- Accueil
- volume 12 (2008)
- Protoplast fusion technology for somatic hybridisation in Phaseolus
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Protoplast fusion technology for somatic hybridisation in Phaseolus

Notes de la rédaction
Received on April 25, 2007, accepted on September 7, 2007
Résumé
La technologie de fusion de protoplastes comme outil pour l’hybridation somatique chez Phaseolus. Le succès d’un croisement interspécifique entre Phaseolus vulgaris L. (PV) et les deux espèces donneuses, Phaseolus coccineus L. (PC) ou Phaseolus polyanthus Greenm. (PP), nécessite l’utilisation de ces dernières en tant que parents femelles. Bien que les barrières d’incompatibilité soient post-zygotiques, le succès de tels croisements F1 est très limité en raison d’un avortement précoce de l’embryon. Les techniques de sauvetage d’embryon globulaire et cordiforme jeune ont été améliorées, mais la régénération de plantes hybrides reste très difficile. Dans cette étude, nous décrivons l’utilisation de techniques de fusion de protoplastes au sein du genre Phaseolus comme une alternative au succès des croisements entre PP ou PC et PV. Un nombre élevé d’hétérocaryons a été produit en utilisant différents génotypes et différentes procédures de fusion, basées essentiellement sur l’électro-fusion (750 ou 1500 V.cm-1), ou sur l’utilisation d’une technique micro-chimique, le polyéthylène glycol (PEG 6000) étant l’agent de fusion. Tant la division des hétérocaryons que la formation de microcals dérivés de ces hétérocaryons ont été observées.
Abstract
The success of interspecific breeding between Phaseolus vulgaris L. (PV) and the two donor species Phaseolus coccineus L. (PC) or Phaseolus polyanthus Greenm. (PP) requires the utilization of the donor species as female parents. Although incompatibility barriers are post-zygotic, success in such F1 crosses is very limited due to early hybrid embryo abortion. Rescue techniques for globular or early heart-shaped embryos have been improved but hybrid plant regeneration remains very difficult. In this study we describe the use of protoplast fusion techniques within the genus Phaseolus, as an alternative to succeed crosses between PP or PC and PV. Large numbers of heterokaryons have been produced using different genotypes and procedures for fusion, based either on electro-fusion (750 or 1500 V.cm-1), or on the use of a chemical micro-method with polyethylene glycol (PEG 6000) as the fusing agent. Both divisions of heterokaryons and the formation of heterokaryon-derived microcalli were observed.
Table des matières
1. Introduction
1Major production constraints of the common bean Phaseolus vulgaris L. (PV) in Latin America and Africa are Ascochyta leaf blight, Bean Golden Mosaic Virus (BGMV), and Bean Fly (Obando et al., 1990; Baudoin, 1992). Sources of resistance have been identified in secondary gene pools, especially in Phaseolus coccineus L. (PC) and Phaseolus polyanthus Greenm. (PP) (Baudoin, 1992). To succeed interspecific crosses between PV and PP or PC, Camarena et al. (1987) and Baudoin et al. (1992) underlined the importance of using PP or PC as female parent to avoid a quick reversal to the recurrent parent PV. However, when using PP or PC cytoplasm in interspecific crosses with PV, incompatibility barriers are expressed at the globular or early heart-shaped embryo stages (Geerts et al., 1999; 2002). Previously, Shii et al. (1982) and Kuboyama et al. (1991) demonstrated that these barriers are post-zygotic and not due to pre-fertilization events.
2Therefore, investigations have been carried out to improve embryo rescue techniques. One of the most efficient techniques in Phaseolus was based on micropod culture: investigations were initiated by Geerts et al. (2000; 2001) and improved by Schryer et al. (2005). Although those techniques are now in an advanced stage of development, success in hybridisation remains extremely low.
3As Zambre et al. (2001) reported the regeneration of PP plants from callus, focus was made on the possibility to use protoplast fusion techniques and somatic hybridization to overcome incompatibility barriers between maternal tissue of PV and hybrid embryo (Geerts et al., 2001).
