Synergistic effects of polyploidization and elicitation on biomass and hyoscyamine content in hairy roots of Datura stramonium
Received on November 24, 2015; accepted on April 13, 2016
Résumé
Effets synergiques de la polyploïdisation et de l’élicitation sur la biomasse et le contenu en hyoscyamine des chevelus racinaires de Datura stramonium
Description du sujet. L'hyoscyamine, un alcaloïde tropanique largement utilisé en médecine, peut être produit à partir de Datura sp. (Solanaceae). Cependant, son contenu dans les racines spontanées reste faible. Par conséquent, les chevelus racinaires (CRs) ont été envisagés comme une alternative potentielle pour améliorer sa biosynthèse. Les chevelus racinaires se caractérisent par une bonne stabilité génétique et une croissance rapide. En effet, les CRs de Datura stramonium ont été largement étudiés dans la perspective de l'amélioration du rendement en hyoscyamine.
Objectifs. Ce document vise à étudier les effets de la polyploïdisation des CRs induits par la colchicine en synergie avec l’élicitation (avec des acides acétylsalicylique ou salicylique) sur la teneur en hyoscyamine de D. stramonium.
Méthode. La colchicine a été appliquée à différentes concentrations et périodes, sur une lignée sélectionnée de chevelu racinaire (LDS) de D. stramonium obtenu par une infection par la souche A4 d’Agrobacterium rhizogenes. La sélection des lignées de CRs tétraploïdes a été réalisée par l'analyse cytogénétique en utilisant la microscopie optique. Les effets de la polyploïdisation et de l’élicitation ont été étudiés sur la biomasse (poids sec) et la teneur en hyoscyamine des CRs.
Résultats. La lignée de CR non traitée (témoin) montre un niveau de diploïde avec 2n = 24 chromosomes. Cependant, les lignées de CRs traitées avec la colchicine montrent, dans la plupart des cas, une endoréplication de leur matériel génétique. Le taux de survie des lignées endorépliquées varie entre 30 % et 93 %, en fonction de la concentration et du temps d'exposition à la colchicine. En outre, la lignée de CR tétraploïde montre une augmentation de sa biomasse et de son contenu en hyoscyamine par rapport à la lignée diploïde de CR (LDS). En outre, l’élicitation des CRs par AAS ou AS à la concentration de 10-4 M provoque une faible diminution ou une augmentation du poids sec, respectivement. Cependant, les mêmes traitements montrent une augmentation significative du rendement en hyoscyamine dans les lignées de CRs élicitées. Par conséquent, notre travail indique que la combinaison de la polyploïdie et de l’élicitation peut conduire à des améliorations significatives dans la biosynthèse et le contenu en hyoscyamine en raison de leurs effets synergiques.
Conclusions. L’élicitation de lignées tétraploïdes de chevelus racinaires améliore considérablement leur teneur en hyoscyamine.
Abstract
Description of the subject. The hyoscyamine, a tropane alkaloid, widely used in medicine, can be produced from Datura sp. (Solanaceae). However, its content in the spontaneous roots remains low; therefore, hairy roots (HRs) were envisaged as a potential alternative to improve its biosynthesis. The hairy roots are characterized by a good genetic stability and a rapid growth. Indeed, Datura stramonium HRs have widely been studied in the perspective of improving the yield of hyoscyamine. This study is part of this same perspective.
Objectives. This paper aims to study the effects of polyploidization of HRs induced by colchicine in synergy with elicitation (with acetylsalicylic [ASA] or salicylic acids [SA]) on the hyoscyamine content in D. stramonium.
Method. Colchicine was applied at different concentrations and periods, on a selected hairy root line (LDS) of D. stramonium obtained by infection with Agrobactrium rhizogenes strain A4. The selection of tetraploid HR lines was performed by the cytogenetic analysis using light microscopy. The effect of polyploidization and elicitation was studied on the biomass (dry weight) and hyoscyamine content of HRs.
