- Accueil
- Volume 21 (2017)
- Numéro 4
- Effect of temperature on growth and fatty acids profile of the biodiesel producing microalga Scenedesmus acutus
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Effect of temperature on growth and fatty acids profile of the biodiesel producing microalga Scenedesmus acutus

Notes de la rédaction
Received on June 5, 2016; accepted on June 9, 2017
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
Résumé
Effet de la température d’incubation sur la croissance et le profil en acides gras de la microalgue Scenedesmus acutus à mettre en rapport avec une production potentielle de biodiesel
Description du sujet. L'étude examine l'effet de la température d'incubation (15, 20, 25, 30, 35 et 40 °C) sur la biomasse, la productivité en acides gras de la microalgue Scenedesmus acutus, potentielle productrice de biodiesel.
Objectifs. Le travail a pour objectif d'étudier l'effet d'une variation de température sur le contenu lipidique de S. acutus, la productivité lipidique et la production de biomasse de l'algue.
Méthode. L'algue a été cultivée sous différentes températures de croissance et la teneur en lipides, la composition en acides gras et la biomasse de S. acutus ont été déterminés.
Résultats. L'incubation de S. acutus à 30 °C donne la meilleure croissance, bien qu'il n'y ait pas de différence significative pour la production de biomasse entre 25 et 30 °C (0,41 et 0,42 g·l-1·j-1, respectivement). La plus haute teneur en acides gras (104,1 mg·g-1 CDW) a été obtenue à 15 °C et décroit lorsqu'on augmente la température. En raison de la forte production de biomasse, la productivité des acides gras a montré les valeurs les plus élevées à 25 et 30 °C (41,27 et 42,10 mg·l-1·j-1, respectivement). En ce qui concerne le profil en acides gras, les proportions d'acides gras saturés et mono-insaturés se sont accrues, tandis que celle des acides gras poly-insaturés a chuté avec l'augmentation de la température d'incubation. Les proportions d'acides gras saturés et mono-insaturés sont montées respectivement de 13,72 à 23,79 % et de 11,13 à 33,10 % dans la représentation des acides gras totaux, lorsque la température d'incubation est passée de 15 à 40 °C, tandis que pour ce même écart thermique, la proportion des acides gras poly-insaturés au sein des acides gras totaux a diminué de 75,15 % à 43,10 %.
Conclusions. On peut conclure de la présente étude que la température d'incubation est un paramètre critique pour la composition quantitative et qualitative des acides gras de S. acutus. De plus, le type et la proportion de chaque acide gras sont des paramètres qui interfèrent dans la qualité du biodiesel, mais qui peuvent être modifiés par la température d'incubation de manière à rencontrer les normes internationales du biodiesel.
Abstract
Description of the subject. The present study examined the effect of temperature (15, 20, 25, 30, 35 and 40 °C) on biomass, esterified fatty acids content and fatty acid productivity of Scenedesmus acutus.
Objectives. This work aimed to study the effect of variation in temperature on lipid productivity and fatty acid profiles of S. acutus as a feedstock for biodiesel production.
Method. The alga was grown under different temperatures and its biomass, as well as fatty acid content and composition, were determined.
Results. The maximum growth rate of S. acutus was achieved at 30 °C , but there was no significant difference in biomass productivity at 25 and 30 °C (0.41 and 0.42 g·l-1·d-1), respectively. The highest fatty acid content (104.1 mg·g-1 CDW) was recorded at low temperature (15 °C) and decreased with increasing temperature. As a result of high biomass production, fatty acids productivity showed the highest values (41.27 and 42.10 mg·l-1·d-1) at 25 and 30 °C, respectively. The proportion of saturated and mono-unsaturated fatty acids increased from 13.72 to 23.79% and from 11.13 to 33.10% of total fatty acids when the incubation temperature was raised from 15 to 40 °C, respectively. The increase of temperature from 15 to 40 °C decreased the poly-unsaturated fatty acids from 75.15% to 43.10% of total fatty acids, respectively.