4Techniques for protoplast isolation and fusion are poorly studied within grain legumes (Ochatt et al., 2005; 2007), and no paper reports the use of protoplast fusion technology between PV and PP or PC. To initiate our research, the protocol described by Durieu et al. (2000) for intergeneric fusion of pea (Pisum sativum L.) and grass pea (Lathyrus sativus L.) protoplasts was adopted. In a previous study, Ochatt et al. (2000) compared the effects of different enzymatic mixtures dissolved in various media on the efficiency of protoplast isolation and subsequent plant regeneration. Notably, they reported large differences between cellulases (Cellulase Fluka, Cellulase Onozuka RS or cellulase Onozuka YC) for the isolation of pea protoplasts and the favourable role of picloram in the regeneration medium.
5In this study, we report the first results on protoplast fusion between PP or PC and PV using a protocol derived from that of Durieu et al. (2000).
2. Materials and methods
2.1. Plant materiel and growing conditions
6From the Phaseolineae active collection held at Gembloux Agricultural University (Belgium), we selected one PP cultivar (NI1015), two PC cultivars (NI0016 and NI0229) and two PV cultivars (NI637 and NI638), according to their ability to grow in vitro (Lecomte, 1997).
7Seeds were surface-sterilized in 12% calcium hypochlorite for ten minutes, and then immersed in 70% ethanol for 30 sec and rinsed three times in sterile de-ionised water. Seed scarification, humidification and pre-germination were carried out in sterile Petri dishes during 10 days.
8Germinated seeds were first transferred into standard Bottles (Weck) containing 100 ml vermiculite and a standard Gamborg et al. (1968) half-strength medium solidified with 2 g.l-1 phytagel (Sigma) until lateral buds developed. Plantlets were then transferred into a new standard Bottle (Weck) onto a solidified MS medium (Murashige et al., 1962) containing 20 g.l-1 sucrose and 5 g.l-1 agar (Pastagar B).
9Growing conditions were 24°C/21°C day/night temperatures with a 16 h photoperiod (Sylvania Gro Lux light, 54 µE.m-2.s-1).
2.2. Standard protoplasts isolation
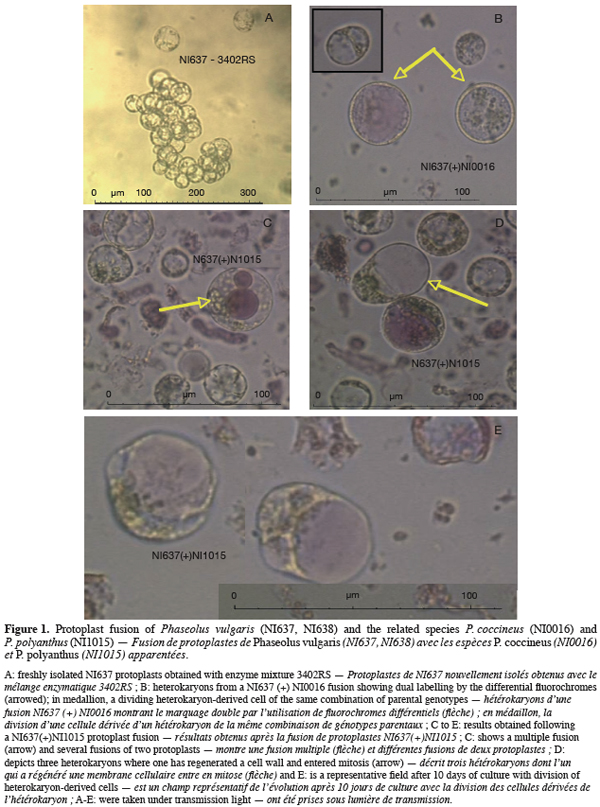
10Protoplasts of PC (NI0016 and NI0229) were isolated from green leaves. Fresh green leaves were more difficult to obtain with PV and PP genotypes when grown in vitro. Therefore, protoplasts were isolated from 10 day-old hypocotyl explants after pre-germination in Petri dishes for PV (NI637 and NI638) and PP (NI1015) accessions. Material was finely chopped and plasmolysed for 1 h in 10 cm³ CPW medium (Frearson et al., 1973) with 10 mM CaCl2, 13% mannitol and adjusted to pH 5.5 (CPW 13M). Tissues of all accessions (PV, PC, PP) were digested overnight on a continuous rotary shaker (60 T.min-1) with an enzyme mixture of 3% Macerozyme R10, 4% cellulase Onozuka RS, and 0.2% Pectolyase Y-23 (described as 3402RS by Ochatt et al., 2000). For PC accessions, we compared the use of cellulase Onozuka YC (described as 3402YC by Ochatt et al., 2000) versus cellulase Onozuka RS in the enzyme mixture. Onozuka YC was tested regarding the difference in source tissues (leaf explants versus hypocotyl).
