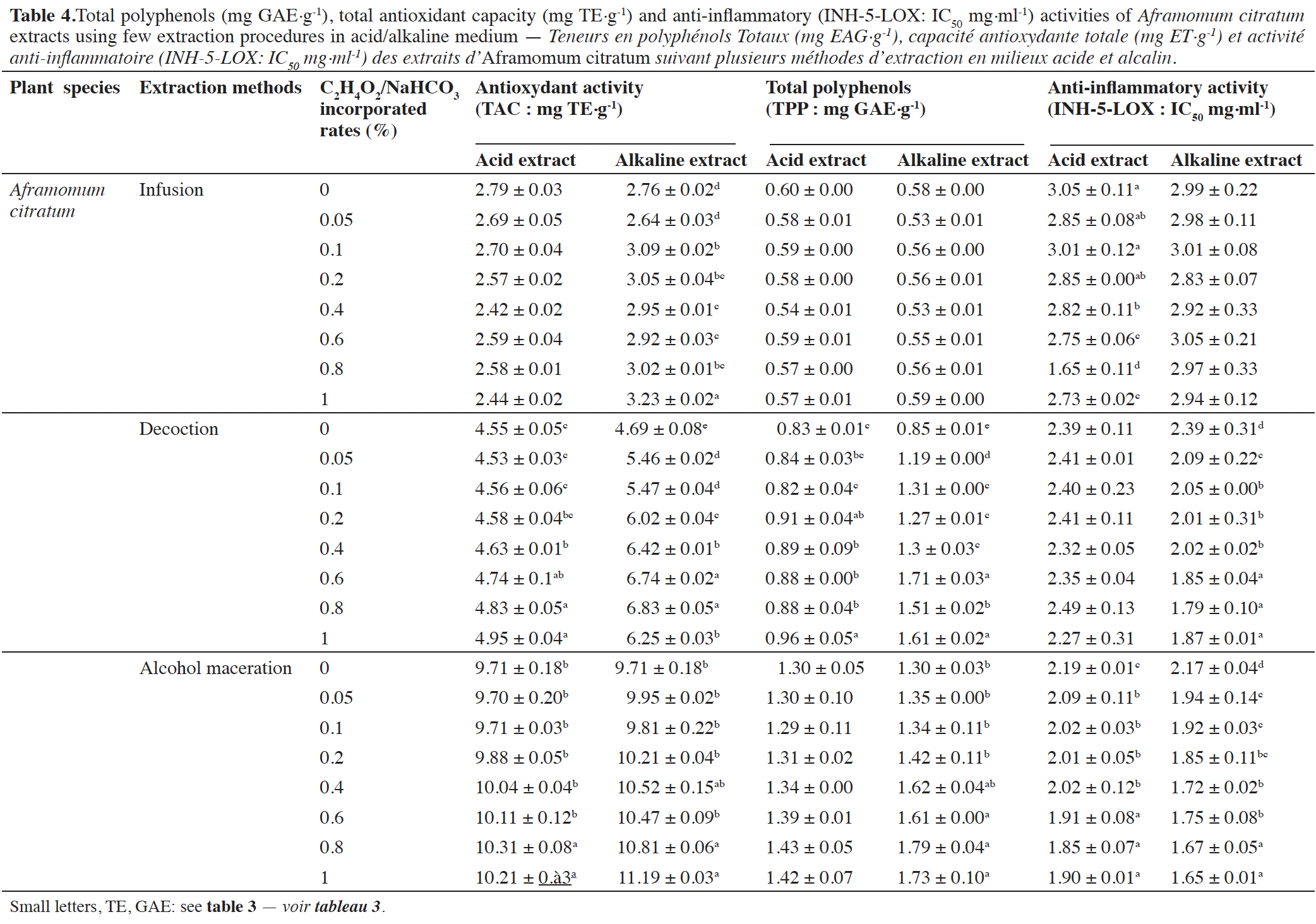
3.4. Effect of the pH medium on the total phenolic content (TPP) and biological activities (TAC) of plant extracts
24The results indicate that TPP contents and the studied in vitro biological activities (Tables 3 and 4) were significantly (p < 0.05) affected by the pH. Indeed, alkaline medium seems to be more effective for TPP recovery than acidic medium in plants extracts obtained by infusion, decoction and alcohol maceration.
3.5. Comparative analysis of phytochemical content, biological activities of plant extracts obtained by different extraction procedures in different medium, extraction methods using PCA
25Principal component analysis was performed (Figure 5) using three quantitative variables (TPP, INH.5-LOX, and TAC) and two qualitative variables (extraction procedures, pH) distributed into the two plant species (A. citratum and T. tetraptera).