Results. The untreated HR line (control) shows a diploid level with 2n = 24 chromosomes. However, the HR lines treated with colchicine show, in most cases, an endoreduplication of their genetic material. The survival rate of endoreduplicated lines varies between 30% and 93%, depending on concentration and exposure time to colchicine. Moreover, the tetraploid HR line shows an increase in its biomass and hyoscyamine content in comparison to the diploid HR line (LDS). Further, elicitation of HRs by ASA or AS at the 10-4 M concentration causes a low decrease or increase in dry weight, respectively. However, the same treatments show a significant increase in the yield of hyoscyamine in elicited HR lines. Consequently, our work indicates that the combination of polyploidy and elicitation can lead to significant improvements in hyoscyamine biosynthesis and content due to their synergistic effects.
Conclusions. Elicitation of tetraploid hairy root lines improves significantly their content of hyoscyamine.
1. Introduction
1The hyoscyamine, a secondary metabolite widely used in medicine, is obtained from Datura sp. (Solanaceae). However, its content within spontaneous roots remains low (Ollagnier et al., 1998; Wu et al., 2005); therefore, the use of hairy roots (HRs) to improve its expression has been investigated (Namdeo, 2007; Amdoun et al., 2009; Amdoun et al., 2010; Harfi et al., 2011). The production of hyoscyamine through biotechnology using genetically transformed HRs is of great interest and has many advantages (Flores et al., 1999; Pitta-Alvarez et al., 2000). The hairy roots are characterized by genetic stability as well as rapid growth, and have been largely used for secondary metabolite production (Srivastava et al., 2007), and even for functional gene promoter analysis (Makhzoum et al., 2011) and plant pharmaceutical recombinant proteins studies (Makhzoum et al., 2013; Makhzoum et al., 2014). Indeed, the HRs of Datura stramonium L. have been widely studied in the quest for ameliorating hyoscyamine yield. One of the methods employed to enhance production is the use of elicitors. Exposure to biotic or abiotic elicitors generally induces the synthesis of secondary metabolites in plants (Benhamou, 1996; Chashmi et al., 2010; Malik et al., 2011; Malik et al., 2013). The three major plant secondary signaling molecules are jasmonate or its derivatives (JAs) (Balbi, 2008), ethylene (Wang et al., 2002) and salicylic acid (SA) (Shah, 2003). This strategy mimics the plant’s natural response to pathogen attack or wounding by activating signal molecules biosynthesis which induces alkaloid biosynthetic gene expression.
2On another hand, clone improvement through genomic manipulation is an attractive strategy to potentially supply the necessary demand of hyoscyamine. In most of the plant species, artificial polyploidy (the phenomenon where species possess more than two complete sets of chromosomes in their somatic cells) has enhanced the vigor of determinate plant parts (Dhawan et al., 1996). For medicinal plants, polyploids are usually more valuable because they exhibit increased biomass and content of bioactive compounds (Gao et al., 1996). Chromosome doubling can be artificially induced by using the toxic natural product, colchicine, which acts to inhibit chromosome segregation during cell division (Otto et al., 2000). Moreover, the alkaloid content in tetraploid seeds of Datura innoxia and D. stramonium was reported to be about 2-fold higher than their diploid seeds (Berkov, 2001). The autotetraploid plants of D. stramonium, obtained by artificial polyploidization with colchicine, were also found to possess higher alkaloid contents than diploid plants (Berkov et al., 2002). Autotetraploid hairy root cultures of D. stramonium induced by direct transformation of autotetraploid plants produced more alkaloids than the diploid ones (Berkov et al., 2003; Pavlov et al., 2009a)
3There are only a few reports concerning exploitation of polyploidization of diploid hairy root cultures for secondary metabolite production (Jesus-Gonzalez et al., 2003; Weber et al., 2008; Dehghan et al., 2012). The main goals of this research were to induce tetraploidy in D. stramonium hairy roots and to study the effects of polyploidization of HRs (induced by colchicine), either alone or in combination with elicitation using acetylsalicylic (ASA) or salicylic acids (SA) with the aim of increasing hyoscyamine content. To our knowledge, there is no previous report studying the combined effect of elicitation and tetraploidization on the production of hyoscyamine alkaloids.