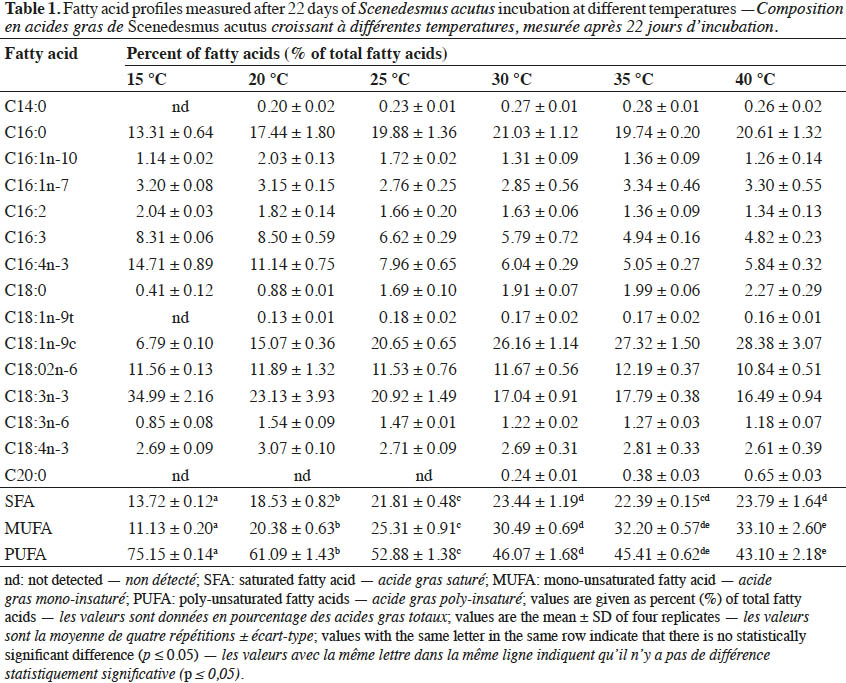
Conclusions. The present study concluded that incubation temperature was a critical parameter for quantitative and qualitative fatty acid compositions of S. acutus. In addition, the type and proportion of individual fatty acids, which interfere with biodiesel quality, can be modified using different incubation temperatures in order to meet the biodiesel international standards.
Table des matières
1. Introduction
1Contemporary global energy usage is based on utilization of fossil fuels including natural gas, coal and oil. Barbir (2009) concluded that the use of fossil fuels has several problems, such as:
2– pollution at local, regional and global scales;
3– risk of complete depletion of fossil fuel energy, as the worldwide fossil oil reserves will be exhausted in shorter than 30 years due to the quick development of anthropogenic activities and overconsumption (Abomohra et al., 2016);
4– increasing of greenhouse gas emissions (i.e. NOx, CO2 and SOx) that cause global warming and climate problems.
5Biofuel (fuel derived from biomass) receives considerable attention because it is a renewable, biodegradable and non-toxic fuel (Mutanda et al., 2010). Biodiesel from microalgae is a promising renewable energy that might completely replace the fossil diesel without influencing the human food supply (Chisti, 2008). In fact, algae have a much higher yield as a biodiesel feedstock than crop plants (Abomohra et al., 2014). The annual oil yield of algae is 7 to 31 times larger than palm oil, for the same given land area, due to their ability to accumulate lipids and their very high actual photosynthetic yield (Li et al., 2008; Abomohra et al., 2014). The total yield of biodiesel depends on the lipid content of the algal strain and also on its growth rate. For biodiesel production, the economic biomass production of microalgae has to be taken into consideration, so microalgal species with a high lipid content and a high cell growth are used (Lv et al., 2010). Abomohra et al. (2013) reported two categories of microalgae for high lipid production:
6– high lipid content (43%) with low growth rate (30 mg·l-1·d-1), such as Botryococcus braunii,
7– high growth rate (250 mg·l-1·d-1) with low lipid content (15%) such as Scenedesmus obliquus.
8Several studies demonstrated that the quantity and quality of intracellular lipids and the cellular growth rate can vary as a result of changes in growth conditions including light intensity, temperature, CO2 concentrations, nitrogen or phosphate limitation, silicon deficiency, and iron supplementation (Tzovenis et al., 2003; Liu et al., 2008; Griffiths & Harrison, 2009; Ho et al., 2010; El-Sheekh et al., 2013; Kumar et al., 2014). Most of the previous studies investigated the impact of those factors on cell growth and/or lipid content of various microalgal species separately. However, to get better understanding of the relationship between cell growth and lipid accumulation, they have to be measured as biomass and lipid productivities. Furthermore, among the factors mentioned above, temperature is a sensitive limiting factor for microalgal growth and metabolic activities. Moreover, it is an easy-to-control factor in the practical operation of microalgae cultivation. In this study, the effects of different incubation temperatures on S. acutus were carried out in a batch cultivation mode. The variation in fatty acids content, fatty acids productivity, and fatty acids profiles in response to different temperatures was measured. The influence of incubation temperature on cell growth and biomass production was also assessed.
