- Accueil
- Volume 26 (2022)
- Numéro 3
- Susceptibility of fall armyworm Spodoptera frugiperda (JE Smith) to microbial and botanical bioinsecticides and control failure likelihood estimation
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Susceptibility of fall armyworm Spodoptera frugiperda (JE Smith) to microbial and botanical bioinsecticides and control failure likelihood estimation

Document(s) associé(s)
Version PDF originaleRésumé
Susceptibilité de la chenille légionnaire d'automne Spodoptera frugiperda (JE Smith) aux bioinsecticides microbiens et botaniques et estimation de la probabilité d'échec du contrôle
Description du sujet. La chenille légionnaire d'automne Spodoptera frugiperda (JE Smith) est devenue l'un des ravageurs les plus dévastateurs du maïs et d'autres cultures d’importance économique en Afrique depuis 2016. Parmi les alternatives de lutte aux insecticides chimiques, les bioinsecticides constituent une option intéressante qui doit être explorée.
Objectifs. La sensibilité de la chenille légionnaire d'automne à sept bioinsecticides disponibles sur le marché ouest-africain a été évaluée au Burkina Faso.
Méthode. L'essai a été réalisé en suivant le protocole IRAC 020.
Résultats. Le spinetoram (LC80 = 85,3 µg·l-1) et le spinosad (LC80 = 437,9 µg·l-1) ont été les plus toxiques à des concentrations inférieures à celles recommandées par le fabricant, et ont présenté des probabilités d'échec du traitement proches de 0 %. Le Bacillus thuringiensis et les produits à base d'extraits d'Azadirachta indica et de Carapa procera ont été moins efficaces (aux doses recommandées par les fabricants), même s'ils ont montré des niveaux significatifs de toxicité sur les jeunes stades.
Conclusions. Une liste de bioinsecticides efficaces devrait être communiquée pour une gestion durable de la chenille légionnaire d'automne en Afrique de l'Ouest.
Abstract
Description of the subject. The fall armyworm Spodoptera frugiperda (JE Smith) has become one of the most devastating pests of maize and other important economic crops in Africa since 2016. Among the alternatives to chemical insecticides, bioinsecticides are an interesting option that needs to be explored.
Objectives. The susceptibility of fall armyworm to seven bioinsecticides available on the West African market was evaluated in Burkina Faso.
Method. Bioassays were conducted following the approved IRAC 020 protocol.
Results. Spinetoram (LC80 = 85.3 µg·l-1) and spinosad (LC80 = 437.9 µg·l-1) were the most toxic at concentrations below those recommended by the manufacturer, and had control failure likelihoods close to 0%. Bacillus thuringiensis and products based on Azadirachta indica and Carapa procera extracts were less effective (at the manufacturers' recommended doses), even though they showed significant levels of toxicity on young instars.
Conclusions. A list of effective bioinsecticides should be communicated for sustainable management of fall armyworm in West Africa.
Table des matières
Received 10 February 2022, accepted 24 May 2022, available online 7 June 2022
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1The fall armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) is one of the most important polyphagous pests of maize and other important economic crops, in tropical and subtropical regions of the Americas. It was first reported on the African continent in 2016 (Goergen et al., 2016). Favorable climatic conditions, year-round availability of host plants, high reproductive capacity, and dispersal of adults have allowed the fall armyworm to establish itself permanently in Africa (Montezano et al., 2018; Prasanna et al., 2018).
2The fall armyworm is currently managed mainly by the application of chemical insecticides (Kansiime et al., 2019; Houngbo et al., 2020). Their widespread and sometimes indiscriminate use in the Americas has resulted in high levels of resistance in fall armyworm populations to the major classes of insecticides such as carbamates, organochlorines, organophosphates and pyrethroids (Gutiérrez-Moreno et al., 2019). Not surprisingly, treatment failures have been reported by farmers as has already been the case in Mexico and Puerto Rico (Gutiérrez-Moreno et al., 2019).
