- Startpagina tijdschrift
- Volume 23 (2019)
- Numéro 4
- Impact of insecticides on the efficacy of entomopathogenic nematodes against the diamondback moth Plutella xylostella (L.) (Lepidoptera: Plutellidae) on cabbage in northern Benin
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Impact of insecticides on the efficacy of entomopathogenic nematodes against the diamondback moth Plutella xylostella (L.) (Lepidoptera: Plutellidae) on cabbage in northern Benin

Nota's van de redactie
Received 5 July 2017, accepted 3 September 2019, available online 4 October 2019
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
Résumé
Effet des insecticides sur l’efficacité des nématodes entomopathogènes contre les larves de la teigne des crucifères Plutella xylostella (L.) (Lepidoptera : Plutellidae) sur le chou au nord Bénin
Description du sujet. L’article traite de l’étude de la compatibilité des nématodes entomopathogènes avec les insecticides utilisés contre la teigne des crucifères Plutella xylostella (L.) au nord du Bénin.
Objectifs. L’objectif de l’étude est d’évaluer l’effet de cinq insecticides sur l’efficacité des nématodes entomopathogènes contre les larves de P. xylostella.
Méthode. Des larves infestantes (IJs) de deux espèces de nématodes entomopathogènes (Steinernema sp. 83a et Heterorhabditis sonorensis KF723827) ont été mises en contact pendant 48 h avec cinq insecticides utilisés contre les larves de P. xylostella à Djougou. Ensuite les IJs exposées aux insecticides ont été utilisées pour infester les larves de P. xylostella en conditions de laboratoire et en milieu semi-contrôlé sur les plants de chou.
Résultats. Les expérimentations conduites en laboratoire montrent que le taux de survie des nématodes exposés à KARATE 2.5 WG, LAMBDA SUPER 2.5EC ou huile de neem est compris entre 95 % et 98 %. Le taux de mortalité le plus élevé des larves de P. xylostella a été observé avec Steinernema sp. 83a (87 % à 50 IJs·cm-2 24 h après traitement), tandis que seulement 35 % de mortalité des larves a été enregistré au niveau de H. sonorensis avec la même dose d’application. Le nombre d’IJs ayant pénétré chaque larve de P. xylostella est de 9 ± 3 IJs·larve-1 pour H. sonorensis KF723827 et 6 ± 2 IJs·larve-1 pour Steinernema sp. 83a. L’exposition des nématodes entomopathogènes aux insecticides n’a pas affecté leur reproduction à l’intérieur des larves mortes.
Conclusions. Ces résultats montrent que les trois insecticides testés contre P. xylostella n’affectent pas la survie ni l’efficacité des nématodes entomopathogènes et peuvent être utilisés en combinaison pour lutter contre P. xylostella.
Abstract
Description of the subject. The article deals with the study of compatibility of entomopathogenic nematode (EPN) species with insecticides currently used against diamondback moth (DBM) Plutella xylostella (L.) in northern Benin
Objectives. The aim of this work was to determine the impact of five insecticides on the efficacy of entomopathogenic nematodes against DBM larvae.
Method. Infective juveniles (IJs) of two EPN species (Steinernema sp. 83a and Heterorhabditis sonorensis KF723827) were exposed to five insecticides used against P. xylostella larvae in Djougou for 48 h. The number of surviving nematodes was used to infest DBM larvae. The experiment was carried out under laboratory and semi-field conditions.
Results. The bioassays carried out in laboratory showed that the survival rate of nematodes exposed to KARATE 2.5 WG, LAMBDA SUPER 2.5EC or neem oil ranges between 95% and 98%. In the treated plots, Steinernema sp. 83a was the most virulent with the highest P. xylostella mortality (87% at 50 IJs·cm-2 after 24 h) while only 35% larval mortality was recorded for H. sonorensis applied at the same dose. Population density of nematodes which penetrated DBM larvae reached 9 ± 3I IJs·larva-1 for H. sonorensis KF723827 and 6 ± 2 IJs·larva-1 for Steinernema sp. 83a. In cadaver of DBM, nematode reproduction did not appear to be affected by the contact with insecticides.
Conclusions. Based on our research, we conclude that the three insecticides did not affect EPNs efficiency and could be used in combination against DBM.
Inhoudstafel
1. Introduction
1The diamondback moth (DBM), Plutella xylostella (L.) (Lepidoptera: Plutellidae), is the most destructive insect pest of cruciferous plants throughout the world (Sarfraz et al., 2006; Syed et al., 2012). Management costs and yield losses are estimated up to US$ 4-US$ 5 billion (Zalucki et al., 2012). In Benin, the DBM causes considerable economic losses to cabbage and other crops such as egg-plant, black mustard, broccoli, kale, radish, turnip and watercress (James et al., 2010). In West Africa the control of this pest lies mostly on synthetic hazardous insecticides (James et al., 2010). The frequent application of these insecticides leads to development of pest resistance particularly in sub-tropical and tropical countries, where farmers apply mixtures of chemical insecticides sometimes more than twice a week (Wright, 2004). Zalucki et al. (2012) suggested that greater efforts at implementation of integrated pest management (IPM) would reduce insecticide inputs considerably, reducing health problems, negative environmental impacts and saving many millions of dollars annually.
2Entomopathogenic nematodes (EPN) of the Steinernematidae and Heterorhabditidae families have achieved a place in biological control programs because of their effectiveness, speed of action, innocuousness to non-target insects and mammals and their relative simplicity in mass production (San-Blas, 2013). They are symbiotically associated with bacteria of the genera Xenorhabdus (Steinernema) and Photorhabdus (Heterorhabditis) (Thanwisai et al., 2012). Only the resistant stage of EPN (infective juveniles or IJs) is free living and in the soil. At this development stage, the IJ seeks a host insect and enters the insect usually through a natural opening, such as the mouth, anus, or spiracles (Shapiro-Ilan et al., 2009). After entering the host’s hemocoel, nematodes release their symbiotic bacteria. Once in the hemolymph, the bacteria multiply rapidly, producing a wide range of toxins and exo-enzymes that cause host mortality within 24 to 48 h. The bacteria grow rapidly in the haemolymph, which serves as a nutrient source for the infective juveniles. After 2-3 generations within the insect cadaver, second-stage juvenile nematodes develop into specialized third-stage, which leave the insect cadaver in search for a new host (Spence et al., 2011).
