- Portada
- Volume 23 (2019)
- Numéro 3
- Aerobic fermentation prior to pasteurization produces a selective substrate for cultivation of the mushroom Pleurotus pulmonarius
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Aerobic fermentation prior to pasteurization produces a selective substrate for cultivation of the mushroom Pleurotus pulmonarius

Notes de la rédaction
Received 23 May 2018, accepted 22 July 2019, available online 6 September 2019
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
Résumé
La fermentation aérobie avant la pasteurisation produit un substrat sélectif pour la culture du champignon Pleurotus pulmonarius
Description du sujet. Les espèces de Pleurotus sont cultivées sur des substrats lignocellulosiques, où des champignons contaminants tels que Trichoderma spp. sont communs. Un substrat sélectif pour Pleurotus doit fournir les conditions nécessaires à la protection contre les contaminants. Des études ont montré que Paenobacillus polymyxa et d'autres bactéries thermophiles peuvent participer à la sélectivité du substrat de culture pour Pleurotus, mais on sait peu de choses sur les communautés bactériennes impliquées dans ces différents substrats.
Objectifs. Évaluer l'effet de l'inoculation de P. polymyxa dans un substrat de culture sur la productivité de P. pulmonarius et sur la protection contre Trichoderma.
Méthode. De la paille d'orge inoculée avec P. polymyxa et de la paille non inoculée (témoin) ont été utilisées après 0, 3 ou 5 jours de fermentation avant un traitement thermique pour produire le substrat de culture. Le contenu microbiologique avant et après traitement thermique, la colonisation mycélienne par P. pulmonarius et Trichoderma viride en compétition et le rendement de P. pulmonarius ont été évalués.
Résultats. Nous avons observé que l'inoculation avec P. polymyxa augmentait le nombre de bactéries cultivables et modifiait la composition de la communauté microbienne. Elle diminue la capacité de colonisation de T. viride et favorise la croissance mycélienne. Cependant, le rendement en champignons a été affecté. Les rendements plus élevés de P. pulmonarius ont été obtenus dans le substrat témoin où aucune contamination de Trichoderma spp. n’a été observée.
Conclusions. L'ajout de P. polymyxa a modifié la succession microbiologique naturelle lors d’une fermentation de paille d'orge pendant 5 jours, favorisant la compétitivité de P. pulmonarius par rapport à T. viride. La fermentation de la paille d'orge pendant les 3 jours qui suivent un traitement thermique est bénéfique pour le rendement et la protection de P. pulmonarius contre T. viride.
Abstract
Description of the subject. Pleurotus species are cultivated on lignocellulosic substrates, in which contaminant fungi such as Trichoderma spp. are common. A selective substrate for Pleurotus provides the necessary conditions for protection against contaminants. Studies show that Paenibacillus polymyxa and other thermophilic bacteria benefit from the selectivity of Pleurotus cultivation substrate, however, little is known regarding these bacterial communities.
Objectives. To evaluate the effect of substrate inoculation with Paenibacillus polymyxa on the productivity of Pleurotus pulmonarius and its protection against Trichoderma.
Method. Barley straw inoculated with P. polymyxa and non-inoculated straw (control) was used following 0, 3 or 5 days of fermentation prior to heat treatment in order to produce the cultivation substrate. The microbiological content before and after the heat treatment, the mycelial colonization by P. pulmonarius and Trichoderma viride in competition and the yield of P. pulmonarius were all evaluated.
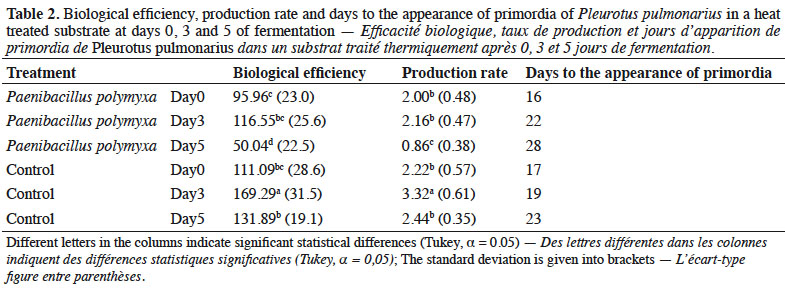
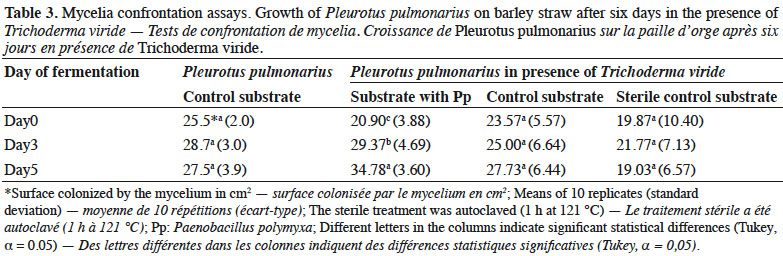
Results. We observed that inoculation with P. polymyxa increased the number of cultivable bacteria and changed the composition of the community. The inoculation decreased the colonization ability of T. viride and favored the mycelial growth, although the yield of mushrooms was affected. Higher yields of P. pulmomarius were obtained in the control substrate where no contamination of Trichoderma spp. was observed.