2. Materials and methods
2.1. Algal strain and cultivation conditions
9Scenedesmus acutus was isolated from municipal wastewater at Tanta, Egypt, and cultivated axenically in 1 l Erlenmeyer flasks with 700 ml KC medium (Kessler & Czygan, 1970) at an initial OD680 of 0.05 nm. Sterile filtered air enriched with 3% CO2 (v/v) was continuously applied to the cultures. Cultures were illuminated from the top using tubular fluorescent lamps (PHILIPS Master TL-D 85 W / 840) with light intensity of 130 ± 10 μmol photons·m-2·s-1 at the surface of the flasks with a photoperiod of 14:10 h light:dark at different incubation temperatures (15, 20, 25, 30, 35 and 40 ˚C). The optical density of the algal suspension was measured at 680 nm every other day. Dry weight and esterified fatty acids (EFAs) were measured after 22 days of incubation and allowed calculation of biomass and fatty acids productivities. Cell number showed a strong positive correlation with the optical density (R2 = 0.991), which can be calculated from the equation:
10Cell number (×106 cell·ml-1) = 1.423 × OD680 - 0.002 (R2 = 0.991)
2.2. Biomass assay
11Algal growth was monitored by measuring the optical density at 680 nm (OD680) and by determination of the cellular dry weight (CDW). From the values of CDW, biomass productivity was calculated according to Abomohra et al. (2013) as follows:
12Biomass productivity (g CDW·l-1·d-1)= CDWL - CDWE)∙(tL-tE)-1
13where CDWE and CDWL represent the CDW (g·l-1) at the days of early exponential phase (tE) and late exponential phase (tL), respectively.
2.3. Lipid extraction
14To analyze the cellular fatty acids composition, 5 ml aliquots of each culture were collected after 22 days of incubation at specified times. Lipids were extracted following the method of Bligh & Dyer (1959). Prior to extraction, trinonadecanoylglycerol was added to the samples as an internal standard for esterified fatty acids.
2.4. Fatty acids profile
15Esterified fatty acids (EFA) from the extract of intracellular lipids were transmethylated for GC analysis as previously described (Scharnewski et al., 2008; Kaczmarzyk & Fulda, 2010). The fatty acids methyl esters were subjected to GC/FID analysis. The GC analysis was performed with a Varian 3900 GC-system equipped with a capillary column (Select Fame, 50 m × 0.25 mm; Varian). Fatty acids content (mg·g-1 CDW) was calculated; in addition, fatty acids productivity was calculated according to Abomohra et al. (2013):
16Fatty acids productivity (mg·l-1·d-1) = (FAL-FAE)∙(tL-tE)-1
17where FAE and FAL represent the total fatty acids content (mg·l-1) at the days of early exponential phase (tE) and late exponential phase (tL), respectively.
2.5. Statistical analysis
18Results are presented as the mean ± standard deviation (SD) of four replicates. Data were statistically analyzed using SAS (v 6.12). The degree of significance using one way analysis of variance and paired-samples t-test at probability level (p) ≤ 0.05 was determined.