3Entomopathogenic nematodes are obligate parasites of insects and are used as biological control agents of economically important insect pests (Shapiro-Ilan et al., 2012). Thirty-seven strains of EPN of the genera Heterorhabditis (30), Steinernema (5) and Heterorhabditoides (2) have been recovered in Benin (first record of EPN in West Africa), characterized and tested in laboratory and field conditions against citrus termites (Zadji et al., 2013). Nyasani et al. (2008) reported that EPN have great potential to control DBM and should be exploited in the management of this pest in Kenya.
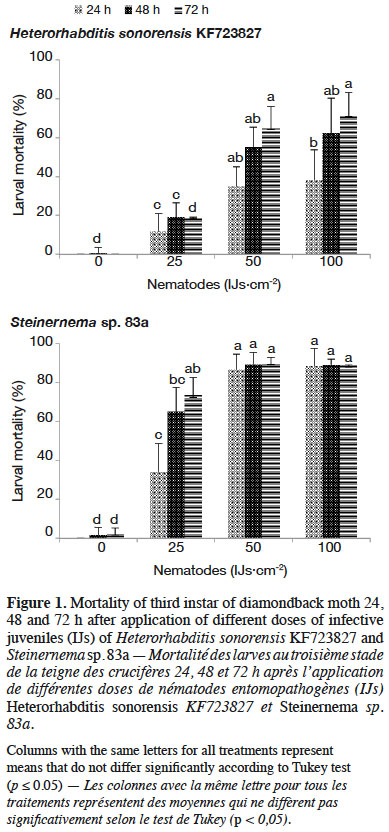
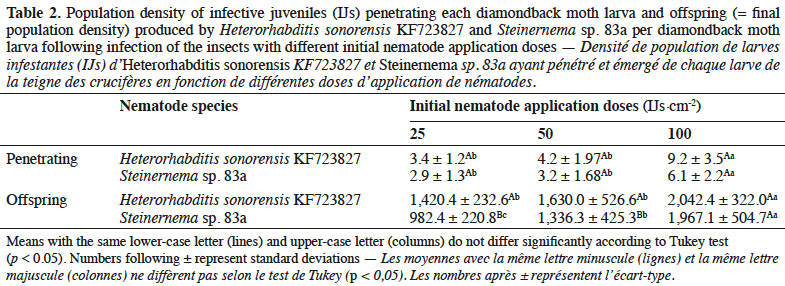
4Combining the use of biological control agents, such as EPN, with chemical insecticides is crucial to promote IPM against many agricultural pests (Laznik & Trdan, 2014). This strategy could facilitate the use of EPN, increase the control efficiency of IPM and reduce the cost of EPN application (Bajc et al., 2017). Furthermore, EPN may also reduce the dependence on chemical insecticides and thus contribute to slowing down the development of insecticide resistance and preventing adverse effects on human health and the environment (Yan et al., 2012). Although the effect of chemical pesticides on EPN has been investigated in many laboratory experiments with exposure of nematodes to pesticides (García-del-Pino & Jové, 2005; Laznik et al., 2012; Atwa et al., 2013; Baimey et al., 2015), it is still necessary to test compatibility of the commonly used pesticides in the area where EPN strains are anticipated to be used. The objectives of this study were:
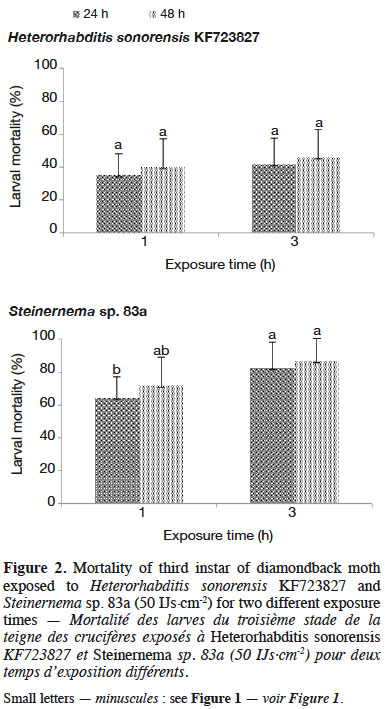
5– to conduct a diagnostic survey in cabbage production fields in northern Benin to obtain information on cabbage production and constraints undermining it;
6– the evaluation under laboratory conditions of the potential of two EPN species (Steinernema sp. 83a and Heterorhabditis sonorensis) to control the DMB;
7– an evaluation of the effects of commonly used insecticides on EPN under laboratory conditions,
8– an evaluation of the effectiveness of the most virulent indigenous EPN species alone or in combination with the insecticide KARATE 2.5 WG against DBM larvae under semi-field conditions on potted cabbage crops.
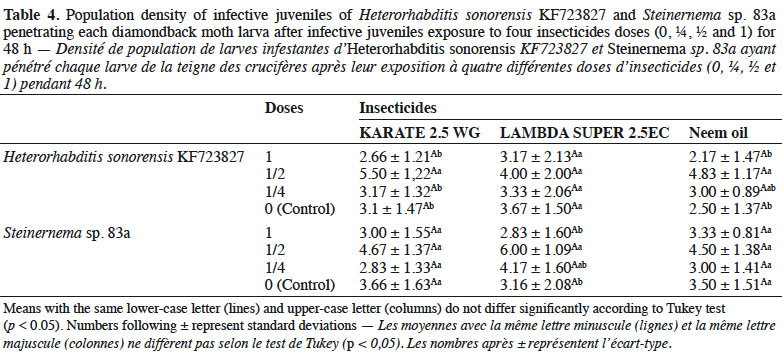
2. Materials and methods
2.1. Diagnostic survey
9The survey was conducted during the mid-dry season in March 2015 on 24 cabbage fields in the communes of Djougou and Ouake, the major cabbage production areas of northern Benin. A questionnaire was used during the survey to collect information from farmers about cabbage production, main pests and diseases and the control methods used. Severity of damage (level of plant attack) was scored using a scale of 1-4, where 1 = weak damage (0-25% of cabbage leaves were damaged per plant) and 4 = considerable damage (75-100% of cabbage leaves were damaged per plant). This scale is a modification of the scale used by Speijer & De Waele (1997) for assessing root-knot nematode galling on banana (Musa spp.).
