- Startpagina tijdschrift
- Volume 26 (2022)
- Numéro 1
- What are the potential sources of on-farm contamination of raw milk butter with Escherichia coli?
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
What are the potential sources of on-farm contamination of raw milk butter with Escherichia coli?

Documenten bij dit artikel
Version PDF originaleRésumé
Quelles sont les principales sources de contamination du beurre au lait cru par Escherichia coli ?
Description du sujet. Selon le Règlement (CE) N° 2073/2005, Escherichia coli doit être dénombré dans le beurre au lait cru avant sa mise sur le marché. L’hygiène des lots est acceptable lorsqu’au maximum deux échantillons sur cinq présentent des niveaux en E. coli compris entre 10 et 100 ufc·g-1. La présence d’E. coli en trop grande concentration dans le beurre au lait cru continue de poser un problème.
Objectifs. Cette étude avait pour objectif d’identifier les sources d’E. coli dans six fermes confrontées à des contaminations récurrentes de leur beurre.
Méthode. Les fermes ont été visitées à trois reprises entre mars et mai 2021. Des échantillons de surface et des échantillons alimentaires ont été collectés tout au long du procédé en vue de subir un dénombrement d’E. coli et de rechercher des corrélations potentielles entre la contamination des équipements et la qualité hygiénique des produits laitiers.
Résultats. Deux sources de contamination majeures ont été identifiées : le manque d’efficacité des procédures de nettoyage et de désinfection des tuyaux transportant le lait, des jointures et des écrémeuses, et l’absence de refroidissement du lait en cas d’intervalle de temps supérieur à 2 h entre la traite et l’écrémage. L’utilisation de ferments lactiques constitue un moyen de contrôle prometteur pour limiter la croissance d’E. coli durant la maturation de la crème.
Conclusions. L’étude a mis en lumière la problématique de la contamination du beurre au lait cru par E. coli, qui est parfois observée à une concentration très élevée. Un nouvel arbre de décision proposé dans ce travail pourrait aider les producteurs et les contrôleurs des agences de sécurité alimentaire à améliorer la qualité hygiénique du beurre au lait cru.
Abstract
Description of the subject. According to Regulation (EC) No 2073/2005, Escherichia coli must be enumerated in raw milk butter before sales. Hygiene of batches is acceptable when a maximum of two samples out of five have levels of E. coli between 10 and 100 cfu·g-1. E. coli is still a threat to the safety of raw milk butter.
Objectives. This study aimed to identify sources of E. coli in six farms facing recurrent contaminations in butter.
Method. Farms were visited three times between March and May 2021. Surface samples and dairy products samples were collected throughout the process for E. coli enumeration and assessment of potential correlations between equipment contamination and hygienic quality of food products.
Results. Two major sources of contamination were identified: the lack of efficacy of cleaning and disinfection practices on milk pipelines, junctions and cream separators, and absence of milk cooling in case of time-lapse of more than 2 h between milking and skimming. The use of lactic acid starters could be a helpful way to control E. coli during cream maturation, in association to adequate good manufacturing practices.
Conclusions. Levels of E. coli in the considered raw milk butter batches were really high. A new decision tree is proposed that could help manufacturers and food controllers to improve hygienic quality of raw milk butter.
Inhoudstafel
Received 16 August 2021, accepted 24 January 2022, available online 8 March 2022
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1Butter was one of the first dairy products and was already manufactured 2,000 years before Christ. In Wallonia (Belgium), butter is still produced from raw milk at an artisanal scale. During a survey performed in 2016, 211 producers of raw milk butter were identified (El-Hajjaji et al., 2019). Manufacture of artisanal butter differs from continuous production in larger factories (Deosarjar et al., 2016). Federal Agency for the Safety of the Food Chain (2012) detailed the production process of artisanal butter. After milking, raw milk is generally directly skimmed, except when milking robot is used. In the latter case, milk is temporarily stored in a buffer tank before further treatment. After separation from skimmed milk, containers are filled with cream for maturation, with or without addition of starters. Both physical and biological maturations occur, modifying cream texture and physicochemical characteristics (Ceylan & Ozcan 2020; Panchal et al., 2021). Lactic acid starters can be used for butter manufacture to promote the development of aromatic compounds, including diacetyl (Bakirci et al., 2002), but also to accelerate acidification rate, allowing to reach faster physicochemical conditions unfavorable for the growth of most pathogens. Maturation procedure differs between farms, in terms of time and temperature cycles. In Wallonia, El-Hajjaji et al. (2019) identified six maturations schemes. Time of maturation was comprised between two and more than seven days. All these factors resulted in somewhat variation and typicity of artisanal butter. In addition to that, influence of seasons on butter characteristics was also reported (Cullinane et al., 1984). Churning is the key step for butter manufacture, consisting in producing a water-in-oil emulsion from cream, which is an oil-in-water emulsion. Fat globules aggregate and butter grains are progressively formed. Butter is then washed to remove buttermilk, before shaping and packaging.