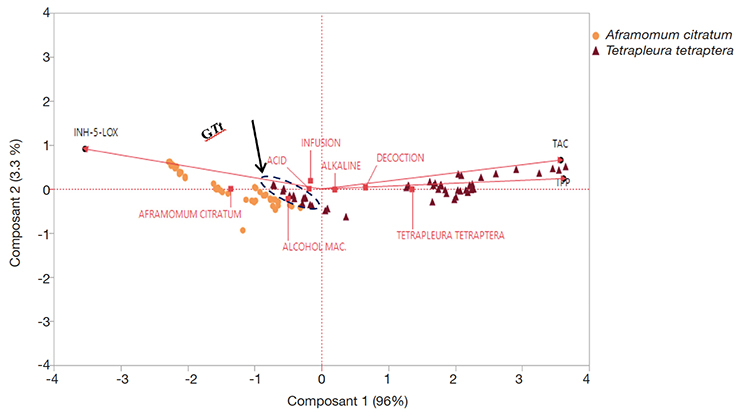
Figure 5. Principal Component Analysis (PCA) applied to polyphenol content and biological activities (quantitative variables) of Tetrapleura tetraptera and Aframomum citratum obtained using three extraction procedures in different pH mediums (qualitative variables) — Analyse en Composantes Principales appliquée à la teneur en polyphenols totaux et aux activités biologiques des extraits de Tetrapleura tetraptera et d’Aframomum citratum obtenus suivants trois modes d’extraction en milieux alcalin et acide.
TPP: total polyphenols — teneur en polyphenols totaux; TAC: total antioxidant capacity — capacité antioxydante totale; INH-5-LOX: lipoxygenase (5-LOX) inhibition — inhibition de la lipoxygénase (5-LOX).
26The applied multivariate analysis helped assess the impact of the qualitative variables on the aqueous extracts, total polyphenols contents and the in vitro biological activities. The simple biplot presents the geographical representation of the plant species according to the studied parameters. The two axes explain a variance of 99.3%. The factor which explains better the component two is INH.5-LOX test while the one which contributes better to the component one is TPP. For both plant species extracts, the total polyphenols content is strongly and positively correlated with the antioxidant activity (r = 0.97; p < 0.05) and negatively correlated to the anti-inflammatory activity (r = -0.94; p < 0.05) of the plant extract.
27Tetrapleura tetraptera extracts present the best TPP, TAC and anti-inflammatory (INH.5-LOX) activities while the highest scores are obtained by decoction using the alkaline medium. Extracts obtained by alcohol maceration and infusion (GTt: figure 5) possess less polyphenols content and are less effective concerning the biological activities studied. Those samples are not different to A. citratum samples treated on the same ways.
28Concerning A. citratum fruit extracts, all the parameters studied are less than the one measured on T. tetraptera extracts. However, the best results (highest TPP content and in vitro biological activities) in general are clearly obtained in the alkaline medium using ethanol maceration.
4. Discussion
29By using 1/4 (m/v) solid liquid ratios during extraction, polyphenol characterization showed the presence of various compounds. Amongst them, resveratrol, oleuropein, luteolin, trans-chalcone, flavone, cyanidin chloride, phloridzin, kojic acid were noticed on recent trials (Eyenga et al., 2020a; Dzah, 2022) on T. tetraptera aqueous extracts. Gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, rutin, quercetin, catechin, ellagic acid, epicatechin, rutin, luteolin, apigenin and theobromine have already been identified and quantified (Moukette et al., 2015; Irondi et al., 2016; Odubanjo et al., 2018) on T. tetraptera methanolic extracts. Sokamte et al. (2019) report the presence of ferulic acid, t-cinnamic acid, epicatechin, quercetin on A. citratum extracts. However, for both phenolic compounds, the concentrations registered during this assay are higher than the ones stated on previous reports. The differences within the results noticed can be explained by the various analytical procedures used to quantify and identify the phenolic compounds or by the plant geographical origins. The detected polyphenols are probably responsible for the biological activities (TAC, INH-5-LOX) of T. tetraptera and A. citratum extracts noticed on this trial. Indeed, both plant species extracts have already been reported to be antioxidant and anti-inflammatory by former reports (Onoja et al., 2014; Famobuwa et al., 2016; Eyenga et al., 2020a). The identified compounds on both plant species are also described as hypotensive, antitumor, antiviral, neuroprotective, antimicrobial and anti-ageing (Li et al., 2014; Pei et al., 2016; Jucà et al., 2018; Sie et al., 2018; Teplova et al., 2018; Espindola et al., 2019; Lakshmi et al., 2019).