Conclusions. Addition of P. polymyxa modified the natural microbiological succession in a fermentation of barley straw for 5 days, favoring the competitiveness of P. pulmonarius against T. viride. Fermentation of barley straw for 3 days, followed by heat treatment, benefits the yield and protection of P. pulmonarius against Trichoderma viride.
Tabla de contenidos
1. Introduction
1Pleurotus species hold second place in terms of production of edible mushrooms worldwide, with 19% of the total production, exceeded only by the shiitake mushroom, Lentinula edodes (Berk.) Pegler (22% of total production). The popular white button mushroom, Agaricus bisporus (J.E. Lange) Imbach, now occupies fourth place after the Auricularia species (Royse et al., 2017). Pleurotus spp. are commonly cultivated on lignocellulosic substrates, where a recurring problem is the presence of competing molds of the genus Trichoderma (Sánchez et al., 2007), causing green mold disease. Following addition of water to the lignocellulosic materials, several methods are used by mushroom producers to prepare the substrate in order to eliminate any disease and insect problems that may occur during the culture, including chemical disinfection, sterilization, cooking in hot water, steam pasteurization and aerobic fermentation (Hernández et al., 2003; Sánchez et al., 2012). However, it is not clear which substrate type guarantees high biological selectivity for Pleurotus spp. and prevents the manifestation of competing fungi (Muez-Orobia & Pardo-Nuñez, 2001).
2A substrate with biological selectivity may contain microbial communities rich in bacteria, Actinomycetes and thermophilic fungi as protectors; these are all non-competitors for the cultivated mushroom. In the case of Pleurotus, different studies show the importance of beneficial thermophilic bacteria such as Paenibacillus polymyxa (Prazmowski) Macé (Stolzer & Grabbe, 1991; Velázquez-Cedeño et al., 2006a; Velázquez-Cedeño et al., 2006b), which consume the sugars that could be a resource for contaminating microorganisms or antagonists during incubation. They also produce metabolites that provide selectivity for development of Pleurotus. As well as P. polymyxa, Bacillus spp. are involved in selectivity by inhibiting the growth of Trichoderma harzianum Rifai and stimulating the defenses of Pleurotus ostreatus (Jacq.) P. Kumm. through the induction of laccases (Nagy et al., 2012; Mwangi et al., 2017).
3Bioprotection by inoculation of beneficial microorganisms is a promising way to increase selectivity in Pleurotus substrates. For instance, immersing paddy straw in boiling water for 30 min and inoculating it with Pseudomonas fluorescens Migula or Bacillus subtilis (Ehrenberg) Cohn isolates reduced green mold intensity and enhanced the yield of Pleurotus sajor-caju (Fr.) Singer, compared to non-inoculated straw (Shah & Nasreen, 2011). Aerobic fermentation of the substrate is also known to permit development of a thermophilic bacterial community. The native microorganisms of the substrate metabolize the available components and heat is generated as a consequence. The substrate itself then reaches temperatures of between 50°C and 70°C for several days. This condition provides a favorable microhabitat for thermophilic microorganisms. Thus, short composting is successfully applied in the preparation of selective substrates for the industrial production of Pleurotus spp. (Villa Cruz et al., 1999; Hernández et al., 2003; Castañeda-de-Leon & Leal-Lara, 2007).
4Controlling the microbial community during aerobic fermentation in short composting of substrate for Pleurotus cultivation can optimize its development, increase production and reduce the risk of contamination by Trichoderma spp. (Velázquez-Cedeño et al., 2008). The aim of the present study was to evaluate the effects of a cultivation substrate preparation that combines an aerobic fermentation phase with a P. polymyxa strain and steam pasteurization, in terms of its efficiency at protecting against Trichoderma viride Pers ex S.F. Gray and on the productivity of Pleurotus pulmonarius (Fr.) Quél.
