- Accueil
- Volume 23 (2019)
- Numéro 2
- Oak or chestnut tannin dose responses on silage pH, proteolysis and in vitro digestibility in laboratory-scale silos
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Oak or chestnut tannin dose responses on silage pH, proteolysis and in vitro digestibility in laboratory-scale silos

Notes de la rédaction
Received 12 December 2018, accepted 11 March 2019, available online 8 April 2019
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
Résumé
Effets des doses des tanins de chêne ou de châtaignier sur le pH, la protéolyse et la digestibilité in vitro d’ensilages en micro-silos
Description du sujet. Cette note de recherche documente, en micro-silos, l’utilisation de tannins hydrolysables comme additifs d’ensilage pour réduire la protéolyse.
Objectifs. Étudier les effets dose d’extraits de tannin de chêne (OTE) et de châtaignier (CTE) sur la composition chimique et la conservation d’ensilage d’herbe.
Méthode. Un mélange de dactyle, trèfles blanc et violet a été ensilé en sachets sous vide, avec des doses de 0, 10, 30, 50 et 70 g·kg-1 MS d’OTE et CTE.
Résultats. Les tannins hydrolysables ont réduit la concentration en azote ammoniacal (N-NH3) des ensilages jusqu’à 18 % (p < 0,05). Pour les doses étudiées, l’OTE a montré une diminution linéaire du N-NH3 (R² = 0,76), tandis que le CTE a provoqué une réduction quadratique (R² = 0,68). Les fortes doses de tannins ont réduit la digestibilité in vitro de la matière organique (DMO) de 3 % (p < 0,05).
Conclusions. Les deux tannins réduisent la protéolyse dans l’ensilage, mais de fortes doses diminuent la DMO.
Abstract
Description of the subject. This short note documents the use of hydrolyzable tannins as silage additives to reduce proteolysis thanks to a laboratory-scale ensiling method.
Objectives. To study oak (OTE) and chestnut tannin extract (CTE) dose responses on chemical composition, pH and ammoniacal nitrogen (N-NH3) content of silage.
Method. A mixture of cocksfoot, white and red clovers was ensiled in vacuum packs, with OTE or CTE at doses of 0, 10, 30, 50 and 70 g·kg-1 DM.
Results. Hydrolyzable tannin extracts decreased N-NH3 content of silage up to 18% (p < 0.05). For the investigated range of doses, OTE induced a linear decrease of N-NH3 content (R² = 0.76) whereas CTE resulted in a quadratic decrease (R² = 0.68). High doses of tannin extracts reduced in vitro organic matter digestibility (OMD) by 3% (p < 0.05).
Conclusions. Both tannins reduced proteolysis in silos but highest doses induced a decrease in OMD.
1. Introduction
1Forages are a main source of proteins for ruminants, especially legume forages. Rich in degradable proteins, these forages are highly susceptible to proteolysis during ensiling (Albrecht & Beauchemin, 2003). However, proteolysis intensity is negatively correlated to tannin concentration in silage (Albrecht & Muck, 1991). These natural polyphenols present in some plants make complexes with proteins and can protect them against degradation in silo but also in rumen (Piluzza et al., 2014). Several studies showed a reduction of non-protein N and/or ammonia in silo when adding hydrolyzable tannin as silage additive (Cavallarin et al., 2002; Tabacco et al., 2006; Herremans et al., 2018). In order to study silages, cheap and convenient models of real-size silos are needed. Vacuum packs are commonly used because of their interesting cost and flexible utilization. Comparisons with glass tubes have shown marginal or no differences regarding fermentation parameters for grass and legume silages (Johnson et al., 2005). The objective of this work was to study the effect of increasing doses of oak (OTE) or chestnut tannin extracts (CTE) on conservation of grass/legume mixed silage in laboratory-scale vacuum packs.