2.2. Nematode, plant and insect rearing
10Nematode rearing. Tests were carried out with two species of EPN via Heterorhabditis sonorensis (KF723827) (Nematoda: Steinernematidae) (Zadji et al., 2013) and Steinernema sp. (strain 83a). Nematodes were extracted from soil samples collected in southern Benin and characterized using molecular tools at the University of Ghent, Belgium (Zadji et al., 2013). They were reared on larvae of the wax moth, Galleria mellonella (L.) in the Plant Protection Laboratory of Higher National School of Agronomic Sciences and Techniques of Djougou/University of Parakou/Benin as described by Kaya & Stock (1997). Infective juveniles were stored in small plastic pots containing polyurethane sponge blocks at 12 °C for three to four weeks. Their viability was checked prior to their use under a stereo microscope (Bajc et al., 2017).
11Cabbage plants. Seeds of cabbage, Brassica oleracea (L.) cv. Oxylus, commonly grown in northern Benin were sown in individual plastic pots (2,000 ml) each filled with 2 kg of sterilized soil (80 °C, 72 h). The pots were arranged in cages (100 cm long, 75 cm large, and 100 cm height). Each cage contained six pots and was maintained at 28-30 °C, with a photoperiod of 12 h light. The cages were covered with fine iron mesh to prevent infestation of the plants by P. xylostella or other pests. Cabbage plantlets were transplanted three weeks after sowing. When the plants had 12-16 leaves (about six weeks after transplanting), they were used in experiments. To increase plant development, 20 g of mineral fertilizer NPKSB (14-23-14-5-1) were applied in each pot. Cabbage plants were watered twice a day.
12Diamondback moth rearing. Third instars of DBM were collected on infected cabbage plants in a conventional cabbage field. The larvae were transferred onto leaves of potted cabbage plants contained in cages described above. The third progeny generation of these larvae constituted a stock-culture of DBM used throughout this study. The cages were maintained at 28 ± 2 °C, with a photoperiod of 12 h light and 60–80% relative humidity. Diamondback moth adults were fed on 10% honey solution as described by Kermani et al. (2013).
2.3. Laboratory experiments
13Experimental conditions. Experiments were performed in 9 cm diameter Petri dishes lined with filter paper (Whatman No. 1). Nematodes previously stored at 12 °C were acclimated at 28-30 °C for 12 h before they were applied at the dose of 25, 50 or 100 IJs·cm-2. The concentrations of IJ required for the bioassays were adjusted by volumetric dilutions in distilled water (Navon & Ascher, 2000). Nematode suspensions were thoroughly shaken each time before pipetting. Each experiment was conducted twice.
14Effect of different application doses of entomopathogenic nematodes against diamondback moth larvae. Dose of 25, 50 or 100 IJs·cm-2 were separately applied into Petri dishes in 2 ml tap water with six replicates. Tap water in the same volume was used as control. Then 10 third instar larvae of DBM were transferred into each Petri dish with cabbage leaf disc (9 cm diameter). The dishes were arranged in a completely randomized design replicated six times for each of two nematodes species and kept at room temperature of 28-30 °C with a photoperiod of 12 h light and 60-80% relative humidity. Insect larval mortality was recorded 24, 48 and 72 h after their contact with IJs and dead larvae were removed from Petri dishes. Three dead larvae from each plate were transferred to 250 ml conical flasks containing 50 ml water. The flasks were shaken to remove all nematodes from the surface of the DBM larvae. The rinsed DBM larvae were dissected under stereomicroscope and the numbers of nematodes were counted to evaluate the penetration rate. Three other dead larvae were also randomly selected and transferred into individual modified White traps (White, 1927) to record nematode reproduction after their emergence from their host. Emerged nematode IJs were collected for 10 days and total population density determined.
15Effect of exposure time on diamondback moth mortality. Based on the bioassay, 50 IJs·cm-2 was the best concentration identified. Nematodes were applied as described previously at 50 IJs·cm-2. Then, 10 third instar larvae of DBM were transferred into each dish and the insects were exposed to the nematodes for 1 h or 3 h. Dishes were arranged in a completely randomized design replicated six times. After each exposure time, insect larvae were removed, rinsed in tap water to remove any nematode IJs attached to their bodies. Then, the insect larvae were transferred into other Petri dishes containing 9 cm diameter cabbage leaf disc. Data on insect mortality, IJs penetrating the insects and nematode reproduction were collected as described above.
16Efficacy of entomopathogenic nematodes against diamondback moth on single cabbage leaf discs. Cabbage leaves were removed from uninfected cabbage plants, surface cleaned with 70% alcohol and thoroughly rinsed with distilled water. Excess water on the leaf discs was removed using tissue paper. The leaves were then cut into 9 cm-diameter disks. The leaf discs were then weighed, lined each in one Petri dish with ten replicates and 50 IJs·cm-2 were applied onto each of them in 2 ml tap water. For control leaf discs, the same volume of tap water was applied with the same volume. After 30 min, 10 DBM larvae were transferred into each Petri dish and the dishes were sealed with parafilm (United American Can, WI). Larval mortality was recorded 24 and 48 h after IJs application and each leaf disc was re-weighed to determine larvae consumption.