2. Materials and methods
2.1. Biological material
5The CDBB35 strain of Paenibacillus polymyxa, provided by the Center for Research and Advanced Studies (CINVESTAV) of Zacatenco, Mexico City, was used. The strain was maintained at 4 °C on 2.4% nutrient agar (AN) (DIFCO®) with 10% glycerol. The strain IE-115 of Pleurotus pulmonarius, obtained from the collection of the Institute of Ecology A.C. (INECOL), Xalapa, Veracruz was studied. It was preserved in Potato Dextrose Agar (PDA) medium at 4 °C and propagated to PDA, with incubation for 14 days at 26 °C. Sorghum seeds (Sorghum vulgare Pers.) were used for the preparation of primary and secondary inoculum (Guzmán et al., 2013). Trichoderma viride (strain IE-637), isolated from a Pleurotus substrate produced at INECOL in 1999 was used, and conserved lyophilized in sorghum grains at 4 °C.
2.2. Preparation of the inoculum of Paenibacillus polymyxa
6Paenibacillus polymyxa was first grown on 2.4% AN (DIFCO®) medium and incubated at 28 °C for two days. It was then further developed in 250 ml Erlenmeyer flasks with 80 ml liquid medium of 0.1% AN. The flasks were shaken at 200 rpm for five days and the contents were then centrifuged at 10,000 rpm for 10 min. The supernatant was removed, and the pellet was suspended in 40 ml of 10% semi-skimmed milk (LALA™). A second centrifugation was performed at 10,000 rpm for 10 min. The supernatant was removed and the precipitate with the bacteria was lyophilized (modified from Requena et al., 1996; Korsten & Cook, 1996).
2.3. Fermentation and heat treatment of the substrate
7One hundred kg (wet weight, 70% ± 5) of barley straw (Hordeum vulgare L.) were composted for each treatment. For the experimental treatment, 500 ml of a suspension of P. polymyxa were added at a concentration of 1.73 x 103 units·ml-1 on day 0. For the control, 500 ml of water were added. In both treatments, the substrate was placed into a metal container 2 m in length x 1 m in width x 1 m in height. This was subjected to a fermentation of 5 days, with the substrate mixed on day 3 to promote aeration and a daily superficial irrigation to maintain the humidity at 70%.
8On days 0, 3 and 5, 25 kg of substrate (wet weight) were taken from both the inoculated substrate and the control, and a heat treatment of steam pasteurization for 12 h at 60 °C applied to these samples, followed by a period of 24 h at 48 °C (modified from Castañeda-de-Leon & Leal-Lara, 2007; Velázquez-Cedeño et al., 2008). These substrates were used for culture, microbiological analysis and confrontation tests.
9Maximum and minimum temperatures, moisture content and pH of the substrate were recorded daily. The temperature was recorded 3 times daily with a thermometer introduced into the center of the mound. The moisture content of the substrate was estimated by weight difference, for which 20 g of straw (wet weight) were taken and dried in an oven for 24 h at 50 °C. To measure the pH of the substrate, 10 g of straw were placed in a flask with 20 ml sterile distilled water (v:v 1:2) and the pH determined with a potentiometer (Hanna®). For both measurements (humidity and pH), the samples were processed in triplicate.
2.4. Analysis of bacteria groups
10The presence of microbial populations in the substrate was quantitatively analyzed by counting the Colony Forming Units (CFU) of total microflora (TM), Bacillus, Pseudomonas and Actinomycetes using specific culture media in Petri dishes. For TM, peptone agar medium (0.5% peptone, 0.5% yeast, 1% glucose, 1.5% agar) was used at pH 7.6. For Bacillus, the same medium as for TM was used, but following sterilization: 0.1% of sterile ketoconase was added when the medium reached a temperature of 50 °C. For Pseudomonas, the medium used was 2% peptone, 0.14% MgCl2, 1% K2SO4 and 1.36% agar, and for Actinomycetes, a modified medium of Pochon and Tardieux (1962) was used (1% glycerol, 0.1% L-aspargine, 0.1% K2HPO4, 1.5% agar and 0.1% K2Cr2O7).