2The use of raw milk for manufacture of dairy products is still a debate nowadays. Several foodborne pathogens can be carried by raw milk, including Listeria monocytogenes, Salmonella spp., Staphylococcus aureus, Campylobacter spp. and toxin-producing Escherichia coli (N’Guessan et al., 2015; Verraes et al., 2015; Costanzo et al., 2020). These bacteria can originate from endogenous (i.e. direct transfer from bloodstream, e.g. mastitis) or exogenous sources (i.e. transfer from the environment, e.g. feces, milking material or teats; Verraes et al., 2015). Consequently, handling of raw milk requires precaution in order to guarantee the manufacture of dairy products not representing a threat for food safety. Escherichia coli is a Gram negative facultative anaerobic bacterium which is a commensal host of human and animal gut microbiota. It is thus observed in feces (Tenaillon et al., 2010). Escherichia coli is considered as hygiene indicator, revealing possible fecal contamination of food. Regulation (EC) No 2073/2005 established process hygiene criterion for the manufacture of raw milk butter (European Commission, 2005). Briefly, five samples (n) must be considered. Ideally, levels of E. coli should be < 10 cfu·g-1 in all samples. Nevertheless, a batch remains acceptable if a maximum of two butter samples have contamination between 10 cfu·g-1 (m) and 100 cfu·g-1 (M). In other circumstances, batch hygienic quality is not acceptable. During a survey performed for the period 2006-2009, 53% of 333 batches of raw milk butter were not acceptable regarding food process hygiene criterion (unpublished results). Nowadays, producers have to implement HACCP plans and to follow good hygiene practices and good manufacturing practices (GMP), but contamination of end-products is still an issue. The present study explored potential sources of contamination with E. coli during raw milk butter manufacture, in farms facing recurrent non-compliances regarding food process hygiene criterion.
2. Materials and methods
2.1. Sampling and microbiological analyses
3Six farms (A-F) facing recurrent contamination of butter with E. coli were visited each during three consecutive weeks between March and May 2021. For each farm three butter batches were considered, i.e. three whole manufacturing cycles from milking to final product. Characteristics of manufacturing process for each farm are summarized in table 1. Two farms were using classical milking rooms, while the four other farms made use of milking robots. When starting this experiment, two farms did not use lactic acid starters for cream maturation, namely farms C and D. Nevertheless, due to high levels of E. coli in the first studied batch (D1), producer from farm D decided to add lactic acid starters for cream maturation during the manufacture of subsequent batches (D2-D3). Two commercial lactic acid starters were observed during this work, namely MA 4001/4002 (Danisco, Copenhagen, Denmark) and Flora Danica (Chr. Hansen, Horsholm, Denmark). Churns varied between farms in terms of rotation axis (horizontal or vertical) and surface material (wood or stainless steel). Whole butter manufacture was considered in triplicate for each farm, i.e. from milking to final butter.

4Dairy products and surface samples considered in this study are summarized in table 2, and figure 1 illustrates surface samples collected from milking and skimming equipment. All surfaces were sampled in triplicate during each visit, just before the production of a new butter batch. When possible, surfaces were sampled by contact plating, i.e. Petrifilm Rapid E. coli/Coliform Count Plate (3M, Two Harbors, MN, USA). When surfaces were hardly accessible, e.g. junctions and skimmers, swab samplers with 10 ml of buffered peptone water (BPW) were used (3M, Two Harbors, MN, USA). In this case and for each sample, a surface of approximately 30 cm² was swabbed, i.e. a surface similar to a single contact plate. Tubes were transported to the laboratory and analyzed the day of sampling. Briefly, after vortexing, 1 ml of suspension was spread on Petrifilm Rapid E. coli/Coliform Count Plate.
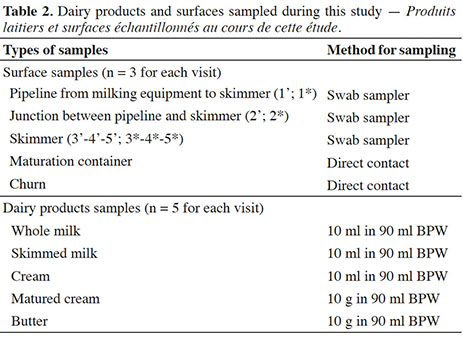

Figure 1. Sampling of skimming equipment — échantillonnage du matériel d’écrémage.
5Dairy products sampled during manufacture included raw milk, skimmed milk, cream, matured cream and butter. Five samples of each product were considered during each visit in farms. Ten g or 10 ml of product were diluted 10-fold in BPW prepared according to method ISO 6687-1:2017 (International Organization for Standardization, 2017). One ml of stock suspension was spread on Petrifilm E. coli/Coliform Count Plate. All films were incubated for 18 to 24 h at 30 ± 1 °C, following instructions provided by the manufacturer. Enumeration was performed according to 3M guide for interpretation (3M, 2018). Blue colonies were considered as E. coli. Other coliforms can also be detected on Petrifilm, under the form of red colonies. Validation tests previously demonstrated that this method did not produce results significantly different from those obtained using method ISO 16649-2:2017 (Bird et al., 2020).
2.2. Statistical analyses
6All statistical treatments were performed using Minitab 19 (State College, PA, USA). In case of number of colonies > 15,000 cfu·g-1 or cfu·ml-1, colonies were uncountable but Petrifilm remained interpretable. In this case, a value of 15,000 cfu·g-1 or cfu·ml-1 was considered in datasheets. Logarithmic transformation was applied to all data prior to analyses. Correlation between variables was tested using Pearson coefficient. Variables associated to surface samples were made qualitative for more readability, by creating four classes, namely (a) 0, absence of E. coli; (b) ≤ 10 cfu·cm-²; (c) ≤ 100 cfu·cm-² but > 10 cfu·cm-²; (d) > 100 cfu·cm-². Their effect on butter hygienic quality was assessed using one-way ANOVA or Kruskal-Wallis tests. Pairwise comparisons were performed using Tukey test.