17Effect of five insecticides on entomopathogenic nematodes’ survival, infection and reproduction potential. Five insecticides commonly used against DBM on cabbage by farmers in northern Benin were used in this experiment (Table 1). Insecticides were diluted to the recommended dose for field application (dose 1). Then, the preparation obtained was successively diluted twice (doses ½ and ¼) to obtain finally three different doses (1, ½ and ¼). Twenty four-well plates were used as experimental arenas as described by Navarro et al. (2014). For each insecticide treatment, 900 µl was pipetted into a well of the plate. Each well received 100 IJs in 100 µl tap water. There were twelve replicates for each insecticide dose/EPN species combination. Control wells received 100 IJs in 100 µl tap water only. The population density of nematodes surviving in each well was recorded 48 h after their exposure to the different treatments. Twenty nematodes were randomly selected per well and observed under stereomicroscope. Dead nematodes were considered as those not reacting when touched with a probe.
18To study the effects of the different treatments on EPN infectivity and reproduction, 10 IJs that survived for 48 h in each well were pipetted and transferred into 1.5 ml Eppendorf tubes previously filled with 3 g of sterile sand (85 °C, 72 h) moistened with water at 10% (w/v). One third larva of DBM was introduced in each Eppendorf tube. For each insecticide dose and for control, there were three replicates of ten Eppendorf tubes (30 replicates). Larval mortality was recorded after 48 h. Nematodes penetration rate and reproduction were recorded as described previously in experiment.
2.4. Infectivity of Steinernema sp. 83a and KARATE 25 WG as foliar spray against diamondback moth larvae on potted cabbage plants under semi-field conditions
19In this experiment, Steinernema sp. isolate 83a was used as observed in previous experiments as more virulent than the H. sonorensis isolate. Five treatments of the Steinernema sp. were used:
20– aqueous suspension of Steinernema sp. 83a at 50 IJs·cm-2 for foliar spray;
21– aqueous suspension of KARATE 2.5 WG (1.33 g·l-1) for foliar spray;
22– aqueous suspension of Steinernema sp. 83a at 50 IJs·cm-2 + KARATE 2.5 WG (0.66 g·l-1),
23– an infested-untreated control for which plants were infested with insects but sprayed with distilled water;
24– an uninfested and untreated control plant.
25For the first four treatments, 10 third instar of DBM were used to infest each plant using a fine brush in the morning for the DBM larvae to establish on cabbage plants before foliar application. Each plant constitutes one replicate and there were six plants per cage. To facilitate nematode survival, each plant was watered 1 h before EPN application in afternoon. To prevent sedimentation while EPN were sprayed in water, the sprayer was constantly shaken during application. Cabbage plants were transferred to laboratory 72 h after application to record DBM mortality. To confirm EPN infectivity and evaluate their penetration rate, three dead larvae were randomly selected per plant and dissected under stereomicroscope. The damage caused by DBM larvae to cabbage plants under various treatments was evaluated by counting the number of feeding spots on first 12 leaves of cabbage and each plant was weighted.
2.5. Data analysis
26Nematode population densities and data on DBM and nematode mortalities were compared with ANOVA using Tukey test, p ≤ 0.05, on MINITAB16. Before the analysis, the mean mortalities were tested for the homogeneity of treatment variances with Levene's test. Mortality rate data were corrected for control mortality, using Abbott’s formula (Abbott, 1925). Prior to analyses, nematode population densities and percentage mortality of DBM larvae were log10(x+1) and arcsine transformed, respectively.
3. Results
3.1. Information about cabbage production in northern Benin and constraints undermining production
27Twenty-four cabbage producers were investigated in northern Benin. Fifty percent of them claim producing cabbage since 5-10 years. More than 90% usually used cv. Oxyllus as cabbage cultivar, which gives the best productivity. In the region, particularly for cabbage crop, farmers used small land areas (ca 0.125 ha by 38% farmers and ca. 0.25 ha by 42% farmers). To install nurseries, farmers usually buy cabbage seeds at cooperative shops and rarely store them. Nursery installation starts end of the rainy season (November–December) and is repeated during all dry season (November–May). After one month in nursery, cabbage seedlings are transferred onto big beds for two-three months before harvesting. Cabbage plants are often attacked by a number of insect pests, viz. the diamondback moth, P. xylostella, the corn earworm, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae), and the cabbage aphid, Brevicoryne brassicae (L.) (Hemiptera: Aphididae). Among these pests, P. xylostella is the most important pest of cabbage in northern Benin. During the field survey, we observed that DBM attacks the growing points, leaves and stems of cabbage plants. Under heavy infestations, the entire plant may become riddled with holes. Cabbage plants develop deformed heads, which encourage soft rots. Diamondback moth incidence in cabbage fields varied between 80-100% and damage severity was between 3 and 4 according to the field, which was very important related to our evaluation scale used. Farmers in this region apply singly and sometimes combination of insecticides such as LAMBDA SUPER 2.5EC (67%), KARATE 2.5 WG (50%) as many as 8–12 times during each cropping period.
3.2 Effect of different application doses of entomopathogenic nematodes against diamondback moth larvae
28Both EPN used infested DBM larvae, but with different mortality rates. When EPN were applied at different rates, the mortality of DBM larvae increased with increasing number of IJs·cm-2 up to a dose of 50 IJs·cm-2 for both nematode species. With H. sonorensis, DBM mortality did not surpass 40% at any rate 24 h after infestation. However, Steinernema sp. 83a caused higher mortality (87%) 24 h after infestation with 50 IJs·cm-2 (Figure 1). Number of nematodes penetrating each DBM larva varies significantly between application doses and EPN species (p ≤ 0.001) (Table 2). Highest number of IJs (ca 9 nematodes per insect larva) of H. sonorensis penetrating insect larva was recorded when 100 IJs·cm-2 were applied. Offspring produced by EPN varied according to nematode species and their application doses. Total population density of Steinernema sp. 83a emerging from DBM larvae differed significantly between application rates and increased with initial nematode application rate (Table 2).
3.3. Effect of exposure time on diamondback moth mortality
29The degree of susceptibility of insect larvae to nematode infection varied with nematode species as well as with exposure time of DBM larvae to EPN. Thirty-five percent larval mortality was observed due to infection with H. sonorensis KF723827 for 1 h exposure time at 50 IJs·cm-2 (Figure 2), whereas Steinernema sp. 83a caused higher larval mortality (64.17%) after 24 h. No significant differences were recorded for H. sonorensis KF723827 when the DBM larvae were exposed for 1 or 3 h to the nematodes. Accordingly, nematode infectivity had occurred within the first hour after application.