11The bacterial groups were analyzed in the substrate with P. polymyxa and in the control on days 0, 3 and 5 of fermentation and following the heat treatment. For each sampling point, a 20 g subsample of the substrate was taken from different parts of the mound and mixed in a blender with 180 ml of 0.05% sterile Tween 80® to obtain a stock solution, which was liquefied twice for 10 seconds at 1 min intervals. Dilutions up to 10-5 (modified from Velázquez-Cedeño et al., 2004) were made from this solution. For Bacillus, the stock solution was incubated in a water bath for 10 min at 80 °C and dilutions (10-5) were carried out. Fifty microliters were taken from each dilution and inoculated on specific culture media, homogeneously spread on the medium with the aid of sterile L-shaped glass rods. This was performed in triplicate for each group. The Petri dishes were incubated at 25 °C and 48 °C to favor development of the mesophilic and thermophilic microflora, respectively. A CFU count was performed at 24 h of incubation and the results were expressed as CFU per gram of dry substrate. The data were subjected to a variance analysis (ANOVA) and the difference in the means was estimated by a Tukey post hoc multiple range test (α = 0.05%) with the software Statistica® ver. 6.1.
2.5. Pleurotus production
12Production of P. pulmonarius was evaluated on cultivation substrates after the heat treatment of barley straw. The culture was carried out using substrate from the mound in fermentation on days 0, 3 and 5, both from the substrate inoculated with P. polymyxa and that of the control.
13Samples of 2 kg of substrate were spawned with 5% barley grains colonized by P. pulmonarius mycelium in bags of 20 × 30 cm. The bags were incubated in the dark at 24 °C for three weeks. The samples were placed in a room with ventilation and artificial lighting (automatic system featuring 12 h of light: 12 h of darkness) at 26 °C and relative humidity between 75% and 80% (automatic nebulization) for fruiting body production. The plastic bags were removed when colonization finished. Fruiting bodies were harvested for 40 days. Biological Efficiency (BE) was calculated as the fresh weight of fruiting bodies (g) produced on 100 g (dry weight) of substrate. Production Rate (PR) was estimated as the biological efficiency per day of production.
14The experimental design was completely random, consisting of six treatments: a substrate inoculated with P. polymyxa and a control substrate on days 0, 3 and 5. Ten replicates were conducted for each treatment. The BE and TP were subjected to an ANOVA and differences in the means identified by Tukey post hoc multiple range test (α = 0.05%) with Statistica® software ver. 6.1.
2.6. Mycelial confrontation of Pleurotus pulmonarius vs Trichoderma viride
15For the reactivation of T. viride, the flask with freeze-dried grains was immersed in a water bath for 10 min at 25 °C, and the seeds then incubated on PDA at 25 °C for three days. Two days prior to using the strain, an implant (0.7 cm Ø) of medium colonized with T. viride was taken and inoculated on 1.5% agar-water medium to obtain mycelium without spores (modified from Pérez-Merlo & Mata, 2002).
16Fermented substrates (with P. polymyxa and the control) were used after the heat treatment on days 0, 3 and 5 of fermentation. In addition, some parts of the same substrates were sterilized (121 °C for 1 h) in order to observe the effect of absence of the bacterial community on the development of P. pulmonarius and T. viride. For the confrontations, 10 g of straw from each treatment were placed in a Petri dish under sterile conditions. One barley grain of spawn of P. pulmonarius was placed at 1 cm from the border and, 1 cm from the edge at the opposite side, a T. viride implant (0.7 cm Ø) developed in agar-water was placed. Ten replicates were conducted per treatment.
17For each treatment, the mycelial growth of P. pulmonarius and T. viride was measured, estimating the area occupied by each on days 3, 5 and 7 of incubation at 25 °C. The areas occupied were digitized and measured using Adobe Photoshop CS3 extended 10.0 software. The data were subjected to an ANOVA and the difference in the means identified by Tukey post hoc multiple range test (α = 0.05%) with Statistica® software ver. 6.1.
3. Results
3.1. Physicochemical parameters during the fermentation process
18The treatment with P. polymyxa (Pp) showed a faster increase of temperature in 24 h compared to the control. However, the maximum temperature was 55 °C on day 4 while the control reached a maximum of 62 °C on day 5. The pH was gradually alkalized in both treatments, but the inoculated substrate presented a higher pH than the control. The moisture content was maintained at between 76-83% (Table 1).
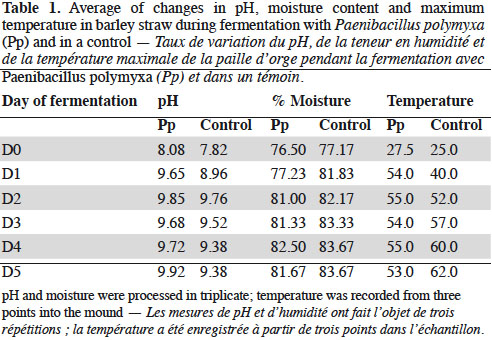
3.2. Development of microorganisms
19The fermentation progressively increased the amount of microorganisms; however, the heat treatment promoted the development of thermophilic groups (Figures 1 and 2) and decreased the mesophilic groups (Figures 3 and 4). The inoculated substrate showed significant differences in the thermophilic Bacillus after heat treatment on days 1 and 5 (Figure 2), while no differences were observed in the control substrate (Figure 1).