2.3. Isolation of protoplasts for fusion
11Isolation was carried out following Durieu et al. (2000) procedure. Briefly, protoplasts were sieved (40 µ for PV and PP and 50 µ for PC) and centrifuged successively at 35 g (5 min, 10°C) and 70 g (5 min, 10°C). Each pellet was resuspended in 200 mm³ CPW13M. Pellets were mixed together and labelled with five drops (about 150 mm³) of fluorescein diacetate (green, described as FDA) for PV accessions, while rhodamine B isothiocyanate (red, described as RBi) was used for PP and PC accessions. The use of both fluorochromes is described by Durieu et al. (2000). Stock solutions of fluorochromes were made from 5 mg for FDA or 30 mg for RBi per cm³ acetone solution. Pellets were finally layered on top of 6 cm³ of CPW solution containing 21% sucrose (CPW21S) and spun at 80 g (10 min, 10°C, maximum acceleration). Under UV light, protoplasts with FDA staining gave a yellow-green fluorescence allowing density and viability evaluation (Widholm, 1972), while those with RBi gave a red fluorescence (Durieu et al., 2000). Density is determined using a Bürker cell (Marienfeld, Germany). Optimum plating density is between 5 x 104 and 1 x 106, maximising wall regeneration and concomitant daughter cell formation (Davey et al., 2005). Viability expressed as percentage is determined as the number of protoplasts that fluoresced yellow-green under UV light out of the total number of isolated protoplasts observed in the same microscopic field under normal light.
2.4. Protoplast fusion
12Regarding the low density of protoplasts obtained for NI637 as PV accession (Table 1), electro-fusion could not be easily realized. Therefore, chemical fusion was conducted to perform protoplast fusion between NI637 with all PP and PC accessions. The micro-method described by Durieu et al. (2000) where PEG 6000 is the fusing agent was adopted.
13For NI638 as PV accession, giving high protoplasts yield (Table 1), electro-fusion was tested with all PP and PC accessions. Electro-fusion was made following the method of Durieu et al. (2000) using 2 ml cuvettes of an Electro cell Manipulator ECM® 630 (BTX, California) with electrodes 1 mm apart. Three pulses at 750 V.cm-1 or 1500 V.cm-1 were delivered at 10 s intervals (capacitor of 75 µF, resistance of 50 Ω).
14The efficiency of protoplast fusion with the two tested method was evaluated under UV light, as the fluorochromes are linked to different parental protoplasts, whereby heterokaryons can be observed and counted through their double fluorescence, green and red.
2.5. Culture
15Protoplasts were cultured at 105 cm-3 on a medium based on KM (Kao et al., 1975) with 0.1 mg.l-1 2,4-D, 0.2 mg.l-1 zeatin and 1 mg.l-1 NAA (described as KP). After one week a dilution was performed with the same medium and, as soon as the majority of cells had regenerated their wall, weekly dilutions (adding weekly 1 ml media per ml initial protoplast culture) were carried out with the culture medium containing 20 g.l-1 sucrose and 10 g.l-1 glucose.