30Population density of IJs observed inside DBM larvae after each exposure time varied significantly (p ≤ 0.05) with EPN species (Table 3). The exposure time affected the percent of insect cadavers producing progeny of Steinernema sp. 83a. Twenty percent of dead larvae of DBM did not produce progeny at 1 h exposure time with Steinernema sp. 83a. However, more than 95% of the insect cadavers infected by H. sonorensis KF723827 had progeny at the same exposure time. Mean number of progeny emerged from DBM cadavers did not differ significantly with Steinernema sp. for both exposure time tested (p > 0.05) (Table 3).
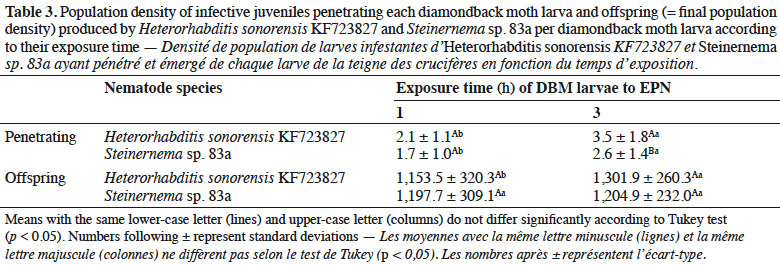
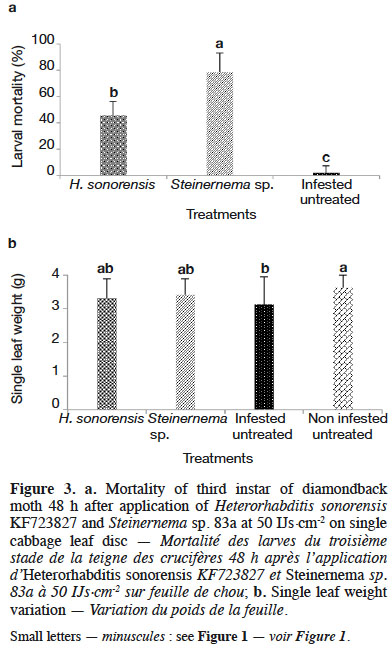
3.4. Efficacy of entomopathogenic nematodes against diamondback moth on single cabbage leaf discs
31Significant differences in DBM mortality were observed between both EPN species tested and the control (i.e. treated only with water) (p = 0.000). Highest DBM mortality (78%) was recorded with Steinernema sp. 83a (Figure 3a). When comparing leaf tissues consumption by DBM larvae, significant differences (p = 0.003) were observed for leaf disc weight following the treatment (Figure 3b).
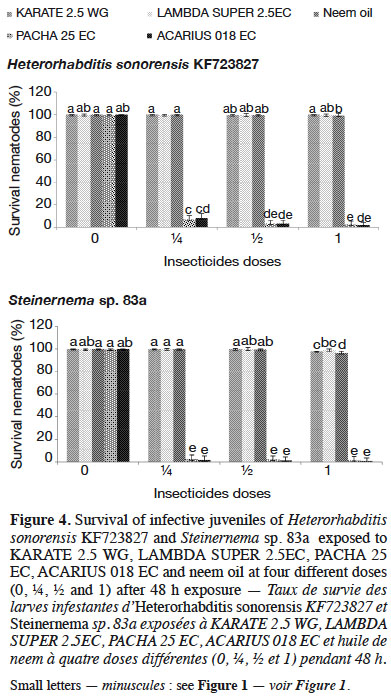
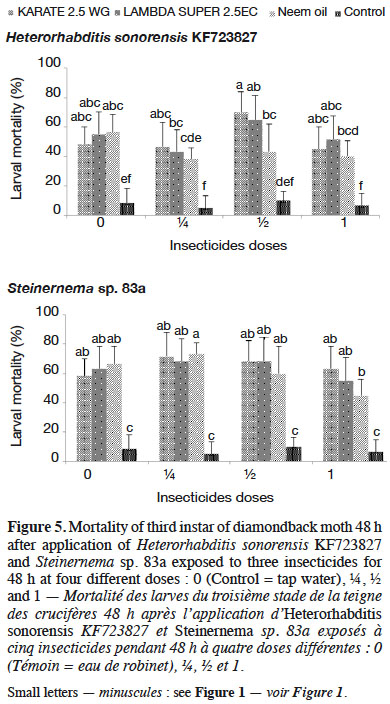
3.5. Effect of five insecticides on entomopathogenic nematodes’ survival, infectivity and reproductive potential
32Among the insecticides studied, PACHA 25EC (lambda-cyhalothrin + acetamiprid) and ACARIUS 018 EC (abamectin) negatively affected both H. sonorensis KF723827 and Steinernema sp. 83a survival 48 h after nematodes’ exposure to different insecticides doses. The percentage of IJs that survived in KARATE 2.5 WG, LAMBDA SUPER 2.5EC and neem oil was highest (> 95%) and was not significantly different from the control (Figure 4). These insecticides did not affect the virulence of H. sonorensis KF723827 and Steinernema sp. 83a. More than 60% larval mortality of DBM was caused by H. sonorensis KF723827 and Steinernema sp. 83a after their exposure to the insecticides at ½ strength of the recommended dose (Figure 5).
33With respect to IJ establishment, these insecticides did not affect the ability of EPN species to infest DBM larvae. For both nematodes, the average number of penetrating IJs was similar to the average number observed in the controls (Table 4).
3.6. Infectivity of Steinernema sp. 83a and KARATE 2.5 WG as foliar spray against diamondback moth larvae on potted cabbage plants under semi-field conditions
34The combined application of KARATE 2.5 WG and Steinernema sp. 83a significantly increased the virulence of this nematode 72 h after application (Figure 6). A 20% increment on DBM mortality was achieved with tested combination compared with the application of either nematodes or KARATE 2.5 WG alone. Population density of nematode IJs observed inside DBM larvae after each treatment was quite different when Steinernema sp. 83a species was used alone or in combination with KARATE 2.5 WG at 0.66 g·l-1 (p < 0.05). The highest value of invading nematodes per DBM larva (3 IJs per larva) was obtained when Steinernema sp. 83a was sprayed in combination with KARATE 2.5 WG.