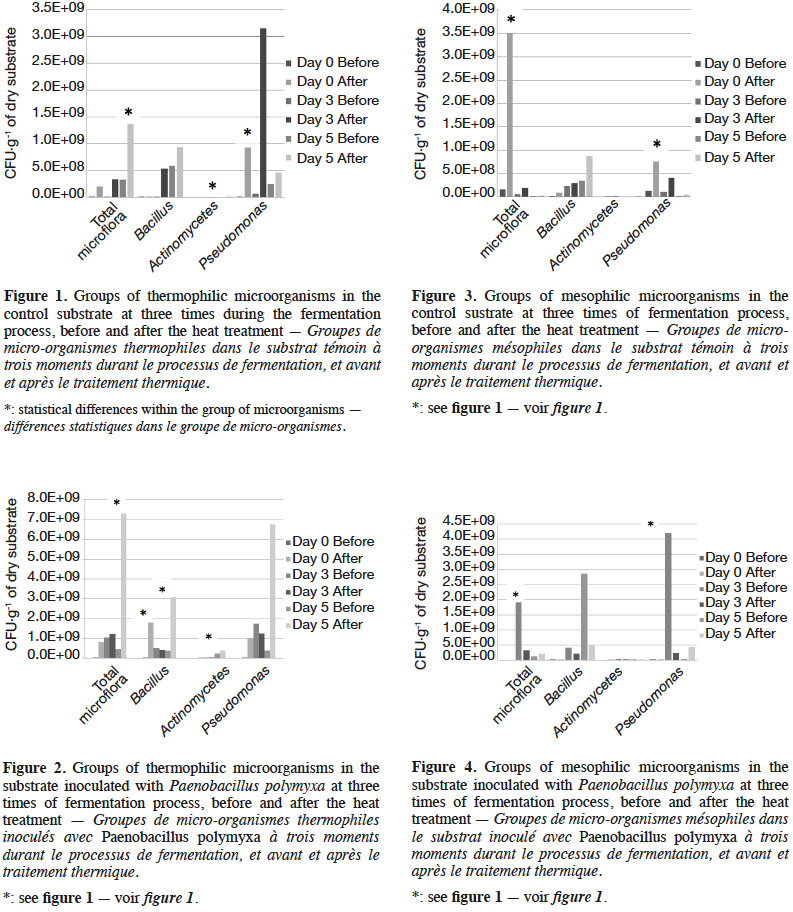
20The development of microorganisms was different in the control compared to the inoculated substrate. The highest amount of the thermophilic Pseudomonas was presented on day 5 in the inoculated substrate, but with no statistical differences (Figure 2). The control substrate showed an increase in this group after heat treatment, and significant differences were observed on day 3 (Figure 1).
21Mesophilic microorganisms also increased during the fermentation process. The inoculated substrate showed a significant increase on day 3 for total microflora, Actinomycetes and Pseudomonas, compared with the control substrate. However, after heat treatment, these groups decreased. On the other hand, in the control substrate, the heat treatment led to a significant increase of the total microflora and Pseudomonas on day 1 (Figures 3 and 4).
22Thermophilic microflora increased clearly (Figures 1 and 2), with significant differences on day 5 after the heat treatment compared to other days in both the inoculated and the control substrates. Thermophilic Actinomycetes increased on day 5 after heat treatment in the inoculated substrate.
3.3. Production of Pleurotus pulmonarius
23The highest Biological Efficiency (BE) value was 169% in the control (Table 2) at 3 days of fermentation (169%), while the lowest was 50% in the treatment with Pp at 5 days of fermentation. Both presented significant differences from the rest of the treatments (p < 0.05). The BE of day 0 and 5 of the control substrate was statistically similar to day 3 of the inoculated substrate. The highest Production Rate (PR) was 3.32 in the control substrate at 3 days of fermentation and the lowest was 0.86 in the treatment with Pp at 5 days of fermentation. Days 0 and 3 in the treatment with Pp substrate and days 0 and 5 of the control did not differ statistically.