3Research and development of alternatives are high on the agenda for sustainable management of this pest in West Africa (Prasanna et al., 2018; Harrison et al., 2019). Bioinsecticides have the advantage of being less toxic to non-target organisms and human health (Bateman et al., 2018; Sisay et al., 2019). On one hand, plant extracts have demonstrated some potential insecticidal activities against the fall armyworm in field and laboratory conditions (Sisay et al., 2019; Phambala et al., 2020). On the other hand, a recent analysis of national pesticide and biopesticide lists from 19 African countries identified 29 biopesticides that could be approved for use in fall armyworm management (Bateman et al., 2018), subject to their efficacy being proven against this new pest. In this study, we evaluated the susceptibility of fall armyworm collected in Burkina Faso to seven bioinsecticides available on the West African market.
2. Materials and methods
4Insect collection and rearing. A starter colony of fall armyworm was established from a maize field located in Nasso (11°13'11''N, 4°26'11''W), Houet province in Burkina Faso. Approximately 600 fourth-instar larvae were collected in November 2020. Larvae were reared in the laboratory on maize leaves as described by Ahissou et al. (2021a). The F1 generation was used for all bioassays.
5Insecticides. We evaluated seven commercial insecticide formulations: spinetoram (Radiant 120SC, Dow AgroSciences, recommended concentration (RC): 60 mg·l-1) and spinosad (Laser 480SC, Dow AgroSciences, RC: 160 mg·l-1), Bacillus thuringiensis (Bio K 16, Savana, RC: 8.107 IU), Carapa procera oil (16 ml·l-1) and various concentrations of Azadirachta indica extracts (HN, HN+, HN++, Bioprotect, 14 ml·l-1).
6Insecticide assay. Bioassays were conducted according to the adapted IRAC 020 protocol, by leaf dipping using first, second and third instar F1 larvae (http://www.irac-online.org/). The first and second stages were used for plant extract-based bioinsecticides that were less toxic. They were performed as described by Ahissou et al. (2021a) and mortality was assessed after 72 h for all bioinsecticides except for spinetoram and spinosad (48 h). Each insecticide was tested using at least five concentrations, after dilution with distilled water containing Triton X-100 (0.2 g·l-1). Non-treated maize leaves were collected, washed with tap water and dried. Then, they were immersed for 10 seconds in the insecticide solution and left to dry for 1 h. Control leaves were treated only with a solution of Triton in water. Leaves were placed in individual Petri dishes (9 cm in diameter) containing blotting paper. A total of 40 larvae were individually exposed to each concentration of each tested product.
7Statistical analysis. Percentage mortality data were corrected for control mortality (Abbott, 1925) and subjected to probit analysis (Finney, 1971) using SPSS software, to calculate slope values, lethal concentrations (LC50; LC80), and fiducial limits (95%). Control failure likelihood (CFL) was calculated by multiplying the achieved mortality percentage by 100, dividing the product by the minimum required efficacy (e.g. 70%) and subtracting the result from 100 (Guedes, 2017). Additionally, an ANOVA was performed to compare mortality rates between different concentrations of a bioinsecticide (Tukey's test, p < 0.05).
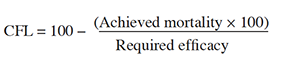
3. Results
8The observed control mortality rate was found to be less than 3% and was used to correct mortality. For the seven bioinsecticides tested, the theoretical values were not significantly different from the observed values, so the Probit model was considered appropriate (Tables 1 and 2). The LC50 and LC80 values and their confidence intervals and CFL are presented in table 1.