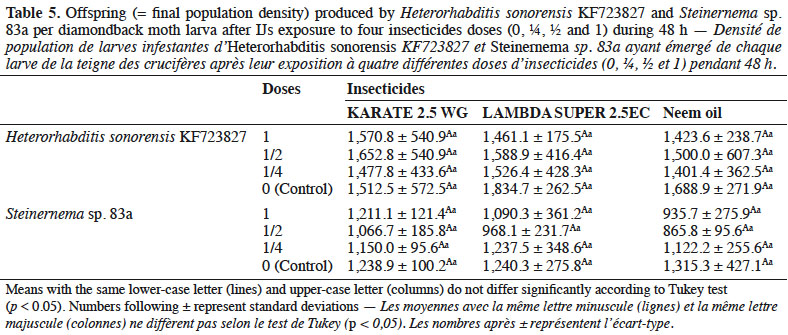
35The trends observed in the data suggested that control plants sprayed with water only recorded maximum damage with 323 feeding spots per plant. The treatments receiving foliar spray of Steinernema sp. 83a at 50 IJs·cm-2 and IJs in combination with KARATE 2.5 WG at 0.66 g·l-1 recorded 122 and 66 feeding spots, respectively (Table 5). Reduction in plant damage was 62 and 34% by treatments with 50 IJs·cm-2 and KARATE 2.5 WG at 2.5%, respectively; the maximum damage reduction (80%) was recorded with 50 IJs·cm-2 in combination with KARATE 2.5 WG at 0.66 g·l-1.
4. Discussion
36In Benin, yield loss of cabbage crops due to DBM has not been estimated. Notwithstanding this, DBM has been identified as the pest of most concern to cabbage farmers (James et al., 2010). Our investigation in northern Benin supports this and even though fields were treated with a variety of agrochemical insecticides, crop loss was frequently recorded by cabbage producers. Most of the time, cabbage farmers used two different insecticides, which contain lambda-cyhalothrin as active substance. This overuse of lambda-cyhalothrin could lead to the problem of DBM developing resistance (Phani Kumar & Gujar, 2005).
37This study provides the first evaluation of efficacy of EPN against DBM larvae in West Africa. Results from the IJs doses screening showed that DBM larvae are highly susceptible to infection by EPN at 50 and 100 IJs·cm-2, this implies that DBM larvae were favorable hosts of the two EPN species tested and that the symbiotic bacteria associated with the nematodes are lethal to the larvae. These findings are in agreement with data from the literature on Steinernema spp. and Heterorhabditis spp. against DBM larvae (Correa-Cuadros et al., 2014; Sunanda et al., 2014). However, major differences between EPN species were revealed. Indeed, Steinernema sp. strain 83a is more infective against DBM larvae than H. sonorensis strain KF723827. Current results appear in contrast to the results of Baur et al. (1995) and Mason & Wright (1997) who found that a Heterorhabditis sp. caused higher mortality of DBM larva than a Steinernema sp. Whereas, Sunanda et al. (2014) reported that the mortality rate of DBM larvae after 72 h was 100 % with Steinernema carpocapsae (1,000 IJs per Petri plate) although this application rate was lower than those used in this study.
38As it is often the case, DBM larval mortality caused by EPN in our semi-field experiment was lower than that seen in the laboratory when single cabbage leaf disc was used in Petri dish. Nyasani et al. (2008) explained that in leaf disc assays nematodes were confined in close proximity with the DBM larvae whereas this was not the case in the semi-field conditions. A further reason for lower semi-field efficacy may be that DBM larvae cease feeding and may even close their spiracles when sprayed with excessive amounts of water (Baur et al., 1995). However heat and desiccation are two major stress factors that reduce survival and efficiency of EPN and lower their shelf life (Zadji et al., 2013).
39The results from our studies on the invasion time of P. xylostella showed significant differences among Steinernema sp. 83a and H. sonorensis KF723827. This is in agreement with Glazer (1992) who revealed a large variability in invasion capacities among different nematode isolates, suggesting that measuring invasion time could be a useful strategy for the selection of virulent strains of EPN. Hominick & Reid (1990) proposed the use of invasion efficiency as a direct measure of nematode infectivity.
40These authors assumed that the nematodes with the greatest efficacy against a target insect would have the highest invasion efficiency. In this respect, based on DBM mortality Steinernema sp. 83a could be the most virulent. As the application rate of Steinernema sp. 83a and H. sonorensis KF723827 increased, the number of invader IJs per larva also increased. The treatment with 25 IJs·cm-2 recorded the lowest penetration rate of IJs, whereas the highest number of invader IJs per larva was recorded at 100 IJs·cm-2. According to Bastidas et al. (2014), the number of invading IJs per larva increases as the number of application rate increases.
41The data in this study suggest that following application rate and types of experiment, both EPN tested were able to infect and propagate within DBM larvae and produce IJs. However, the highest production of IJs was obtained with H. sonorensis KF723827, which yielded 2 x 103 IJs·larva-1 at 100 IJs·cm-2. Differences in reproduction between the two nematode species recorded at the 25 and 50 IJs·cm-2 have been documented for many insect hosts (Ali et al., 2006). According to Yadav & Lalramliana (2012), Heterorhabditis indica and Steinernema thermophilum exhibited a linear relationship between the rates of IJs applied and total number of IJs produced per infected larva but it was not the case when Steinernema glaseri was used. Loya & Hower (2003) reported that the size and behaviour of nematode species may account for differences in nematode ability to reproduce in the host. A higher production of IJs by H. sonorensis KF723827 in this study may also be attributed, in part, to the fact that heterorhabditids being hermaphroditic are likely to contribute to more progeny than steinernematids which are amphimictic (Mannion & Jansson, 1992).