24The shortest time for appearance of primordia (16 days) was in the inoculated substrate on day 0 of fermentation. The days of occurrence of primordia increased in line with the days of fermentation, where the longest time for primordia appearance (28 days) was observed in the treatment inoculated with P. polymyxa at 5 days of fermentation (Table 2).
3.4. Confrontation of P. pulmonarius and T. viride
25Pleurotus pulmonarius in the presence of T. viride showed the highest surface colonization (34.78 cm2) on the substrate with Pp on day 5, followed by day 3 (29.37 cm2) and day 0 (20.9 cm2), with significant differences (Table 3). No statistical differences were observed in the other treatments (the control and sterile control substrates). However, the average mycelial extension of P. pulmonarius in the presence of T. viride on the control substrate was similar to the treatment where P. pulmonarius was cultivated alone. The lowest mycelial growth of P. pulmonarius in the presence of T. viride was recorded in the sterilized control.
26Mycelial growth of T. viride showed greater inhibition in the substrate with Pp and control than in the sterile substrates (Table 4). Specifically, days 3 and 5 of fermentation presented the highest inhibition in both substrates, which differed statistically from that of day 0 of fermentation and the sterile substrate.
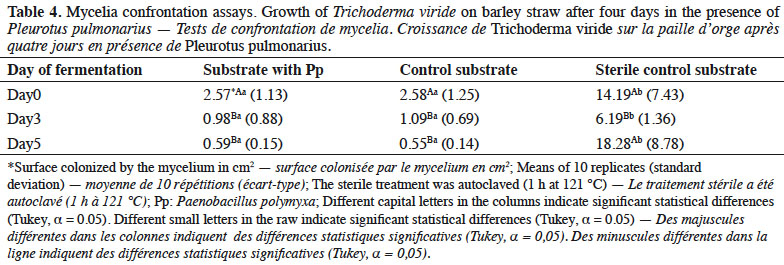
4. Discussion
27In this study, we obtained a substrate that confers competiveness to P. pulmonarius in the presence of T. viride. The inoculation of P. polymyxa allowed the development of a microbial community of thermophilic Pseudomonas and Actinomycetes (Figure 2). This is an unexpected effect that serves to improve the competitiveness of P. pulmonarius against of T. viride. Mycelial growth of Pleurotus was higher on day 5 of fermentation in the substrate with Pp compared to that of days 0 and 3. On day 5, we observed the highest quantity of thermophilic microorganisms, including total microflora and Pseudomonas. A similar result has been reported previously by Kim et al. (2008), in which a strain of Pseudomonas significantly increased the mycelial growth of Pleurotus eryngii. Kang & Cho. (2014) subsequently revealed that auxin produced by Pseudomonas was the main compound stimulating the growth of the mycelia. In the present study, we observed a high quantity of Pseudomonas on day 5; this could have stimulated the mycelial growth compared to days 0 and 3, but slowed the formation of primordia. The quantity of Pseudomonas is an important factor for faster mycelial development in commercial production, but has not shown any benefit in terms of primordia formation.
28It should be noted that fermentation of the barley straw before the heat treatment limited colonization by T. viride in treatments both with and without inoculation of P. polymyxa. The relationship between the decrease in mycelial growth and the presence of any group of bacteria was not determined. However, several studies have reported the benefits of bacterial community in terms of limiting the growth of Trichoderma compared to a sterile substrate (Stolzer & Grabbe, 1991; Muez-Orobia & Pardo-Nuñez, 2001; Velázquez-Cedeño et al., 2004; Velázquez-Cedeño et al., 2006a; Velázquez-Cedeño et al., 2006b; Colavolpe et al., 2013). The importance of microorganisms (including P. polymyxa) in selectivity mechanisms has been demonstrated in vitro, showing a strong inhibition against Trichoderma (Velázquez-Cedeño et al., 2004; Gbolagade, 2006; Velázquez-Cedeño et al., 2008).
29Another explanation could be the accumulation of secondary metabolites, such as iturin, produced by Bacillus subtilis. This bacterium showed strong antimycotic activity (Ohno et al., 1993; Ohno et al., 1996). This genus and other thermophilic microorganisms obtained by the heat treatment (12 h at 58 °C) and conditioning (24 h at 48 °C), favored inhibition of the growth of T. viride.
30Interestingly, on day 5 of fermentation, the pH was at its most alkaline, at 9.9. This value is higher than optimal pH reported for mycelial growth of Pleurotus, which is from 6.5 to 7.0 (Kalmis et al., 2008), and Yadav & Chandra (2014) determined that a pH of between 7-8 increases mycelial development in cultivated Pleurotus species. Moreover, Téllez-Téllez et al. (2008) showed that substrate fermentation helps to regulate an optimum pH for the development of Pleurotus. However, under certain conditions (high number of microorganisms), the pH probably negatively affects the yield of Pleurotus but not the mycelial growth.