30According to Wong et al. (2013) and Herman et al. (2021), the solid-liquid ratio factor has a critical role during extraction and is correlated to the contact area between the sample and the solvent used for extraction. In fact, that area “can reach optimum conditions when the liquid phase is saturated to solid”. In this study, the influence of the solid-liquid ratio on TPP contents and the extracts antioxidant activity is similar to the results previously mentioned (Wong et al., 2013; Predescu et al., 2016; Herman et al., 2021). Indeed, the phenolic compounds yields and the extract antioxidant activity increased gradually with the increase of the solid-to-solvent ratio.
31Concerning T. tetraptera extracts and by comparing the solvent used (water and ethanol), the results obtained are the same as those previously published by Josiah et al. (2017) when studying the comparative antioxidant capacity of aqueous and ethanol fruit extracts. In fact, the aqueous extract was more efficient than the ethanol extract for both biological activities and phytochemical analysis. The suitable explanations are the strongest extractability of phenolic content by water and the positive correlation between the phenolic content and the biological activities studied.
32Concerning A. citratum extracts and among the extraction methods and the solvent tested, ethanol maceration seems to be more efficient for extracting polyphenols. The observation made could be due by neo-formation of phenolic compounds with more phenol groups or with higher molecular weights comparing to the phenolic in water. These compounds seem to be soluble in hydro alcoholic extract (Moldovan et al., 2017; Rachmat & Wulandari, 2021). Another explanation can be the increase in the medium of non-phenolic compounds (terpene, organic acids, sugars, soluble proteins) during infusion which could interfere with the TPP recovery (Nguikwie et al., 2013).
33A comparison between infusion and decoction shows that decoction is the most efficient for both plant species. The highest quantity of phenolic compounds obtained by decoction could have few justifications according to Moldovan et al. (2017): the release of cellular constituents by hydrolysis (as well as tannins); “the degradation of complex phenolic tannins as well as the enzymatic or non-enzymatic oxidation process leads to supplementary content of phenolic compounds”; the production of new phenolic compounds during Maillard reactions.
34The pH has also an influence during the extraction of polyphenols. The results obtained in this trial are in close agreement to those obtained by Bouras et al. (2015). Similarly to our results, these authors found that alkaline medium extraction was more efficient for phenolic compounds recovery than acidic medium. Adding hydroxide sodium (NaOH) in the medium is increasing the extraction rate by the removal of tannins; the breakage of ester bonds and lignin physical barrier; the reduction of plant material viscosity; the increase of the extract reactivity. But Wiyono et al. (2020) reported that the optimum pH varies from one extraction to another. It was pH 6 for polyphenols extraction from Phoenix dactylifera leaves and pH 4 from Litchi chinensis fruit peel. On the contrary, another report mentioned that the pH did not affect the polyphenol extraction from Cyperus esculentus by-product (Ruenroengklin et al., 2008 cited by Wiyono et al., 2020). This shows that the polyphenol extraction efficiency is also closely related to the polyphenol class of the samples studied.
5. Conclusions
35Tetrapleura tetraptera and A. citratum fruits aqueous extracts are good sources of bioactive compounds (phenolic acids, flavonoids, tannins, etc.) as it has been revealed after the chromatographic analysis. The solid-liquid ratio, the pH and the extraction methods used affect the effectiveness of polyphenol extraction and the in vitro biological activities studied of both fruits’ extracts. Indeed, solid-liquid ratio 1/4 (m/v), alkaline medium and decoction method were the best features concerning T. tetraptera extracts. Concerning A. citratum fruits, the solid-liquid ratio 1/4 (m/v), the alkaline medium and the alcohol maceration extraction method recorded the highest results (total polyphenols content and biological activities). Because the total polyphenols content is positively correlated to the biological activities tested, the various factors (solid-liquid ratio, pH, extraction mode) should be carefully handled by herbalists, traditional healers and common users during the extraction process. Complementary studies on the same topic can also be investigated to compare the phenolic compounds profiles of the plant extracts after the three extraction procedures tested.