42Some of the insecticides tested in this study may be harmful to the EPN. For instance in the current study PACHA 25 EC (lambda-cyhalothrin + acetamiprid) and ACARIUS 018 EC (abamectin) affect significantly Steinernema sp. 83a and H. sonorensis KF723827 survival when IJs were exposed to these active substances for 48 h. Laznik & Trdan (2014) showed that abamectin influenced the mortality of Heterorhabditis bacteriophora. Moreover, the ingredients abamectin have a well-known nematicide effect (Mahfooz et al., 2008), which may explain the relatively high mortality values observed for both EPN species used. In their laboratory experiment, Head et al. (2000) reported that direct exposure of S. feltiae to abamectin reduces significantly IJs infectivity which rendered the nematodes unsuitable for commercial use. However, neem oil (azadirachtin and other compounds), LAMBDA SUPER 2.5EC and KARATE 2.5 WG (lambda-cyhalothrin) have negligible effects on EPN survival comparatively to the control where only distilled water was used. These results are in agreement with the findings of other authors (Yan et al., 2012; Laznik & Trdan, 2014) who reported that azadirachtin had no adverse effects on EPN survival and infectivity. In contrast, Laznik & Trdan (2014) mentioned that lambda-cyhalothrin affects EPN survival at moderate rate. Nevertheless the same author concluded that compatibility of EPN with pesticides is not only a species-specific, but also a strain-specific characteristic. This is in agreement with Radová (2011) who mentioned that it is hard to explain the differential reaction of EPNs with different pesticides, but these findings show that different nematodes species/strain can react to the same chemicals differently. Atwa et al. (2013) reported that no toxic effect of several insecticides, fungicides and mineral oils on nematodes survival, infectivity and reproduction were observed.
43In semi-field experiments, the combination of Steinernema sp. 83a and lambda-cyhalothrin at 1.25% had a synergistic or additive effect on DBM mortality. The feasibility of this combination is enhanced by the compatibility of these agents in sprayer mixes and the lack of insecticide effect on nematode reproduction and fitness with DBM larvae. Furthermore, combination caused mortality to occur more rapidly than for the single Steinernema sp. 83a application. Negrisoli et al. (2010) reported that KARATE ZEON 250 CS (lambda-cyhalothrin formulated in microcapsules) when combined at double doses caused the highest IJs mortality (52.6%) of H. indica. However, the association of KARATE ZEON 250 CS insecticide with S. glaseri caused the highest infectivity (91.6%) of EPN.
44It can be concluded from this study that EPN can offer effective control of DBM larvae and prevent serious damage to cabbage crops. Moreover, some of insecticides used against DBM in northern Benin do not affect EPN effectiveness, which can therefore be used in IPM programs. Because this study was conducted under laboratory and semi-field conditions, effectiveness of EPN might need to be verified in extensive field.
Acknowledgements
45This work was supported by Pesticide Science Laboratory, Gembloux Agro-Bio Tech/University of Liege/Belgium and Plant Protection Laboratory of Higher National School of Agronomic Sciences and Techniques of Djougou/University of Parakou/Benin. Thanks and gratitude to farmers and extension officers who made the study a success.
Bibliographie
Abbott W.S., 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol., 18(2), 265-267.
Ali S.S., Perveza R., Hussain M.A. & Ahmad R., 2006. Susceptibility of three lepidopteran pests to five entomopathogenic nematodes and in vivo mass production of these nematodes. Arch. Phytopathol. Plant Prot., 41, 300-304.
Atwa A.A., Shamseldean M.M. & Yonis F.A., 2013. The effect of different pesticides on reproduction of entomopathogenic nematodes. Turk Entomol. Dergisi, 37(4), 493-502.
Baimey H. et al., 2015. Influence of pesticides, soil temperature and moisture on entomopathogenic nematodes from southern Benin and control of underground termite nest populations. Nematology, 17, 1057-1069.
Bajc N., Držaj U., Trdan S. & Laznik Ž., 2017. Compatibility of acaricides with entomopathogenic nematodes (Steinernema and Heterorhabditis). Nematology, 19(8), 891-898.
Bastidas B., Portillo E. & San-Blas E., 2014. Size does matter: the life cycle of Steinernema spp. in micro-insect hosts. J. Invertebr. Pathol., 121, 46-55.
Baur M.E., Kaya H.K. & Thurston G.S., 1995. Factors affecting entomopathogenic nematode infection of Plutella xylostella on a leaf surface. Entomol. Exp. Appl., 77, 239-250.
Correa-Cuadros J.P., Rodríguez-Bocanegra M.X. & Sáenz-Aponte A., 2014. Susceptibility of Plutella xylostella (Lepidoptera: Plutellidae; Linnaeus 1758) to Beauveria bassiana Bb9205, Metarhizium anisopliae Ma9236 and Heterorhabditis bacteriophora HNI0100. Univ. Sci., 19(2), 277-285.
Garcia-del-PinoF. & Jove M., 2005. Compatibility of entomopathogenic nematodes with fipronil. J Helminthol., 79, 333-337.
Glazer I., 1992. Invasion rate as a measure of infectivity of steinernematid and heterorhabditid nematodes to insects. J. Invertebr. Pathol., 59, 90-94
Head J., Walters F.K.A. & Langton S., 2000. The compatibility of the entomopathogenic nematode Steinernema feltiae, and chemical insecticides for the control of the South American leafminer, Liriomyza bhuidobrensis. Biol. Control, 45, 345-353.
Hominick W.M. & Reid A.P., 1990. Perspectives on entomopathogenic nematology. In: Gaugler R. & Kaya H.K., eds. Entomopathogenic nematodes in biological control. Boca Raton, FL, USA: CRC Press, 327-345.
James B. et al., 2010. Integrated pest management in vegetable production: a guide for extension workers in West Africa. Ibadan, Nigeria: International Institute of Tropical Agriculture (IITA).
Kaya H.K. & Stock S.P., 1997. Techniques in insect nematology. In: Lacey L.A., ed. Manual of techniques in insect pathology. Elsevier, 281-324.
Kermani N. et al., 2013. Pathogenicity of Nosema sp. (Microsporidia) in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). PloS One, 8(5), e62884.