3. Results
3.1. Butter microbial quality
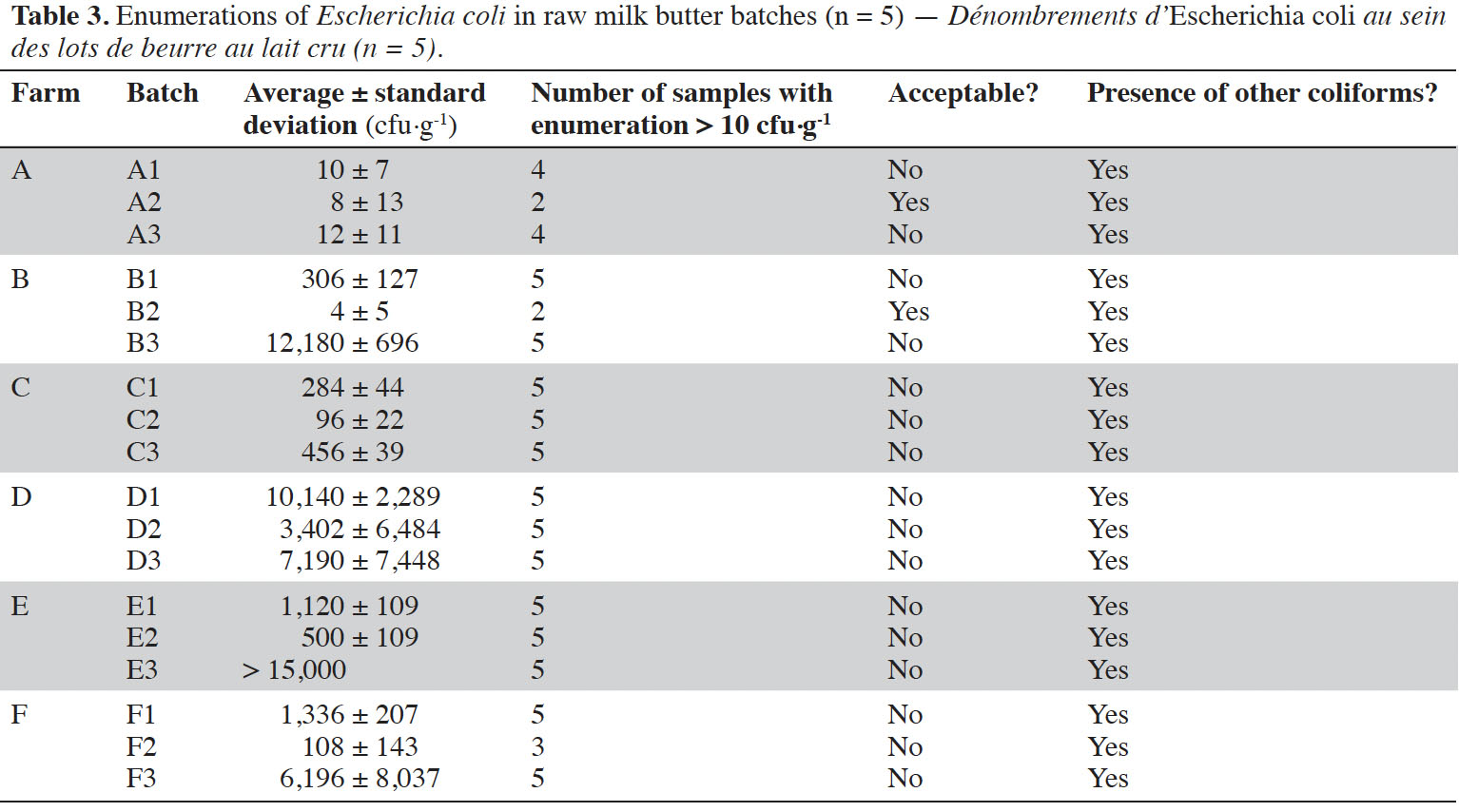
7Escherichia coli levels were enumerated in 18 batches of raw milk butter, considering five samples per batch (Table 3). Only one batch (B2) had levels ≤ 10 cfu·g-1 in five samples. Another batch (A2) was acceptable, i.e. a maximum of two samples out of five had levels of E. coli between m and M. It means that 16 batches were unacceptable, with E. coli contamination sometimes above the limit of enumeration of Petrifilm, i.e. > 15,000 cfu·g-1. Other coliforms were observed on all Petrifilms.