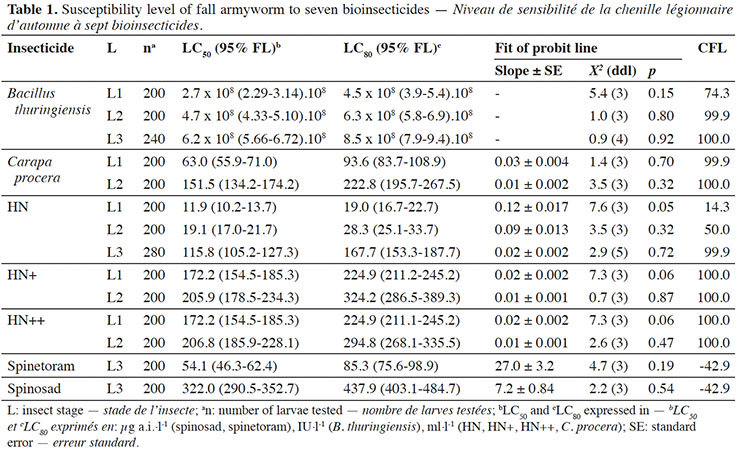
9Spinetoram and spinosad were the most toxic of the insecticides tested with LC80 values of 85.3 µg·l-1 and 437.9 µg·l-1 respectively. These values are 99% lower than recommended by the manufacturer.
10Bacillus thuringiensis LC50 and LC80 values increased significantly with the developmental stage of the fall armyworm, as the confidence intervals did not overlap. Lethal concentration values were 5.6 to 10.6 times higher than the manufacturer's recommended dose, so the CFL is very high (74.3-100%).
11Plant extract-based insecticides tested were less toxic to the fall armyworm larvae. Lethal concentrations values were 6 to 23 times higher than the manufacturers' recommended concentrations, meaning that the CFL is high.
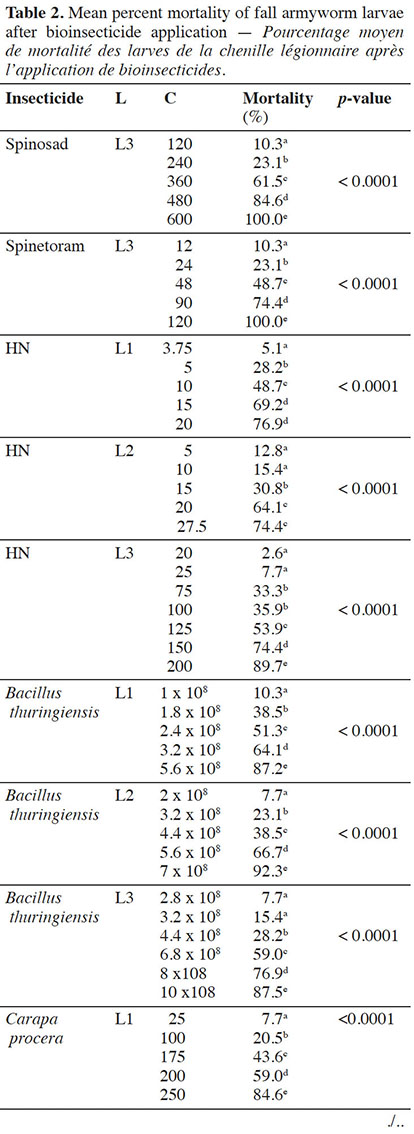
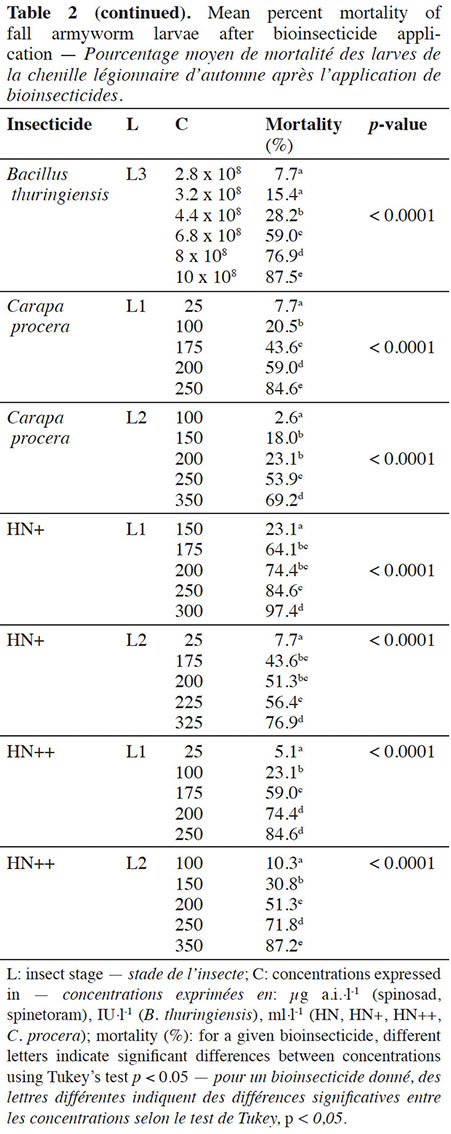
12For each bioinsecticide, the observed mortality rates were always affected by the tested concentrations (p < 0.0001) (Table 2).