Koppenhöfer A.M. & Grewal P.S., 2005. Compatibility and interactions with agrochemicals and other biocontrol agents. In: Grewal P.S., Ehlers R. & Shapiro-Ilan D.I., eds. Nematodes as biocontrol agents. Wallingford, UK: CABI Publishing, 363-381.
Laznik Z., Vidrih M. & Trdan S., 2012. The effects of different fungicides on the viability of entomopathogenic nematodes Steinernema feltiae (Filipjev), S. carpocapsae Weiser, and Heterorhabditis downesi (Nematoda: Rhabditida) under laboratory condition. Chil. J. Agric. Res., 72(1), 62-67.
Laznik Z. & Trdan S., 2014. The influence of insecticides on the viability of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) under laboratory conditions. Pest Manage. Sci., 70(5),784-789.
Loya L.J. & Hower A.A., 2003. Infectivity and reproductive potential of the Oswego strain of Heterorhabditis bacteriophora associated with life stages of the clover root curculio, Sitona hispidulus. J. Invertebr. Pathol., 83, 63-72.
Mahfooz A. et al., 2008. Prevalence and athelmintic efficacy of abamectin against gastrointestinal parasites in horses. Pak. Vet. J., 28, 76-78.
Mannion C.M. & Jansson R.K., 1992. Comparison of ten entomopathogenic nematodes for control of sweet potato weevil (Coleoptera: Apionidae). J. Econ. Entomol., 85, 1642-1650.
Mason J.M. & Wright D.J., 1997. Potential for the control of Plutella xylostella larvae with entomopathogenic nematodes. J. Invertebr. Pathol., 70, 234-242.
Navarro P.D., McMullen J.G. & Stock S.P., 2014. Effect of dinotefuran, indoxacarb, and imidacloprid on survival and fitness of two Arizona-native entomopathogenic nematodes against Helicoverpa zea (Lepidoptera: Noctuidae). Nematropica, 44, 64-73.
Navon A. & Ascher K.R.S., 2000. Bioassays of entomopathogenic microbes and nematodes. CABI Publishing.
Negrisoli A., Garcia M.S. & Barbosa Negrisoli C.R.C., 2010. Compatibility of entomopathogenic nematodes (Nematoda: Rhabditida) with registered insecticides for Spodoptera frugiperda (Smith, 1797) (Lepidoptera: Noctuidae) under laboratory conditions. Crop Prot., 29(6), 545-549.
Nyasani J.O., Kimenju J.W., Olubayo F.M. & Wilson M.J., 2008. Laboratory and field investigations using indigenous entomopathogenic nematodes for biological control of Plutella xylostella in Kenya. Int. J. Pest Manage., 54(4), 355-361.
Phani Kumar K. & Gujar G.T., 2005. Baseline susceptibility of the diamondback moth, Plutella xylostella (Linnaeus) to Bacillus thuringiensis Cry1A toxins in India. Crop Prot., 25, 207-212.
Radová Š., 2011. Effect of selected pesticides on survival and virulence of two nematodes species. Pol. J. Environ. Stud., 20(1), 181-185.
San-Blas E., 2013. Progress on entomopathogenic nematology research: a bibliometric study of the last three decades: 1980–2010. Biol. Control, 66(2), 102-124.
Sarfraz M., Dosdall L.M. & Keddie B., 2006. Diamondback moth–host plant interactions: implications for pest management. Crop Prot., 25(7), 625-639.
Shapiro-Ilan D.I. et al., 2009. Characterization of biocontrol traits in the entomopathogenic nematode Heterorhabditis georgiana (Kesha strain), and phylogenetic analysis of the nematode’s symbiotic bacteria. Biol. Control, 51(3), 377-387.
Shapiro-Ilan D.I., Hèan R. & Dolinksi C., 2012. Entomopathogenic nematode production and application technology. J. Nematol., 44(2), 206-217.
Speijer P.R. & De Waele D., 1997. Inibap Technical Guidelines. 1. Screening for Musa germplasm for resistance and tolerance to nematodes. Roma: IPGRI; Montpellier, France: INIBAP.
Spence K.O. et al., 2011. Effect of insect cadaver desiccation and soil water potential during rehydration on entomopathogenic nematode (Rhabditida: Steinernematidae and Heterorhabditidae) production and virulence. J. Invertebr. Pathol., 106(2), 268-273.
Sunanda B.S., Jeyakumar P. & Jacob V.V., 2014. Bioefficacy of different formulations of entomopathogenic nematode Steinernema carpocapsae against diamond back moth (Plutella xylostella) infesting cabbage (Brassica oleracea var. capitata). J. Biopest., 7(2), 209-214.
Syed T.S., Abro G.H., Awan M.S. & Sattar M., 2012. The role of natural enemies to control diamondback moth, Plutella xylostella (L.) population in various seasons. Pak. J. Zool., 44(6), 1479-1485.
Thanwisai A. et al., 2012. Diversity of Xenorhabdus and Photorhabdus spp. and their symbiotic entomopathogenic nematodes from Thailand. PloS One, 7(9), e43835.
White G.F., 1927. A method for obtaining infective nematode larvae from cultures. Science, 66, 302-303.
Wright D., 2004. Biological control of DBM: a global perspective. In: Bordat D. & Kirk A.A., eds. Proceedings of the International Symposium in Montpellier, Improving biocontrol of Plutella xylostella, 21-24 Oct 2002, Montpellier, France. Montpellier, France: CIRAD, 9-14.
Yadav A.K. & Lalramliana, 2012. Soil moisture effects on the activity of three entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) isolated from Meghalaya. J. Parasitic Dis., 36(1), 94-108.
Yan X. et al., 2012. Effects of selected insecticides on osmotically treated entomopathogenic nematodes. J. Plant Dis. Prot., 119(4), 152-158.
Zadji L. et al., 2013. First record on the distribution of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Southern Benin. Russ. J. Nematol., 21(2), 117-130.
Zalucki M.P. et al., 2012. Estimating the economic cost of one of the world's major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): just how long is a piece of string? J. Econ. Entomol., 105(4), 1115-1129.