Bibliographie
Adesina S.K., Iwalewa E.O. & Johnny I.I., 2016. Tetrapleura tetraptera Taub- Ethnopharmacology, chemistry, medicinal and nutritional values - a review. J. Pharm. Res. Int., 12, 1-22, doi.org/10.9734/BJPR/2016/26554
Alade G.O. et al., 2016. Indigenous knowledge of herbal medicines among adolescents in Amassoma, Bayelsa State, Nigeria. Glob. J. Health Sci., 8, 217-237, doi.org/10.5539/gjhs.v8n1p217
Alara O.R., Nour H.A. & Chininso A.U., 2021. Extraction of phenolic compounds: a review. Curr. Res. Food Sci., 4, 200-214, doi.org/10.1016/j.crfs.2021.03.011
Amadi S.W., Zhang Y. & Wu G., 2016. Research progress in phytochemistry and biology of Aframomum species. Pharm. Biol., 54, 2761-2770, doi.org/10.2174/157340310793566172
Atchan A.P.N. et al., 2023. Anti-inflammatory, antioxidant activities, and phytochemical characterization of edible plants exerting synergistic effects in human gastric epithelial cells. Antioxidants, 12, 591, doi.org/10.3390/antiox12030591
Bouras M. et al., 2015. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crops Prod., 77, 590-601, doi.org/10.1016/j.indcrop.2015.09.018
Dzah C.S., 2022. Optimized ultrasound-assisted recovery, HPLC/LC-MS identification and biological activities of Tetrapleura tetraptera L. dry fruit polyphenols. Food Chem. Adv., 1, 100093, doi.org/10.1016/j.focha.2022.100093
Espíndola K.M. et al., 2019. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front Oncol., 9, doi.org/10.3389/fonc.2019.00541
Eyenga M. et al., 2020a. Hypoglycaemic activity of preheated (roasting) Aframomum citratum (C.Pereira) K. Schum and Tetrapleura tetraptera (Schumach & Thonn.) fruits beverage on streptozotocin-induced rats. J. Pharmacogn. Phytother., 12, 44-61, doi.org/10.5897/JPP2019.0570
Eyenga M. et al., 2020b. Optimisation of phenolic compounds and antioxidant activity extraction conditions of a roasted mix of Tetrapleura tetraptera (Schumach & Thonn.) and Aframomum citratum (C.Pereira) fruits using Response Surface Methodology (RSM). Saudi J. Biol. Sci., 27, 2054-2064, doi.org/10.1016/j.sjbs.2020.05.003
Fadjare F.T., Owusu B.N. & Badu M., 2021. Optimization of extraction conditions for polyphenols from the stem bark of Funtumia elastica (Funtum) utilizing Response Surface Methodology [version 2; peer review: 3 approved]. AAS Open Res., 4:46, doi.org/10.12688/aasopenres.13284.2
Famobuwa O. et al., 2016. Antioxidant activity of the fruit and stem bark of Tetrapleura tetraptera Taub (Mimosaceae). J. Pharm. Res. Int., 9, 1-4, doi.org/10.9734/BJPR/2016/21462
Fankam A.G. et al., 2011. Antibacterial activities of selected Cameroonian spices and their synergistic effects with antibiotics against multidrug-resistant phenotypes. BMC Complementary Altern. Med., 11, 104, doi.org/10.1186/1472-6882-11-104
Gunathilake K.D., Ranaweera K.K. & Rupasinghe H.P., 2018. In vitro anti-inflammatory properties of selected green leafy vegetables. Biomedicines, 64, 1-10, doi.org/10.3390/biomedicines6040107
Hafiz A.R.S., Barrow C.J. & Dunshea F.R., 2020. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods, 9, 1206, doi.org/10.3390/foods9091206
Herman I.A. et al., 2021. Single factor effect of natural deep eutectic solvent citric acid-glucose based microwave-assisted extraction on total polyphenols content from Mitragyna speciosa Korth. Havil leaves. Pharmacogn. J., 13, 1109-1115, doi.org/10.5530/pj.2021.13.143
Hildebrant K.L., 2016. The guide to pH measurement in food and drink. Our Daily Brine Version 1.0. Portland, Oregon, USA.
Irondi E.A et al., 2016. Phenolics extract of Tetrapleura tetraptera fruit inhibits xanthine oxidase and Fe2+ -induced lipid peroxidation in the kidney, liver, and lungs tissues of rats in vitro. Food Sci. Hum. Wellness, 5, 17-23, doi.org/10.1016/j.fshw.2015.11.001
Josiah S.J. et al., 2017. Comparative antioxidant capacity of aqueous and ethanol fruit extracts of Tetrapleura tetraptera. J. Biol. Sci., 17, 185-193, doi.org/10.3923/jbs.2017.185.193
Jucá M.M. et al., 2018. Flavonoids: biological activities and therapeutic potential. Nat. Prod. Res., 34, 692-705, doi.org/10.1080/14786419.2018.1493588
Karishma R., Himanshu D. & Usha M., 2015. Polyphenols: methods of extraction. Sci. Rev. Chem. Commun., 5, 1-6.