3. Results and discussion
3.1. Protoplast isolation
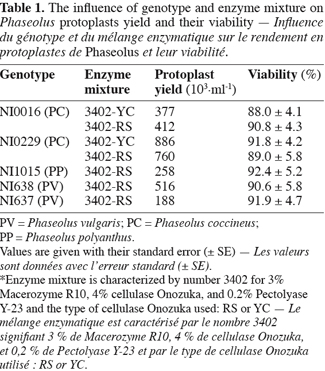
16Table 1 presents the results concerning the influence of genotypes and enzyme mixtures on protoplast yield and viability. A high dependency on genotype is observed whereas the influence of the tested enzyme mixture, containing either Cellulase Onozuka RS or YC, was not significant. To initiate protoplast isolation, we tested successfully enzyme mixture 3402 (Ochatt et al., 2000) as shown in figure 1A. Those results are largely supported by the literature (Davey et al., 2005) on protoplast culture pointing out that success in protoplast isolation is mostly genotype-dependent. Protoplast viability was also very high (Table 1) suggesting that CPW13M medium, characterised by the presence of high level of Calcium and Mannitol, is as well adapted to Phaseolus protoplasts as it is for pea protoplasts (Ochatt et al., 2000). Although our results allowed an optimal culture density of 105 protoplast per ml, some improvement can be achieved through the adjustment of various parameters, such as plasmolysis, enzyme concentration, the time of incubation and/or mannitol concentration aiming at a larger yield and coupled with an improved initial culture response (Davey et al., 2005).
3.2. Protoplast fusion
17Both chemical and electrofusion techniques led us to form heterokaryons as shown in figure 1. Chemical fusion with protoplasts of PV genotype NI638 was more efficient than electrofusion with protoplasts of PV genotype NI637 (Table 2): the number of viable heterokaryons was above 10% in some cases and all combinations provided heterokaryons. However, the latter obtained by this method were not able to divide correctly compared to electro-fused protoplasts (Table 2). Table 2 also shows that the use of a high voltage (1500 V.cm-1) was more effective to obtain heterokaryon-derived colonies. These heterokaryons could evolve to at least 10 cells microcallus within 25 days after fusion with some combinations, which underlines once a putative genotype dependence.
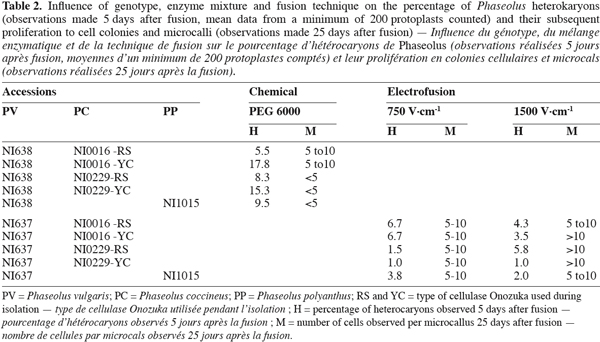
18NI637(+)NI0016-YC gives the most interesting microcalli in terms of viability and growth. Cytogenetics and flow cytometry studies (not shown) have attested that DNA content was more similar between NI637 and NI0016 than between other accessions, suggesting that such cytological studies could be used for a first screening of accessions. It is largely recognized that protoplast fusion between species having a significantly different nuclear DNA content is so far very difficult to obtain (Davey et al., 2005; Ochatt et al., 2007). Forthcoming experiments will look deeply into this aspect and will also address the sustained subsequent proliferation of the heterokaryon-derived tissues towards the ultimate regeneration of somatic hybrids between Phaseolus species.
4. Conclusions and perspectives
19These first results using protoplast fusion technology for Phaseolus enlarge breeding perspectives for the improvement of common bean through interspecific hybridisation of a high interest. They describe a method allowing to screen rapidly and at an early stage genotypes showing potential ability to be fused through protoplasts and to generate microcalli.