31The yield of P. pulmonarius showed no increase in the inoculated substrate relative to the control. This is probably because P. polymyxa negatively affected the yield of Pleurotus. A similar report by Velázquez-Cedeño et al. (2008) showed no significant difference in the yield of P. ostreatus following inoculation of P. polymyxa and Actinomycetes in the substrate.
32Beyond the use of P. polymyxa, the fermentation and heat treatment significantly promote the development of thermophilic microorganisms. Both of these substrates presented the best BE of P. pulmonarius on day 3 of fermentation, with a subsequent decrease by day 5. This means that the fermentation and proposed heat treatment offer an advantage for cultivation of Pleurotus in short fermentation processes. This agrees with the findings of Castañeda-de-Leon & Leal-Lara (2007), in which a short fermentation (3 days) for the substrate is recommended, followed by heat treatment to obtain a high performance in Pleurotus. The fermentation and heat treatment conditions used in this study represent an alternative to obtain a selective substrate for Pleurotus cultivation.
5. Conclusions
33In conclusion, the addition of P. polymyxa modified the natural microbiological secession in a five-day fermentation with barley straw. It favored the competitiveness of Pleurotus pulmonarius against Trichoderma viride on day 5 compared to a control substrate, although it did not favor the yield. The fermentation and heat treatment are therefore important components in the production of a selective substrate for Pleurotus.
Acknowledgements
34The authors thank the support of the following institutions: Universidad Veracruzana, Instituto de Ecología, A.C. and CONACYT (Mexico) and Institut National de la Recherche Agronomique and ANR (France) for financing their joint research.
Bibliographie
Castañeda-de-Leon V.T. & Leal-Lara H., 2007. Factores que influyen en la producción de sustratos selectivos para el cultivo de Pleurotus ostreatus. In: Sánchez J.E., Martínez-Carrera D., Mata G. & Leal-Lara H., eds. El cultivo de setas Pleurotus spp. en México. Tapachula, México: El Colegio de la Frontera Sur, 81-90.
Colavolpe M.B., Mejía S.J. & Albertó E., 2013. Efficiency of treatments for controlling Trichoderma spp. during spawning in cultivation of lignicolous mushrooms. Braz. J. Microbiol., 45(4), 1263-1270
Gbolagade J.S., 2006. Bacteria associated with compost used for cultivation of Nigerian edible mushrooms Pleurotus tuber-regium (Fr.) Singer, and Lentinus squarrosulus (Berk.). Afr. J. Biotechnol., 5(4), 338-342.
Guzmán G. et al., 2013. El cultivo de hongos comestibles con especial atención a especies tropicales y subtropicales en esquilmos y residuos agroindustriales. México: Instituto Politécnico Nacional, 245.
Hernández D., Sánchez J.E. & Yamasaki K., 2003. A simple procedure for preparing substrate for Pleurotus ostreatus cultivation. Bioresour. Technol., 90(2), 145-150.
Kalmis E., Azbar N., Yıldız H. & Kalyoncu F., 2008. Feasibility of using olive mill effluent (OME) as a wetting agent during the cultivation of oyster mushroom, Pleurotus ostreatus, on wheat straw. Bioresour. Technol., 99(1), 164-169.
Kang Y.M. & Cho K.M., 2014. Identification of auxin from Pseudomonas sp. P7014 for the rapid growth of Pleurotus eryngii mycelium. Korean J. Microbiol., 50(1), 15-21.
Kim M.K. et al., 2008. Effect of Pseudomonas sp. P7014 on the growth of edible mushroom Pleurotus eryngii in bottle culture for commercial production. Bioresour. Technol., 99(8), 3306-3308.
Korsten L. & Cook N., 1996. Optimizing culturing conditions for Bacillus subtilis. South African Avocado Growers’Association Yearbook, 19, 54-58.
Muez-Orobia M.A. & Pardo-Nuñez J., 2001. La preparación del substrato. In: Sánchez J.E. & Royse D.J., eds. La biología y el cultivo de Pleurotus spp. Tapachula, México: El Colegio de la Frontera Sur, 157-186.
Mwangi R.W., Kariuki S.T. & Wagara I.N., 2017. Biocontrol of green mould disease of oyster mushroom (Pleurotus ostreatus) using Bacillus amyloliquefaciens. J. Biol., 7(10), 25-30.