3.2. Surface samples
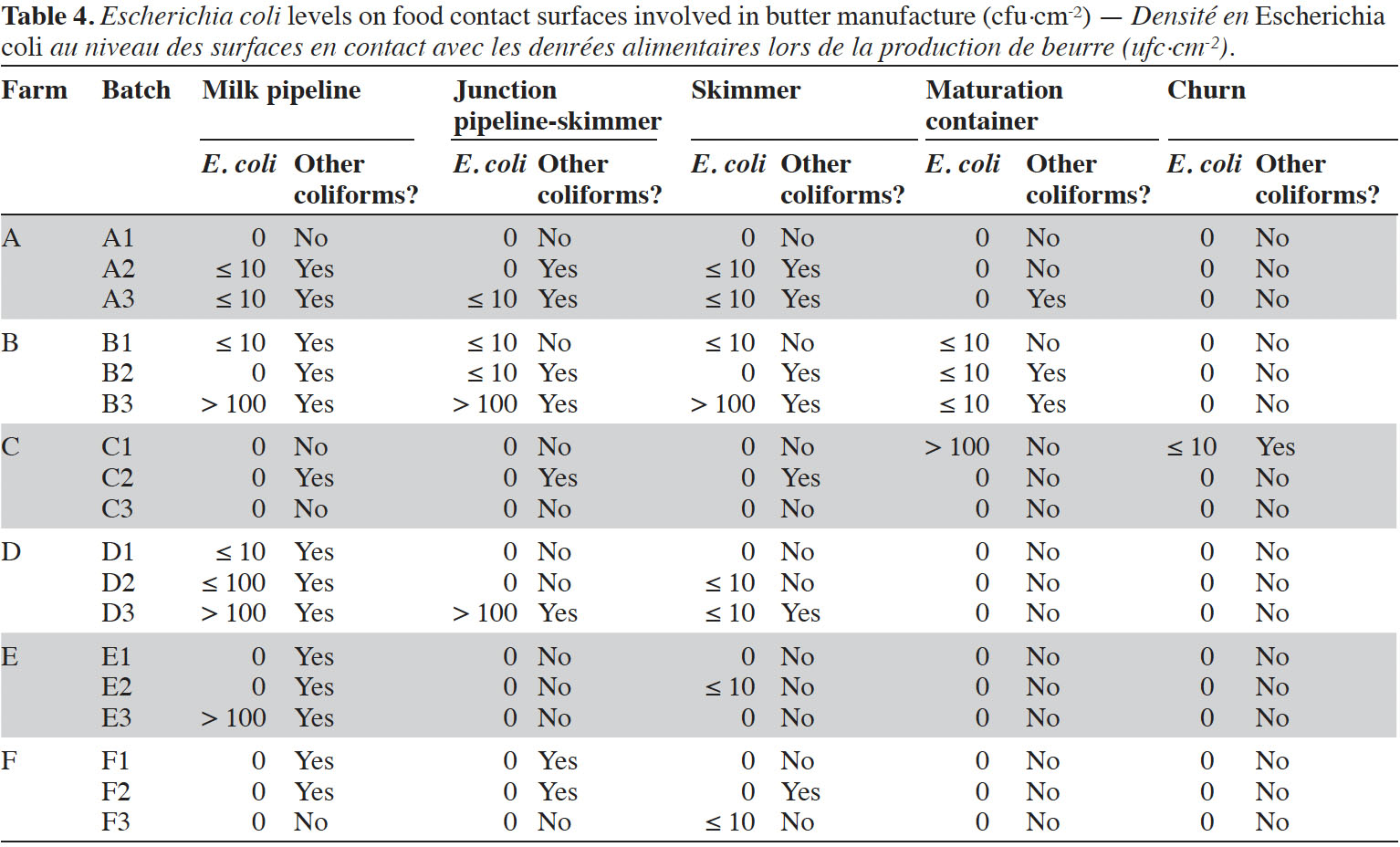
8Enumerations of E. coli were performed in triplicates on five food contact surfaces involved in butter manufacture, namely milk pipeline to skimmer, junction between pipeline and skimmer, skimmer, maturation container and churn (Table 4). It was observed that milk pipelines were often contaminated (at least during one batch manufacture in four farms out of six). Junctions between pipeline and skimmer (three farms out of six) as well as skimmers themselves (five farms out of six) were frequently contaminated. Maturation containers were generally not contaminated, except in farms B (batches B1-B2-B3) and C (batch C1). No E. coli were identified inside churns, except for butter batch C1.
3.3. Dairy products samples
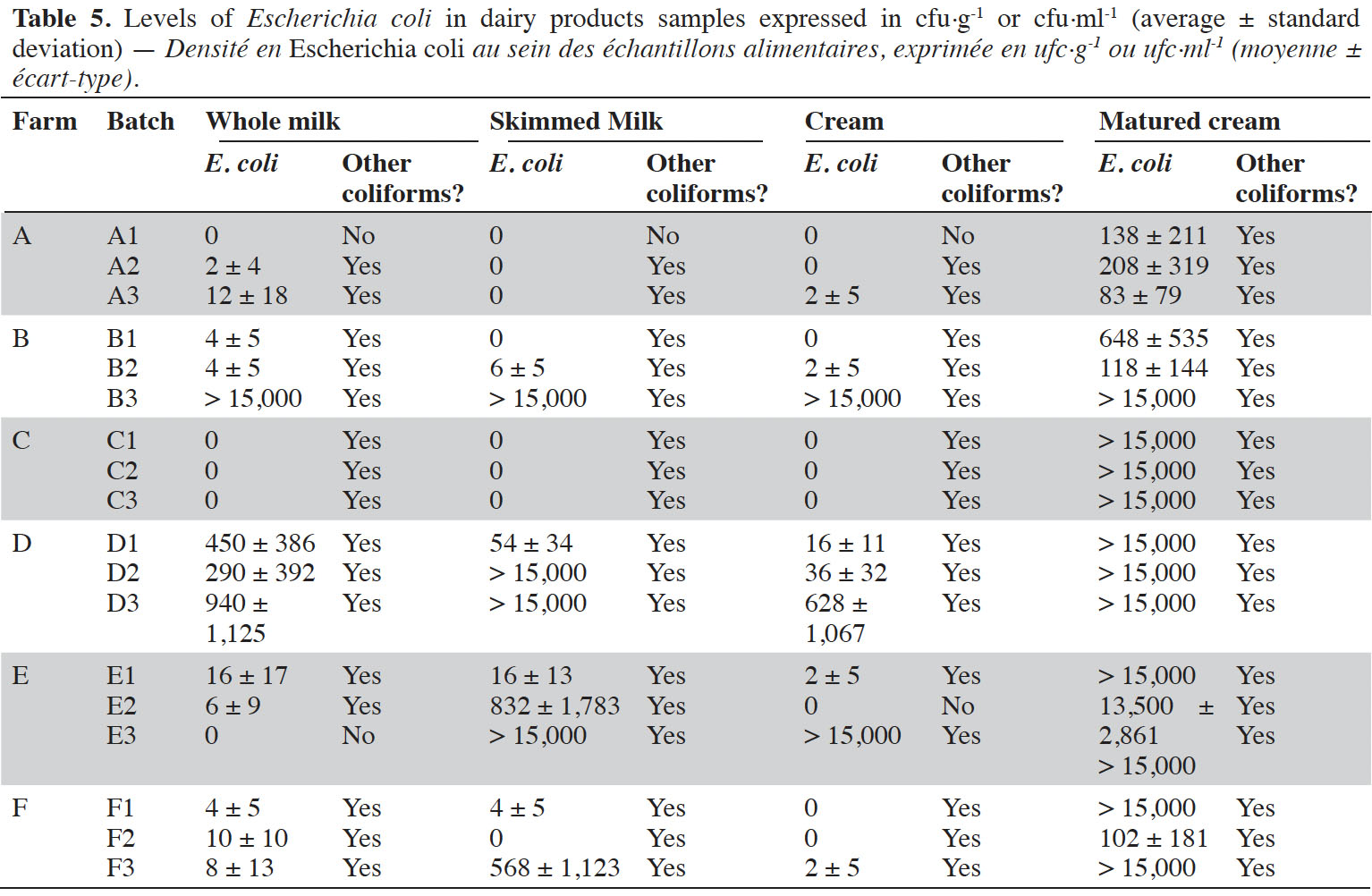
9Enumerations of E. coli in dairy products are summarized in table 5. Globally, dairy products samples with levels of E. coli considered as low (i.e. < 10 cfu·g-1) were scarce. All milk samples from farm D were highly contaminated (up to more than 1,000 cfu·ml-1). It was also the case of milk from batch B3 (> 15,000 cfu·ml-1). It was observed that, in case of contamination at the beginning of the process, end-products were also contaminated (r² = 0.30, p-value = 0.040). On the opposite, absence of contamination of whole milk did not necessarily result in butter with satisfying hygienic quality, as it was for instance observed in farms A and C.
3.4. Factors impacting milk and butter hygienic quality
10One-way ANOVA revealed significant differences in milk hygienic quality between farms (p-value = 0.001). Escherichia coli levels were higher in milk from farm D in comparison with other farms, and significantly lower in milk from farm C. Interestingly, contamination was high in butter from farm C, although manufactured from milk exempted of E. coli. Significant differences were observed in E. coli levels in whole milk regarding milking equipment (p-value = 0.030), with