2. Materials and methods
2A mature stand of cocksfoot (Dactylis glomerata), white clover (Trifolium repens) and red clover (Trifolium pratense) was harvested in Gembloux, Belgium, as a second cut in July 2018. Forage contained 615 g of cocksfoot and 385 g of mixed white and red clovers per kg of DM. Forage was cut and chopped before being ensiled in plastic packs (10 l capacity, Brouwland, Beverlo, Belgium) according to two additive treatments: OTE or CTE. For each tannin type, five doses were investigated (0, 10, 30, 50 and 70 g·kg-1 forage DM). OTE and CTE extracts contained 468 ± 6 g·kg-1 DM and 492 ± 7 g·kg-1 DM respectively of total tannins (dosed by methyl cellulose precipitable method). Each treatment-dose combination was replicated five times and packs were filled with 600 g of fresh forage. Where appropriate, powdered tannin extracts were added and mixed directly in packs. The 50 packs were sealed with a vacuum packaging machine (model UNICA GAS, Lavezzini, Fiorenzuola, Italy) and stored at room temperature. After 28 days, laboratory-scale silos were opened and homogenized. Forages were oven-dried at 60 °C until constant weight and ground in a Cyclotec mill (1 mm screen, FOSS, Hillerød, Denmark). Chemical composition and in vitro organic matter digestibility (OMD; de Boever et al., 1986) were determined by near infrared reflectance spectroscopy (database of Minet et al., 2016; XDS-system spectrometer, FOSS, Hillerød, Denmark). Measurement of pH was carried out with a conventional glass electrode in the water extract. Silage N-NH3 content was measured in the acidified (pH < 3) water extract by the Kjeldahl method (Kjeltec, FOSS, Hillerød, Denmark). Using Minitab® Statistical Software, all data were processed through a general linear model with tannin type and dose as fixed factors. Dunnett post-hoc test was used to compare dose means to dose 0 mean. A regression analysis on tannin dose response of N-NH3 content was performed with Minitab Assistant. The regression model with the highest-order significant term (p < 0.05) was considered as the best.
3. Results
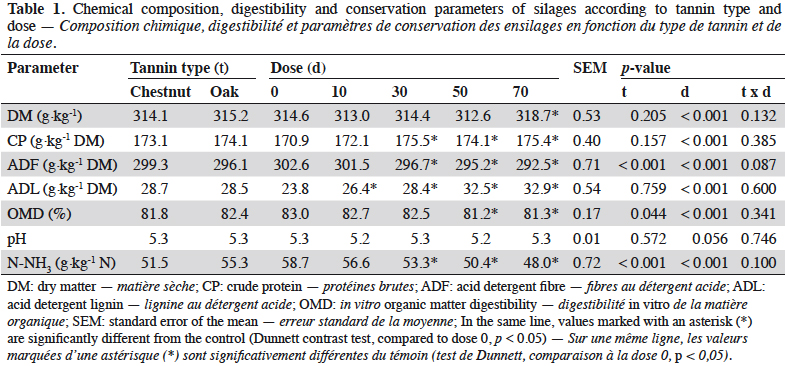
3After ensiling, DM content reached 314 g·kg-1 on average. Tannin dose significantly affected all composition parameters (Table 1, p < 0.05). Dry matter (DM) and crude protein (CP) content showed significant but minor variations. From a dose of 30 g·kg-1 DM, acid detergent fibre (ADF) fell when increasing tannin dose whereas acid detergent lignin (ADL) increased. ADF was the only composition parameter influenced by the type of tannin, showing a lower lever with OTE compared to CTE. OMD was affected by tannin from a dose of 50 g·kg-1 forage DM. CTE induced a lower OMD than OTE (p < 0.05). Tannins did not influence pH but ammoniacal nitrogen content was lower in CTE than in OTE silages (-7%). At 30 g·kg-1 forage DM, tannin extracts reduced N-NH3 content by 9% compared to 14% and 18% reductions at 50 and 70 g·kg-1 forage DM respectively. No interactions were found between tannin type and dose. N-NH3 content was negatively correlated to tannin dose (Figure 1). N-NH3 content decreased linearly with OTE dose (R² = 0.76) whereas it seems to decrease along a quadratic relation with CTE (R² = 0.68). With CTE, the linear model showed a lower R² (y = 56.14 – 0.1440 x; p < 0.001; R² = 0.58).