Nagy A. et al., 2012. Biological control of oyster mushroom green mould disease by antagonistic Bacillus species. Biol. Control Fungal Bact. Plant Pathog., 78, 289-293.
Ohno A., Ano T. & Shoda M., 1993. Production of the antifungal peptide antibiotic, iturin by Bacillus subtilis NB22 in solid state fermentation. J. Ferment. Bioeng., 75(1), 23-27.
Ohno A., Ano T. & Shoda M., 1996. Use of soybean curd residue, okara, for the solid state substrate in the production of a lipopeptide antibiotic, iturin A, by Bacillus subtilis NB22. Process Biochem., 31(8), 801-806.
Pérez-Merlo R. & Mata G., 2002. Selección de cepas de Pleurotus ostreatus (Jacq. ex Fr.) Kumm. y Pleurotus pulmonarius (Fr.) Quél. y la factibilidad de reutilizar la madera de Pinus spp. para su cultivo. For. Veracruzana, 4(1), 31-34.
Pochon J. & Tardieux P., 1962. Techniques d’analyse en microbiologie du sol. Paris : Éditions de la Tourelle.
Requena N., Azcon R. & Baca T., 1996. Chemical changes in humic substances from compost due to incubation with ligno-cellulolytic microorganism and effects on lettuce growth. Appl. Microbiol. Biotechnol., 45, 857-863.
Royse D.J., Baars J. & Tan Q., 2017. Current overview of mushroom production in the world. In: Cunha Zied D. & Pardo-Giménez A., eds. Edible and medicinal mushrooms. Wiley-Blackwell, 5-13.
Sánchez J.E., Marínez Carrera D., Mata G. & Leal Lara H., 2007. El cultivo de setas Pleurotus spp. en México. Tapachula, Mexico: El Colegio de la Frontera Sur.
Sánchez J.E., Moreno L. & Andrade R., 2012. Low input technology for pasteurizing substrate for oyster mushroom production. In: Petre M. & Berovic M., eds. Mushroom biotechnology and bioengineering. Bucarest: Editura CD Press, 195-204.
Shah S. & Nasreen S., 2011. Evaluation of bioagents against the infection of green mould (Trichoderma spp.) in Pleurotus sajor-caju cultivation. Int. J. Plant Pathol., 2, 81-88.
Stolzer S. & Grabbe K., 1991. Mechanisms of substrate selectivity in the cultivation of edible fungi. Mushroom Sci., 13(1), 30.
Téllez-Téllez M. et al., 2008. Growth and laccase production by Pleurotus ostreatus in submerged and solid-state fermentation. Appl. Microbiol. Biotechnol., 81(4), 675-679.
Velázquez-Cedeño M.A., Farnet A.M., Ferré E. & Savoie J.M., 2004. Variations of lignocellulosic activities in dual cultures of Pleurotus ostreatus and Trichoderma longibrachiatum on unsterilized wheat straw. Mycologia, 96(4), 712-719.
Velázquez-Cedeño M., Farnet A.M., Mata G. & Savoie J.-M., 2006a. Wheat straw management to produce a substrate improving the culture conditions of Pleurotus. In: Poggi-Varaldo H.M. et al., eds. Proceedings of the Second International Meeting on Environmental Biotechnology and Engineering (2IMEBE), 26-29 September 2006, CINVESTAV, México City, Mexico.
Velázquez-Cedeño M., Mata G., Farnet A.M. & Savoie J.-M., 2006b. Estudio preliminar de la microflora bacteriana termotolerante de la pulpa de café y la paja de trigo con potencial de inhibición contra Trichoderma viride en el cultivo de Pleurotus spp. Rev. Mex. Micologia, 22, 33-39.
Velázquez-Cedeño M., Farnet A.M., Mata G. & Savoie J.-M., 2008. Role of Bacillus spp. in antagonism between Pleurotus ostreatus and Trichoderma harzianum in heat-treated wheat-straw substrates. Bioresour. Technol., 99(15), 6966-6973.
Villa Cruz V., Huerta-Palacios G. & Sánchez Vázquez J., 1999. Fermentation of a mixture of corn-cobs and coffee pulp for the cultivation of Pleurotus ostreatus. Micologia Neotrop. Apl., 12, 67-74.
Yadav M.K. & Chandra R., 2014. Evaluation of culture media, pH and temperature for mycelial growth of different strains of Pleurotus sp. Agric. Sci. Dig., 34(4), 299-302.