3. Results
3.1. Growth and biomass productivity
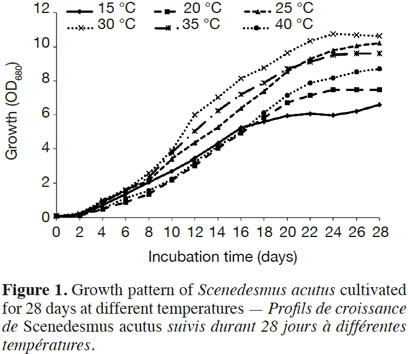
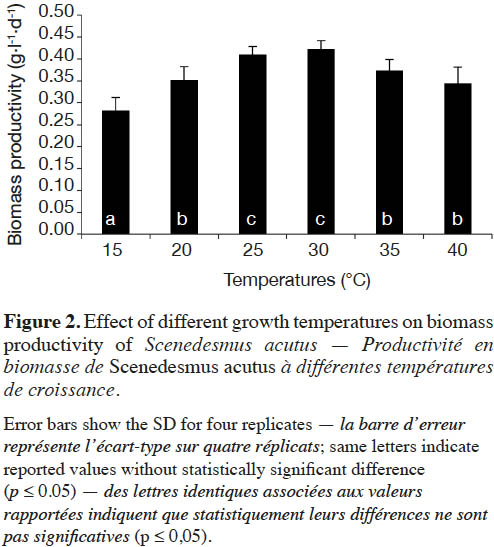
19Effect of incubation temperature on the growth rate of S. acutus was examined based on cell density (measured as optical density) and cellular dry weight (biomass productivity) for 28 days of incubation (Figure 1). At late exponential phase (after 22 days), incubation at 30 ˚C showed the maximum growth, while the lowest growth was recorded at 15 ˚C. The growth reduction was less pronounced at 40 ˚C. Biomass productivity showed the same pattern, with significantly less (one way ANOVA, p ≤ 0.05) biomass productivity (0.28 g·l-1·d-1) at 15 ˚C (Figure 2), while the highest biomass productivity (0.42 g·l-1·d-1) was recorded at 30 ˚C. Differences in biomass productivity did not vary significantly between incubation temperatures of 25 and 30 ˚C. Incubation at 40 ˚C showed significant reduction in biomass productivity by 18% as compared with that at 30 ˚C (Figure 2).
3.2. Fatty acids content
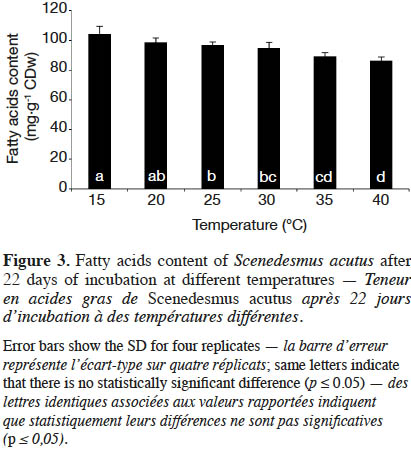
20The fatty acids contents per microalgal biomass (mg·g-1 CDW) of S. acutus after 22 days of cultivation at different incubation temperatures are shown in figure 3. At 30 ˚C, i.e. at maximum growth rate, the fatty acids content was 94.90 mg·g-1 CDW. At lower temperature (15 ˚C), the fatty acids content was 104.12 mg·g-1 CDW, significantly (paired-samples t-test, p ≤ 0.05) higher than the one at 30 ˚C by 10%. At higher temperature (40 ˚C), the fatty acids content significantly decreased to 86.34 mg·g-1 CDW.
3.3. Fatty acids productivity
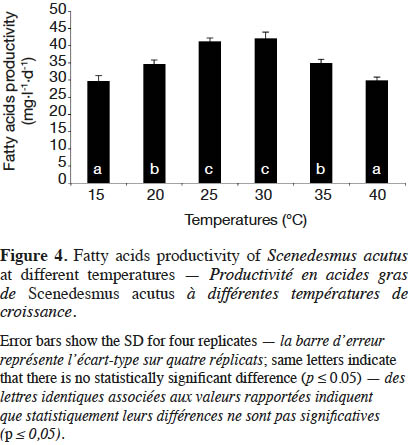
21Esterified fatty acids (EFA) productivity of S. acutus at different cultivation temperatures is shown in figure 4. Due to the high microalgal biomass, the highest EFA productivity was recorded at 30 ˚C (42.10 mg·l-1·d-1). The lowest EFA productivity (~ 30 mg·l-1·d-1) was achieved at 15 and 40 ˚C. The EFA productivity (~ 35 mg·l-1·d-1) by S. acutus cultivated at 20 and 35 ˚C decreased by 29 and 17%, respectively, compared to that at 30 ˚C. Therefore, the optimal cultivation temperature to produce microalgal biomass (S. acutus) and fatty acids for biodiesel production ranges from 25 to 30 ˚C.
3.4. Fatty acids profile
22Fatty acids composition of S. acutus grown at different temperatures is shown in table 1. The ratio of the sum of saturated and monounsaturated fatty acids increased by increasing the temperature, while under the same conditions, the ratio of polyunsaturated fatty acids was reduced. At relatively low temperature (15 °C), approximately 35% of total fatty acids of S. acutus was composed of polyunsaturated fatty acid (C18:3n-3). While at high temperature (40 °C), the fatty acids were mainly composed of the monounsaturated fatty acids (C18:1n-9c) and saturated fatty acid (C16:0) with 28 and 21%, respectively.