Kumari R. et al., 2024. An insight into quantitative, qualitative, and analytical methods for the measurement of antioxidant activity through various assays. Vegetos, doi.org/10.1007/s42535-024-00961-w
Lakshmi A., Vishnurekha C. & Baghkomeh P.N., 2019. Effect of theobromine in antimicrobial activity: an in vitro study. Dent. Res. J. (Isfahan), 16, 76-80, doi.org/10.4103/1735-3327.250975
Li A.N. et al., 2014. Resources and biological activities of natural polyphenols. Nutrients, 6, 6020-6047, doi.org/10.3390/nu6126020
Moldovan Z. et al., 2017. Studies on chemical composition and antioxidant activity of Rudbeckia triloba. J. Anal. Methods Chem., 27, doi.org/10.1155/2017/3407312
Moukette B.M. et al., 2015. In vitro ion chelating, antioxidative mechanism of extracts from fruits and barks of Tetrapleura tetraptera and their protective effects against fenton mediated toxicity of metal ions on liver homogenates. Evidence-Based Complementary Altern. Med., 2015, 423689, doi.org/10.1155/2015/423689
Nguikwie S.K. et al., 2013. The chemical composition and antibacterial activities of the essential oils from three Aframomum species from Cameroon, and their potential as sources of (E)-(R)-nerolidol. Nat. Prod. Commun., 8, 829-834, doi.org/10.1177/1934578X1300800638
Odubanjo V.O. et al., 2018. Aqueous extracts of two tropical ethnobotanicals (Tetrapleura tetraptera and Quassia undulata) improved spatial and non-spatial working memories in scopolamine-induced amnesic rats: influence of neuronal cholinergic and antioxidant systems. Biomed. Pharmacother., 99, 198-204, doi.org/10.1016/j.biopha.2018.01.043
Onoja S.O. et al., 2014. Evaluation of the in vitro and in vivo antioxidant potentials of Aframomum melegueta methanolic seed extract. J. Trop. Med., 1214, 1-6, doi.org/10.1155/2014/159343
Pei K. et al., 2016. p-Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric., 96, 2952-2962, doi.org/10.1002/jsfa.7578
Predescu N.C. et al., 2016. The influence of solid-to-solvent ratio and extraction method on total polyphenol content, flavonoid content and antioxidant properties of some ethanolic plant extracts. Rev. Chim. (Bucharest), 67, 1922-1927.
Rachmat H. & Wulandari P., 2021. Methods of extraction: maceration, percolation and decoction. Eureka Herba Indonesia, 2, doi.org/10.37275/ehi.v2i1.15
Sie C.Z. et al., 2018. Synthesis of Kojic ester derivatives as potential antibacterial agent. J. Chem., 1-7, doi.org/10.1155/2018/1245712
Sokamte T.A. et al., 2019. Phenolic compounds characterization and antioxidant activities of selected spices from Cameroon. S. Afr. J. Bot., 121, 7-15, doi.org/10.1016/j.sajb.2018.10.016
Suwal S. & Marciniak A., 2019. Technologies for the extraction and purification of polyphenols: a review. Nepal J. Biotechnol., 6, 74-91, doi.org/10.3126/njb.v6i1.22341
Teplova V.V. et al., 2018. Natural polyphenols: biological activity, pharmacological potential, means of metabolic engineering (review). Appl. Biochem. Microbiol., 54, 221-237, doi.org/10.1134/S0003683818030146
Voukeng I.K. et al., 2012. Antibacterial and antibiotic-potentiation activities of the methanol extract of some Cameroonian spices against Gram-negative multi-drug resistant phenotypes. BMC Res. Notes, 5, 299, doi.org/10.1186/1756-0500-5-299
Wiyono R., Nurhayati E.R. & Herawati U.L, 2020. The effect of time, pH and solvent composition on cocoa shell polyphenol extraction and its antioxidant activity: response surface method approach. In: The 3rd international conference on natural products and bioresource sciences, 23-24 October 2019, Tangerang, Indonesia. Bristol, UK: IOP Publishing.
Wong B.Y., Tan C.P. & Ho C.W., 2013. Effect of solid-to-solvent ratio on phenolic content and antioxidant capacities of “Dukung Anak” (Phyllanthus niruri). Int. Food Res. J., 20, 325-330.
Zakaria A.C., 2012. Études chimiques et biologiques d’Aframomum sceptrum (Zingiberaceae) et de la curcumine. Paris : Université Paris Sud - Paris XI, https://tel.archives-ouvertes.fr/tel-00812878