higher contamination when milking robots were used. The use of lactic acid starters significantly impacted butter hygienic quality (p-value = 0.034). Four batches (C1-C2-C3 and D1) were manufactured without lactic acid starters; average E. coli levels in butter manufactured with or without addition of starters being 2.88 and 4.06 log10 cfu·g-1, respectively. The choice of the commercial starter did not significantly impact E. coli levels in final butter (p-value > 0.050). Churning equipment (rotation axis and surface material) did not significantly impact butter hygienic quality.
4. Discussion
11Globally, considered batches did not satisfy food process hygiene criterion defined by Regulation (EC) No 2073/2005, with 89% of batches presenting unacceptable levels of E. coli. Enumerations revealed that surface samples associated to the beginning of the process, i.e. milk pipelines from milking system to skimmer, were already contaminated in most farms, sometimes with levels > 1,000 cfu·cm-². Generally, these pipelines are treated as part of milking equipment cleaning and disinfection procedure, involving alkaline and acid rinsing. As all junctions, valves or turns, junctions between milk pipelines and skimmers represented a critical point, as already identified by Ostrov et al. (2016). Contamination of these junctions was particularly marked in farm B (≤ 10 cfu·cm-² in B1-B2 and > 100 cfu·cm-² in B3). In farms B and C, E. coli was also identified on cream maturation containers, revealing insufficient cleaning and disinfection. Only one surface sample collected from churn was contaminated (C1). This churn was composed of wood. It is known that wooden surfaces are inclined to be contaminated and colonized by bacterial biofilms, as already reported for traditional wooden cheese vats (Gaglio et al., 2016). Nevertheless, in the case of this study, contamination of this churn was not observed during other visits in the farm, and no apparent scratches on wood were identified.