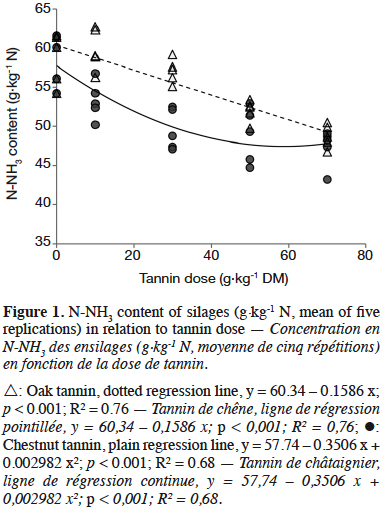
4. Discussion
4As tannin contents higher than 50 g·kg-1 forage DM are known to reduce intake and protein digestibility (Piluzza et al., 2014), our highest dose was used for scientific interest only. According to Hagerman et al. (1992), hydrolyzable tannins such as oak or chestnut tannins should not affect feed digestibility because of their quick degradation in the gut. It seems more likely that the rise observed in ADL content would reflect the presence of lignin in the commercial tannin extracts, as they are wood by-products. This presence could explain most of the OMD decrease.
5The N-NH3 reduction supports the hypothesis that tannin-protein complexes are stable at pH 5 and that tannins reduce proteolysis in this environment. The quadratic relation observed with CTE seems to be confirmed by Tabacco et al. (2006), showing a higher decrease of N-NH3 content with 40 g·kg-1 relative to 20 g·kg-1 forage DM but no differences between 40 and 60 g·kg-1 forage DM doses of CTE in silages. Tabacco et al. (2006) explained the absence of further significant N-NH3 reduction by an already complete inhibition of some silage microorganisms at 40 g·kg-1 DM. The linear relation observed with OTE could be due to a threshold dose higher than the investigated doses. However, the hypothesis of a quadratic relation between hydrolysable tannin dose and silage N-NH3 content should be confirmed by an experiment with higher tannin doses because of the short plateau observed with CTE and its absence with OTE at the tested doses. Globally, CTE had a slightly higher beneficial effect on silage proteolysis than OTE. This is probably due to the specificity of chemical bonds between tannins and proteins, which can differ according to tannin and protein structures. This specificity also induces an interaction between tannin and forage composition, as already shown in a previous work (Herremans et al., 2018).
5. Conclusions
6In order to improve protein conservation in silage, the best dose investigated in the present study seems to be around 30 g·kg-1 forage DM of hydrolyzable tannin extract in silage. Lower dose prevents tannins from lowering proteolysis and doses from 50 g·kg-1 DM seem to reduce OMD and probably dry matter intake. More research is needed on the dose range between 10 and 50 g·kg-1 DM as well as on different forage compositions.
Bibliographie
Albrecht K.A. & Muck R.E., 1991. Proteolysis in ensiled forage legumes that vary in tannin concentration. Crop Sci., 31(2), 464‑469.
Albrecht K.A. & Beauchemin K.A., 2003. Alfalfa and other perennial legume silage. In: Silage science and technology. Madison, WI, USA: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, 633-664.
Cavallarin L. et al., 2002. Effect of chestnut tannin on protein degradation in lucerne silages. In: Proceedings of the 19th General Meeting of the European Grassland Federation, The Grassland Science in Europe, 27-30 May 2002, La Rochelle, France, 68‑69.
de Boever J.L. et al., 1986. The use of an enzymatic technique to predict digestibility, metabolizable and net energy of compound feedstuffs for ruminants. Anim. Feed Sci. Technol., 14(3), 203‑214.
Hagerman A.E. et al., 1992. Tannin chemistry in relation to digestion. J. Range Manage., 45(1), 57.
Herremans S. et al., 2018. Silage additives to reduce protein degradation during ensiling and evaluation of in vitro ruminal nitrogen degradability. Grass Forage Sci., 11, 86-96.
Johnson H.E. et al., 2005. Vacuum packing: a model system for laboratory-scale silage fermentations. J. Appl. Microbiol., 98(1), 106‑113.
Minet O. et al., 2016. La spectrométrie proche infrarouge. Une technologie rapide, précise et écologique pour déterminer la composition et la qualité des produits agricoles et alimentaires. Gembloux, Belgique : ASBL Requasud.
Piluzza G., Sulas L. & Bullitta S., 2014. Tannins in forage plants and their role in animal husbandry and environmental sustainability: a review. Grass Forage Sci., 69(1), 32‑48.
Tabacco E. et al., 2006. Effect of chestnut tannin on fermentation quality, proteolysis, and protein rumen degradability of alfalfa silage. J. Dairy Sci., 89(12), 4736-4746.