20The fusion technique still needs a number of adjustments to increase the viability of heterokaryons and their further evolution to microcalli. In this context, several factors such as the enzyme mixture, the fusion agents, the ratio between protoplasts belonging to each partner and the culture media could all have an influence on the results. Fusion agents could be other chemical fusogens such as PEG 1540 or 4000 or other electrical parameters such as voltage and pulse duration (Davey et al., 2005). However, given that the genotype seems to be one of the most detrimental factors for both fusion and cell proliferation, it seems interesting to concentrate further efforts upon screening first all the available accessions by the electro-fusion technique described here.
21Such screening should be completed by cytogenetic studies in order to characterize the DNA content of each accession and to identify the best source material (hypocotyls, leaves, stems, etc.), knowing that protoplast fusion technology is dependent on both DNA content (Davey et al., 2005) and protoplast origin and size (Ochatt et al., 2005; 2007).
22Finally, these results should also lean on those obtained with the embryo rescue technique using micro-pod culture (Geerts et al., 2000; 2001; Schryer et al., 2005) to improve the culure media that will give the heterokaryon-derived microcalli the perspective to evolve to hybrid somaclones. Somatic embryogenesis could be performed based on an adaptation of the protocol described by Zambre et al. (2001).
23To conclude, this work describes an interesting alternative tool to succeed crosses between PC or PP and PV in order to improve Phaseolus vulgaris L. resistance to diseases, in particular and to abiotic or biotic stresses in general. It shows that protoplast fusion technology for Phaseolus breeding is a reliable interesting approach where classical tools, such as embryo rescue, have limited power in the regeneration process of hybrids. This is particularly the case when PC or PP is used as female parent.
24Acknowledgments
25We thank the Ministry of the Walloon Region (Belgium) for financial support. The authors express sincere thanks to Ochatt S. from INRA (Dijon-France) for his advice, and scientific and technical support. We are also grateful to the Gembloux agricultural University (Belgium) for providing plant material from their Phaseolineae active collection.
Bibliographie
Baudoin J.-P., 1992. L’amélioration génétique des légumineuses alimentaires sous les tropiques. Athena, 81, 1-6.
Baudoin J.-P., Camarena M.F. & Schmit V., 1992. Contribution à une meilleure connaissance de la position phylétique de la légumineuse alimentaire Phaseolus polyanthus Greenm. Bull. Rech. Agron. Gembloux, 27, 167-198.
Camarena M.F. & Baudoin J-P., 1987. Obtention des premiers hybrides interspécifiques entre Phaseolus vulgaris et Phaseolus polyanthus avec le cytoplasme de cette dernière forme. Bull. Rech. Agron. Gembloux, 22(1), 43-55.
Davey M.R., Anthony P., Power J.B. & Lowe C.L., 2005. Plant protoplasts: status and biological perspectives. Biotechnol. Adv., 23, 131-171.
Durieu P. & Ochatt S.J., 2000. Efficient intergeneric fusion of pea (Pisum sativum L.) and grass pea (Lathyrus sativus L.) protoplasts. J. Exp. Bot., 51(348), 1237-1242.
Frearson E.M., Power J.B. & Cocking E.C., 1973. The isolation, culture and regeneration of Petunia leaf protoplasts. Dev. Biol., 33, 130-137.
Gamborg O.L., Miller R.A. & Ojima K., 1968. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell. Res., 50, 151-158.
Geerts P., Mergeai G. & Baudoin J.-P., 1999. Rescue of early heart-shaped embryos and plant regeneration of Phaseolus polyanthus Greenm. and P. vulgaris L. Biotechnol. Agron. Soc. Environ., 3(3), 141-148.
Geerts P., Sassi K., Mergeai G. & Baudoin J.-P., 2000. Development of an in vitro pod culture technique for young pods of Phaseolus vulgaris L. In vitro Cell. Dev. Biol. - Plant., 36, 481-487.
Geerts P., Toussaint A., Mergeai G. & Baudoin J.-P., 2001. Culture of very young Phaseolus vulgaris L. pods and plantlet regeneration. In: Sorvari S., Karhu S., Kanervo E. & Pihakaski S., eds. The 4th International Symposium on in vitro culture and horticultural breeding, 2-7 July 2000. Tempere, Finland. Acta Hortic., 560, 411-417.