12As expected, butter manufactured from milk with insufficient quality cannot satisfy food process hygiene criterion. Indeed, when whole milk was contaminated with E. coli, all subsequent dairy products, namely cream, matured cream and butter were also contaminated and did not satisfy food process hygiene criterion defined by the European regulation. This phenomenon was already reported by Sudhakaran & Minj (2020), revealing the importance of guaranteeing hygiene from farm to fork. As such, butter manufacturing process does not provide sufficient hurdles to inhibit the growth of E. coli. Although El-Hajjaji et al. (2021) demonstrated during challenge studies with L. monocytogenes that cream maturation allowed a decrease in levels of the pathogen, such studies were not performed regarding E. coli, to our knowledge. Results from the present study indicated that multiplication of the pathogen mainly occurred during cream maturation. Both commercial starters met during the study did not have the same composition, in terms of bacterial strains. Choozit MA4001/4002 was composed of Lactococcus lactis subsp. lactis, Lactococcus lactis subsp. cremoris, Lactococcus lactis subsp. lactis biovar. diacetylactis and Streptococcus thermophilus. The same strains were included in Flora Danica, except S. thermophilus, but not in the same proportions. Inability of starters to inhibit the growth of E. coli during cream maturation was surprising, as it was already observed that Lactococcus lactis subsp. lactis inhibited E. coli in vitro (Metlef & Dilmi-Bouras, 2009). Nevertheless, results obtained at laboratory scale could differ from real situation, for which food matrix and complex microbial interactions can prevent expected activities of individual strains. During the present study, churning allowed a decrease in the levels of E. coli in 16 out of 18 batches, but its effect was not sufficient to ensure that final butter complies with Regulation (EC) No 2073/2005. Although the use of starters can be suggested as helpful tool to limit the level of the contamination with E. coli, it does not guarantee the manufacture of butter with acceptable hygienic quality. Consequently, its use should always be associated with an accurate application of GMP and of cleaning and disinfection procedure. Using milk of satisfying hygiene does not guarantee the manufacture of butter with acceptable levels of E. coli, as contamination can occur during manufacturing steps due to inadequate cleaning and disinfection of materials and surfaces. In farm C, it can be supposed that cream was spoiled during maturation, as the presence of E. coli was observed in maturation containers.
13ANOVA revealed higher levels of E. coli in butter manufactured in farm D, with all enumerations > 1,000 cfu·g-1. This farm used robot for milking. For manufacture of the first batch, no lactic starters were added. Due to levels of E. coli > 15,000 cfu·g-1 in butter, farmer decided to mature cream using lactic acid starters for other batches. Farm E was equipped with a robot of the same brand and model but was not concerned with so high levels of E. coli in milk. In fact, the robot manufacturer identified issues at the filtration level regarding the concerned model and changed filter in the robot of farm E. Farm D was still waiting for this modification at the time of the study. Nevertheless, hygienic quality was significantly improved in farms equipped with classical milking rooms in comparison with milking robots. Robots allow continuous milking, in comparison to traditional milking rooms in which all animals are milked together once or twice a day. In the latter case, whole milk batch can directly be skimmed. When a robot is used, milk is temporarily stored in buffer tank without cooling, sometimes for several hours. This step seemed critical, as it could be highly favorable for the growth of E. coli and spoilage or pathogenic microbiota. Vilar et al. (2012) considered a critical control point when milk is not cooled at 3-4 °C within 30 min after milking.
14Regarding these results, authors would like to suggest potential ways to farm producers in order to improve milk and butter quality, regarding levels of E. coli (Figure 2). A major observation was that, despite cleaning and disinfection practices, surfaces were still contaminated with E. coli, especially milk pipelines and junctions with cream separators. Globally, it was concluded that guidelines should be provided to perform adequate cleaning and disinfection, in a uniform way for all farms. It was indeed observed that each producer has its own practices, in terms of frequency of cleaning and disinfection and of types of detergents and disinfectants used. Also, it is not clear if a complete protocol must be applied after each milking or only once a day. Producers should always pay attention to several elements, namely (a) to use products useful for elimination of fatty products, (b) to apply a sufficiently high temperature, especially when only hot water is used, (c) to avoid corrosive chemicals, as damaged surfaces represent suitable places for establishment of biofilms, (d) to make sure flow is turbulent and (e) to double-check critical pieces including turns, junctions, taps and gates. An essential critical point in farms equipped with milking robots was the buffer tank, in which milk was accumulated without cooling throughout the day, waiting for skimming. Food and Agriculture Organization of the United Nations (2004) and European Commission (2004) mentioned that milk should be cooled at temperature < 6 °C if it is not processed within 2 h after milking. Consequently, although it represents extra energetic costs, producers should always cool their milk during this time-lapse, in order to avoid the growth of E. coli, but also to control growth of other foodborne pathogens potentially present in raw milk. Raw milk should thus be heated afterwards to reach optimal temperature for cream separation.