4. Discussion
23Microalgae are one of the potential sources for biodiesel production due to high efficiency of solar energy conversion to chemical energy. The impact of nutrient depletion and physical stress was examined on different algae for enhancement of lipid production. However, Abomohra et al. (2013) and Francisco et al. (2010) reported that biomass productivity and lipid content are inversely related. Therefore, for biodiesel production, the economic feasibility of microalgal mass culture has to be taken into consideration. The selection of microalgal species with high lipid contents and high cell growth is of great importance. In the present study, the cell growth and consequently biomass productivity of S. acutus were closely related with cultivation temperature. The highest biomass productivity was achieved at 25-30 °C, while biomass productivity decreased constantly when increasing the temperature over 30 °C. Westerhoff et al. (2010) reported 20 °C as the optimal cultivation temperature for Nannochloropsis oculata. However, they concluded that the exponential growth rate constant did not vary between 27 and 39 °C for Scenedesmus and Chlorella, while at 42 °C algal growth stops.
24Temperature is a sensitive parameter for microalgal growth and the concomitant metabolic activities, especially lipid biosynthesis. In the coupled system of wastewater treatment and biodiesel production by microalgae, increasing lipid productivity by temperature adjustment has the advantage that the composition of wastewater does not need to be changed or chemically treated for processing and is therefore ecologically safer (Xin et al., 2011). Chen et al. (2008) reported a little effect of cultivation temperature on lipid content of microalgal biomass, with a significant decrease of triglycerides (TAGs) at low temperatures. However, Converti et al. (2009) reported that high temperature enhances the lipid accumulation in Nostoc oculata.
25In our study, the dependence of lipid content of S. acutus on cultivation temperature is in agreement with the findings of Chen et al. (2008). In the present study, low temperature induced the fatty acid accumulation in S. acutus cells and resulted in high lipid content. From an economic viewpoint, enhancement of lipid productivity, which is related to the growth, has more feasibility than lipid content. Li et al. (2010) showed that high lipid contents of Scenedesmus sp. could be obtained under stress conditions; however due to low growth rate, the lipid productivity decreased as well as microalgal biomass productivity.
26Abomohra et al. (2014) and El-Sheekh et al. (2017) studied the efficiency of pilot cultivation of S. obliquus as a feedstock for biodiesel. They recorded that the fatty acids mass fraction in CDW and biomass production of S. obliquus after 21 days of pilot cultivation were 123.3 g·kg-1 and 3.11 g·l-1, respectively. They concluded that the measured fatty acid productivity of 17.4 mg·l-1·d-1 would be nearly 5 and 8 times higher than lipid productivity of Jatropha and rapeseed, respectively. However, fatty acid productivity (≈ 42 mg·l-1·d-1) at the optimum temperature in the present study is about 50% greater than that recorded for S. obliquus by Abomohra et al. (2014). This confirms that microalgae might be a major biofuel feedstock with the potential to completely displace fossil diesel.
27The saturation and chain length of fatty acids would affect the properties of biodiesel. Hu et al. (2008) mentioned that saturated fats produce a biodiesel with higher oxidative stability and cetane number, but rather poor low-temperature properties. However, biodiesel feedstock rich in polyunsaturated fatty acids produces a biodiesel with good cold-flow properties, but with high oxidation ability. In our study, lipids of S. acutus were mainly composed of polyunsaturated fatty acids at a relatively low cultivation temperature, but they mainly contained saturated and monounsaturated fatty acids at high temperatures. The fatty acid profile of S. acutus evolves with incubation temperature. It makes the biodiesel from microalgae suitable for cold or warm areas depending on the cultivation temperature under which the feedstocks are obtained.
5. Conclusions
28In conclusion, the variation of temperature showed minor effects on lipid content of S. acutus and strongly affected the lipid productivity due to its influence on algal biomass production. The optimum temperature range for maximum lipid and fatty acids production was 25-30 °C. The degree of saturation of fatty acids increased with incubation temperature, which allows biodiesel properties from S. acutus to be used in different climates. Research on microalgae-based biofuel and commercial-scale use of microalgae for biofuel production would require massive investments because it is a highly promising way to meet the energy demand through third generation biofuel from the microalgal feedstock.