Geerts P., Toussaint A., Mergeai G. & Baudoin J.-P., 2002. Study of the early abortion in reciprocal crosses between Phaseolus vulgaris L. and Phaseolus polyanthus Greenm. Biotechnol. Agron. Soc. Environ., 6(2), 109-119.
Kao K.N. & Michayluk M.R., 1975. Nutritional requirements for growth of Vicia hajastana cells and protoplasts at a very low population density in liquid media. Planta, 126, 105-110.
Kuboyama T., Shintaku Y. & Takeda G., 1991. Hybrid plants of Phaseolus vulgaris L. and Phaseolus lunatus L. obtained by means of embryo rescue and confirmed by restriction endonuclease analysis of rDNA. Euphytica, 54, 177-182.
Lecomte B., 1997. Etude du développement embryonnaire in vivo et in vitro dans le genre Phaseolus L. Thèse de doctorat : Faculté universitaire des Sciences agronomiques de Gembloux (Belgique).
Murashige T. & Skoog F., 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant., 15, 472-497.
Obando L., Baudoin J-P., Dickburt C. & Lepoivre P., 1990. Identification des sources de résistance à l’ascochytose du haricot au sein du genre Phaseolus. Bull. Rech. Agron. Gembloux, 25(4), 443-457.
Ochatt S.J., Mousset-Déclas C. & Rancillac M., 2000. Fertile pea plants from protoplasts when calluses have not undergone endoreduplication. Plant Sci., 156, 177-183.
Ochatt S.J. et al., 2005. One team, PCMV, and one approach, in vitro biotechnology, for one aim, the breeding of quality plants with a wide array of species. In: Dris R., ed. Crops growth, quality and biotechnology. Helsinki, Finland: WFL Publ. Sci. & Technol., 1038-1067.
Ochatt S.J., Abirached-Darmency M., Marget P. & Aubert G., 2007. The Lathyrus Paradox: « poor men’s diet » or a remarkable genetic resource for protein legume breeding? In: Ochatt S.J. & Jain S.M., eds. Breeding of neglected and under-utilised crops, spices and herbs. Plymouth, UK: Science Press, 41-60.
Schryer P.A., Lu Q., Vandenberg A. & Bett K.E., 2005. Rapid regeneration of Phaseolus angustissimus and Phaseolus vulgaris from very young zygotic embryos. Plant Cell Tissue Organ Cult., 83(1), 67-74.
Shii C.T., Rabakoarihanta A., Mok M.C. & Mok D.W.S., 1982. Embryo development in reciprocal crosses of Phaseolus vulgaris L. and Phaseolus coccineus Lam. Theor. Appl. Genet., 62, 59-64.
Widholm, 1972. The use of fluorescein diacetate and phenosaphranine for determining the viability of cultured cells. Stain Technol., 47, 189-194.
Zambre M. et al., 2001. Regeneration of fertile plants from callus in Phaseolus polyanthus Greenman (Year Bean). Ann. Bot., 88, 371-377.
Pour citer cet article
A propos de : Pascal Geerts
Centre de Recherche agronomique wallon. Département Biotechnologie. Chaussée de Charleroi, 234. B-5030 Gembloux (Belgique). E-mail: p.geerts@cra.wallonie.be
A propos de : Philippe Druart
Centre de Recherche agronomique wallon. Département Biotechnologie. Chaussée de Charleroi, 234. B-5030 Gembloux (Belgique).
A propos de : Sergio Ochatt
Centre de Recherche INRA de Dijon. Unité de Recherches en Génétique et Ecophysiologie des légumineuses à graines (URLEG). BP 86510. F-21065 Dijon Cedex (France).
A propos de : Jean-Pierre Baudoin
Gembloux Agricultural University – FUSAGx. Unité de Phytotechnie tropicale et Horticulture. Passage des Déportés, 2. B-5030 Gembloux (Belgique).