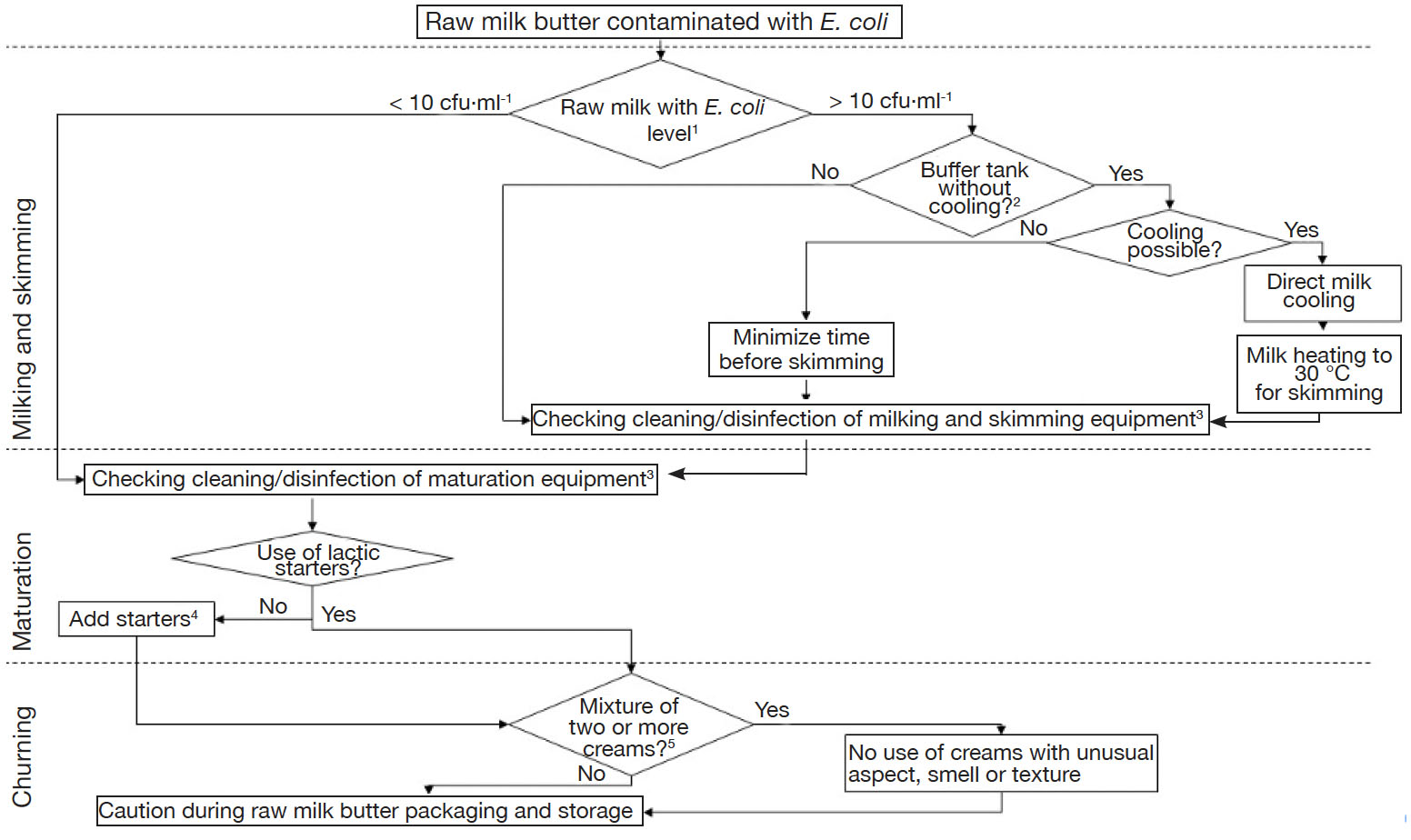 Figure 2. Decision tree in case of contamination of raw milk butter with Escherichia coli — Arbre de décision à suivre dans le cas d’une contamination de beurre au lait cru par Escherichia coli.
Figure 2. Decision tree in case of contamination of raw milk butter with Escherichia coli — Arbre de décision à suivre dans le cas d’une contamination de beurre au lait cru par Escherichia coli.
1: milk microbiological quality based on official results provided by dairies — qualité microbiologique du lait sur base des résultats fournis par les laiteries; 2: when several hours occur before cooling or skimming — lorsque plusieurs heures s’écoulent avant le refroidissement ou l’écrémage; 3: hygiene should also be checked upstream, i.e. breeding — l’hygiène devrait aussi être vérifiée en amont de la fabrication, i.e. au niveau de l’élevage; 4: use of commercial starters with controlled and constant composition, do not use creams from previous batches for seeding — utilisation de ferments commerciaux de composition constante et contrôlée, ne pas utiliser de crème issue de lots précédents pour l’ensemencement; 5: creams from several milkings and skimmings, so which did not undergo the same maturation time and temperature cycles — crèmes issues de plusieurs traites et écrémages, qui n’ont donc pas suivi des cycles temps-température de maturation identiques.
15Sometimes, precautions during breeding, milking and skimming were not sufficient to guarantee the manufacture of butter in compliance with food process hygiene criterion from Regulation (EC) No 2073/2005. It is then important to check the efficacy of cleaning and disinfection procedure of maturation containers. If none of these operations allowed the manufacture of butter with acceptable levels of E. coli, a complementary solution is the addition of lactic acid starters for cream maturation. Nevertheless, as previously discussed, this solution is not the panacea. Furthermore, the use of commercial starters should not be generalized, as it could result in the standardization and in a loss of typicity of Belgian raw milk artisanal butter. Mixture of creams obtained during distinct days could also be questionable. Indeed, the day of butter manufacture, all creams have not undergone the same maturation duration. Consequently, they do not have similar physicochemical and microbiological characteristics, and this could affect general hygiene of butter. Hygiene of maturation containers and churns should also be checked with as much attention as milking and skimming equipment. Finally, caution is necessary during packaging and storage.
5. Conclusions
16The main conclusion of this study was that contamination of raw milk artisanal butter is still an issue. This study revealed contamination pathways are variable. The problem should generally be considered case by case, as situation differed between visited farms. Major pathways of investigations remained to check the efficiency of cleaning and disinfection procedure, and an efficient milk cooling during time-lapse between milking and processing, especially when continuous milking was performed in farms equipped with milking robots. From this study, it also appeared that Petrifilm represents a promising tool in farms and dairy industry. Indeed, their use is easy to implement in comparison with classical agar plates, and they could provide essential information to farmers, producers and operators regarding overall hygienic quality on farms or factories.