29Acknowledgements
30The authors are very grateful to Dr. C. Staley, Department of Soil, Water and Climate, Bio-Technology Institute, University of Minnesota, St. Paul, MN, USA for his valuable comment to improve this manuscript. The financial fund from STDF project no. 4399 was also acknowledged.
Bibliographie
Abomohra A., Wagner M., El-Sheekh M. & Hanelt D., 2013. Lipid and total fatty acid productivity in photoautotrophic fresh water microalgae: screening studies towards biodiesel production. J. Appl. Phycol., 25(4), 931-936.
Abomohra A., El-Sheekh M. & Hanelt D., 2014. Pilot cultivation of the chlorophyte microalga Scenedesmus obliquus as a promising feedstock for biofuel. Biomass Bioenergy, 64, 237-244.
Abomohra A. et al., 2016. Microalgal biomass production as a sustainable feedstock for biodiesel: current status and perspectives. Renewable Sustainable Energy Rev., 64, 596-606.
Barbir F., 2009. Transition to renewable energy systems with hydrogen as an energy carrier. Energy, 34(3), 308-312.
Bligh E.G. & Dyer W.J., 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol., 37(8), 911-917.
Chen G., Jiang Y. & Chen F., 2008. Variation of lipid class composition in Nitzschia laevis as a response to growth temperature change. Food Chem., 109(1), 88-94.
Chisti Y., 2008. Biodiesel from microalgae beats bioethanol. Trends Biotechnol., 26(3), 126-131.
Converti A. et al., 2009. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process, 48(6), 1146-1151.
El-Sheekh M.M., Abomohra A. & Hanelt D., 2013. Optimization of biomass and fatty acid productivity of Scenedesmus obliquus as a promising microalga for biodiesel production. World J. Microbiol. Biotechnol., 29, 915-922.
El-Sheekh M.M., El-Gamal A., Bastawess A.E. & El-Bokhomy A., 2017. Production and characterization of biodiesel from the unicellular green alga Scenedesmus obliquus. Energy Sources Part A, 39(8), 783-793.
Francisco É.C., Neves D.B., Jacob-Lopes E. & Franco T.T., 2010. Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J. Chem. Technol. Biotechnol., 85, 395-403.
Griffiths M. & Harrison S., 2009. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol., 21, 493-507.
Ho S., Chen W. & Chang J., 2010. Scenedesmus obliquus CNW-N as a potential candidate for CO2 mitigation and biodiesel production. Bioresour. Technol., 101(22), 8725-8730.
Hu Q. et al., 2008. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J., 54(4), 621-639.
Kaczmarzyk D. & Fulda M., 2010. Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling. Plant Physiol., 152(3), 1598-1610.
Kessler E. & Czygan F.C., 1970. Physiologische und biochemische Beiträgezur Taxonomie der Gattung Chlorella. Archiv Mikrobiol., 70, 211-216.
Kumar M.S. et al., 2014. Influence of CO2 and light spectra on the enhancement of microalgal growth and lipid content. J. Renewable Sustainable Energy, 6(6), 063107
Li Q., Du W. & Liu D., 2008. Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biotechnol., 80, 749-756.
Li X., Hu H., Ke G. & Sun Y., 2010. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol., 101(14), 5494-5500.
Liu Z., Wang G. & Zhou B., 2008. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol., 99(11), 4717-4722.
Lv J. et al., 2010. Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour. Technol., 101(17), 6797-6804.
Mutanda T. et al., 2010. Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour. Technol., 102(1), 57-70.
Scharnewski M. et al., 2008. Mutants of Saccharomyces cerevisiae deficient in acyl-CoA synthetases secrete fatty acids due to interrupted fatty acid recycling. FEBS J., 275, 2765-2777.
Tzovenis I., De Pauw N. & Sorgeloos P., 2003. Optimization of T-ISO biomass production rich in essential fatty acids: I. Effect of different light regimes on growth and biomass production. Aquaculture, 216(1-2), 203-222.
Westerhoff P., Hu Q., Esparza-Soto M. & Vermaas W., 2010. Growth parameters of microalgae tolerant to high levels of carbon dioxide in batch and continuous flow photobioreactors. Environ. Technol., 31(5), 523-532.
Xin L., Hong-Ying H. & Yu-Ping Z., 2011. Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresour. Technol., 102, 3098-3102.