4. Discussion
13Our study was conducted to identify low toxicity molecules effective against the fall armyworm in West Africa. Spinetoram (LC80 = 85.3 µg·l-1) and spinosad (LC80 = 437.9 µg·l-1) insecticides have the best efficacy profiles against fall armyworm at concentrations significantly lower than manufacturers' recommendations. The high slope values (7.26 to 27.03) mean that a small increase in insecticide concentration is sufficient to significantly increase larval mortality, suggesting that the fall armyworm population is very sensitive to these molecules. At the recommended dose very limited treatment failure should be observed. Similar results were obtained in Brazil, China, Mexico and Puerto Rico (Gutiérrez-Moreno et al., 2019; Lira et al., 2020). With CFL close to zero, both spinosyns are more effective than chemical insecticides such as abamectin (CFL = 66%), deltamethrin (CFL = 80%), and lambda-cyhalothrin (CFL = 96%) (Ahissou et al., 2021a) which are widely used against this pest in West Africa (Kansiime et al., 2019; Ahissou et al., 2021b).
14For the plant extract-based insecticides tested, the required LC80 values were much higher than the manufacturers' recommended concentrations with better results on smaller larvae. However, it is interesting to note that the leaves treated with the botanical insecticides were not consumed by the larvae. Azadirachtin and C. procera are powerful food deterrents and insect growth regulators (Seigler, 1998; Isman, 2006). Their use should be recommended — as it is the case of azadirachtin in China (Zhao et al., 2020) — in fall armyworm IPM programs in combination with other compatible methods.
15With high LC80 values ranging from 4.48 ×108 to 8.50 ×108 IU·l-1 and low slope values (< 0.00001), fall armyworm showed resistance to B. thuringiensis var. kurstaki with a CFL between 74 - 100%. This is consistent with the hypothesis that some Bt-resistant lepidopterans are highly susceptible to spinosad (Xiao et al., 2016), as is the case in this study.
5. Conclusions
16In conclusion, we recommend extending to farmers these results, which show that some bioinsecticides are very effective and could play an important role in IPM programs against fall armyworm. Apart from superior insecticidal activity relative to some chemical insecticides, such bioinsecticides have the advantage of being less toxic to non-target organisms.
Acknowledgements
17This research was funded by the Academy of Research and Higher Education-Commission Development Cooperation (ARES-CDD) as part of the PRD AGRO-ECO project. The authors would like to thank the project coordinators, Prof. Marie-Paule KESTEMONT (UCLouvain, Belgium) and Prof. Enoch G. ACHIGAN-DAKO (University of Abomey-Calavi, Benin).