17Some complementary aspects should be investigated to acquire a deeper knowledge on the contamination of raw milk butter with E. coli. Firstly, it could be interesting to focus on more farms, allowing to confirm pathways of investigation identified during this study, or to identify other potential points of attention. Inclusion of farms manufacturing butter in compliance with food process hygiene criteria detailed by Regulation (CE) No 2073/2005 could also help in the understanding of the fate of E. coli during butter manufacture. Seasonal effect should also be considered. Indeed, in the present paper, all farm visits were performed in the same season. Temperature has indeed an influence on the growth and on the survival of E. coli. Similarly, animal feeding could also play a role. Hygiene during breeding could also be investigated, namely udder hygiene or cleanliness of milking material. Finally, it must be said that reference method for food process hygiene criteria, as well as method involving Petrifilm Rapid E. coli/coliforms Plate Counts, does not allow the identification of the presence of toxin-producing E. coli, including strain O157:H7. It could thus be of interest to focus on this foodborne pathogen and on its fate during butter manufacture and storage, using methods allowing its detection and enumeration.
Acknowledgements
18Authors would like to thank the Walloon Region, through DiversiFerm, for its financial help during this work.
Bibliographie
3M, 2018. Interpretation guide Rapid E. coli/Coliform Count Plate, https://multimedia.3m.com/mws/media/1541346O/3m-petrifilm-rapid-e-coli-coliform-count-plate-interpretation-guide.pdf, (25/06/2021).
Bakirci I. et al., 2002. The effects of commercial starter culture and storage temperature on the oxidative stability and diacetyl production in butter. Int. J. Dairy Technol., 55, 177-181.
Bird P. et al., 2020. Evaluation of the 3MTM PetrifilmTM Rapid E. coli/Coliform Count Plate for the enumeration of E. coli and coliforms: collaborative study, first action: 2018.13. J. AOAC Int., 103, 513-522, doi.org/10.1093/jaocint/qsz013
Ceylan O. & Ozcan T., 2020. Effect of the cream cooling temperature and acidification method on the crystallization and textural properties of butter. LWT Food Sci. Technol., 132, 109806, doi.org/10.1016/j.lwt.2020.109806
Costanzo N. et al., 2020. Foodborne pathogen assessment in raw milk cheeses. Int. J. Food Sci., 2020, 3616713, doi.org/10.1155/2020/3616713
Cullinane N. et al., 1984. Influence of season and processing parameters on the physical properties of Irish butter. Irish J. Food Sci. Technol., 8, 13-25.
Deosarjar S.S. et al., 2016. Butter manufacture. In: Caballero B. et al., eds. The Encyclopedia of food and health. Cambridge, MA, USA: Academic Press, 529-534
El-Hajjaji S. et al., 2019. Overview of the local production process of raw milk butter in Wallonia (Belgium). Int. J. Dairy Technol., 72, 466-471, doi.org/10.1111/1471-0307.12608
El-Hajjaji S. et al., 2021. Study of the bacterial profile of raw milk butter, made during a challenge test with Listeria monocytogenes, depending on cream maturation temperature. Food Microbiol., 98, 103778, doi.org/10.1016/j.fm.2021.103778
European Commission, 2004. Commission Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. Off. J. L., 139, 55.
European Commission, 2005. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. L., 338, 1-26.
Federal Agency for the Safety of the Food Chain, 2012. Guide d’autocontrôle pour la production et la vente de produits laitiers à la ferme, https://www.favv-afsca.be/autocontrole-fr/guides/distribution/g034/_documents/G-034_V1_23-07-2012_Fr.pdf, (25/06/2021.
Food and Agriculture Organization of the United Nations, 2004. CAC:RCP 57-2004 Code of hygienic practice for milk and milk products, http://www.fao.org/fileadmin/user_upload/livestockgov/documents/CXP_057e.pdf, (01/07/2021).
Gaglio R. et al., 2016. Microbial activation of wooden vats used for traditional cheese production and evolution of neoformed biofilms. Appl. Env. Microbiol., 82, 585-595, doi.org/10.1128/AEM.02868-15
International Organization for Standardization, 2017. ISO 6887-1:2017. Microbiology of the food chain – Preparation of test samples, initial suspension and decimal dilutions for microbiological examination – Part 1: General rules for the preparation of the initial suspension and decimal dilutions. Geneva, Switzerland: ISO.
Metlef S. & Dilmi-Bouras A., 2009. Effet antagoniste de Lactococcus lactis, souches extrêmophiles locales, sur des espèces de la flore intestinale résidente. Rev. Nat. Technol., 1, 33-44.
N’Guessan E. et al., 2015. A survey of bacteria found in Belgian dairy farm products. Biotechnol. Agron. Soc. Env., 19, 346-354.
Ostrov I. et al., 2016. Development of a method to determine the effectiveness of cleaning agents in removal of biofilm derives spores in milking system. Front. Microbiol., 7, 1498, doi.org/10.3389/fmicb.2016.01498
Panchal B. et al., 2021. Influence of fat globule size, emulsifiers, and cream-aging on microstructure and physical properties of butter. Int. Dairy J., 117, 105003, doi.org/10.1016/j.idairyj.2021.105003
Sudhakaran A.V. & Minj J., 2020. Basic facts about dairy processing and technologies. In: Minj J. et al., eds. Dairy processing: advances research to applications. Berlin, Germany: Springer, 1-24.
Tenaillon O. et al., 2010. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol., 8, 207-217, doi.org/10.1038/nrmicro2298
Verraes C. et al., 2015. Review of the microbiological hazards of dairy products made from raw milk. Int. Dairy J., 50, 32-44, doi.org/10.1016/j.idairyj.2015.05.011
Vilar M.J. et al., 2012. Implementation of HACCP to control the influence of milking equipment and cooling tank on the milk quality. Trends Food Sci. Technol., 23, 4-12, doi.org/10.1016/j.tifs.2011.08.002