Bibliographie
Abbott S.W., 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol., 18, 265-267, doi.org/10.1093/jee/18.2.265a
Ahissou B.R. et al., 2021a. Baseline toxicity data of different insecticides against the fall armyworm Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) and control failure likelihood estimation in Burkina Faso. Afr. Entomol., 29(2), 435-444, doi.org/10.4001/003.029.0435
Ahissou B.R. et al., 2021b. Integrated pest management options for the fall armyworm Spodoptera frugiperda in West Africa: challenges and opportunities. A review. Biotechnol. Agron. Soc. Environ., 25(3), 192-207, doi.org/10.25518/1780-4507.19125
Bateman M.L. et al., 2018. Assessment of potential biopesticide options for managing fall armyworm (Spodoptera frugiperda) in Africa. J. Appl. Entomol., 142, 805-819, doi.org/10.1111/jen.12565
Finney D.J., 1971. Probit analysis. 3rd ed. Cambridge, UK: Cambridge University Press.
Goergen G. et al., 2016. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS One, 11(10), e0165632, doi.org/10.1371/journal.pone.0165632
Guedes R.N.C., 2017. Insecticide resistance, control failure likelihood and the First Law of Geography. Pest Manage. Sci., 73, 479-484, doi.org/10.1002/ps.4452
Gutiérrez-Moreno R. et al., 2019. Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol., 112(2), 792-802, doi.org/10.1093/jee/toy372
Harrison R.D. et al., 2019. Agro-ecological options for fall armyworm (Spodoptera frugiperda JE Smith) management: providing low-cost, smallholder friendly solutions to an invasive pest. J. Environ. Manage., 243, 318-330, doi.org/10.1016/j.jenvman.2019.05.011
Houngbo S. et al., 2020. Farmers’ knowledge and management practices of fall armyworm, Spodoptera frugiperda (J.E. Smith) in Benin, West Africa. Agriculture, 10(430), 1-15, doi.org/10.3390/agriculture10100430
Isman M.B., 2006. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol., 51, 45-66, doi.org/10.1146/annurev.ento.51.110104.151146
Kansiime M.K. et al., 2019. Farmer perception of fall armyworm (Spodoptera frugiderda J.E. Smith) and farm-level management practices in Zambia. Pest Manage. Sci., 75, 2840-2850, doi.org/10.1002/ps.5504
Lira E.C. et al., 2020. Resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to spinetoram: inheritance and cross-resistance to spinosad. Pest Manage. Sci., 76(8), 2674-2680, doi.org/10.1002/ps.5812
Montezano D.G. et al., 2018. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol., 26(2), 286-300, doi.org/10.4001/003.026.0286
Phambala K. et al., 2020. Bioactivity of common pesticidal plants on fall armyworm larvae (Spodoptera frugiperda). Plants, 9(112), 1-10, doi.org/10.3390/plants9010112
Prasanna B.M., Huesing J.E., Eddy R. & Peschke V.M., eds, 2018. Fall armyworm in Africa: a guide for integrated pest management. 1st ed. Mexico: CIMMYT.
Seigler D.S., 1998. Limonoids, quassinoids, and related compounds. In: Plant secondary metabolism. Boston, MA, USA: Springer, 473-485.
Sisay B. et al., 2019. The efficacy of selected synthetic insecticides and botanicals against fall armyworm, Spodoptera frugiperda, in maize. Insects, 10(45), 1-14, doi.org/10.3390/insects10020045
Xiao Y. et al., 2016. Resistance to Bacillus thuringiensis mediated by an ABC transporter mutation increases susceptibility to toxins from other bacteria in an invasive insect. PLoS Pathog., 12(2), e1005450, doi.org/10.1371/journal.ppat.1005450
Zhao Y.-X. et al., 2020. Susceptibility of fall armyworm, Spodoptera frugiperda (J.E. Smith), to eight insecticides in China, with special reference to lambda-cyhalothrin. Pestic. Biochem. Physiol., 168, 104623, doi.org/10.1016/j.pestbp.2020.104623