2. Materials and methods
2.1. Plant material and in vitro propagation
4Selected HR line of D. stramonium (LDS) was grown for one year on a solid MS medium (Murashige et al., 1962) containing 20 g·l-1 of sucrose. This line is obtained after transforming the hypocotyls of D. stramonium by Agrobacterium rhizogenes (strain A4) based on the method described by Amdoun et al. (2009). Before treatment with colchicine, the same line was grown in liquid B5 (Gamborg et al., 1976) medium at 0.5 g fresh weight per flasks containing about 50 ml of the liquid B5 medium supplemented with 30 g·l-1 of sucrose. The pH is adjusted to 5.6-5.8 in all the media. The HR cultures were kept in darkness on shaker set at 100 rpm and at a temperature of 25 ± 2 °C.
2.2. Confirmation of transgenic hairy root lines
5The integration of T-DNA in putative transformed lines was confirmed by polymerase chain reaction (PCR), where the genomic DNA was extracted from transformed hairy roots (LDS) and non-transformed roots (control) of D. stramonium. Approximately 100 mg of samples were homogenized using a crusher with tungsten balls (30 tour·s-1) for 6 min by using PL1 Kit buffer and then genomic DNA was extracted by NucleoSpin® Plant II Kit (Macherey-Nagel, Germany) and stored at -20 °C until use. Plasmid DNA from A. rhizogenes strain A4 was extracted with thermic shock and then used as a positive control. Isolated DNA was analyzed by PCR for the presence of rolB gene employing the primers, F-5′-GCGACAACGATTCAACCATATCG-3′ and R-5′-TTTACTGCAGCAGGCTTCATGAC-3′. PCR reaction was performed in a 20 l volume containing 100 ng of DNA with a volume of 2 l, 0.8 l of 10 µM of each primer (InvitrogenTM), 2 l 10X Taq buffer with Mg2+ (GenScript, USA), 0.4 l of 10 mM dNTPs (GenScript,USA), 0.4 l (5U·l-1) Green Taq DNA polymerase (GenScript, USA) and 13.6 μl of PCR clean H2O. For PCR reaction, initial denaturation was performed at 94 °C for 4 min, followed by 30 cycles of amplification (each comprising 1 min at 94 °C, 1 min at 57 °C and 1 min at 72 °C) and a final extension at 72 °C for 7 min (Mira, 2009). The PCR products were analyzed with a 1Kb Plus DNA ladder (InvitrogenTM) on 1.2% agarose-ethidium bromide gel (UltraPureTM Agarose, Invitrogen by life technologies) in TBE buffer and visualized under UV light.
2.3. Induction of tetraploid lines
6A factorial experiment was designed with two concentrations of colchicine; 0.1% and 0.5% (w/v) and medium without colchicine (0.0%, w/v) was treated as control. To do this, the liquid B5 medium containing colchicine was sterilized and used for the induction of tetraploid while following the protocol adopted by Jesus-Gonzalez et al. (2003). Fifteen days old unbranched roots (about 2 cm), were removed from B5 liquid culture medium and placed in B5 liquid medium that contains the respective concentrations of colchicine as described above, and incubated for varying exposure times (4, 7 and 11 days). The same culture conditions were applied to the HR line without treatment with colchicines (control). A total of 30 replicates were used for each treatment. At the end of the treatments, the HRs were rinsed three times for 3 to 4 min with sterile liquid B5 medium, blotted dry on sterile filter paper and transferred in a semi-solid B5 medium solidified with agar (5 g·l-1). These cultures were then incubated at 25 °C under darkness for 30 days.
2.4. Determination of ploidy level
7The number of chromosomes in Datura sp. is 2n = 2x = 24 (Berkov et al., 2003). Before polyploidy induction, the number of chromosomes of the untreated plant material (LDS) was determined by a chromosome count performed on root tips to determine the ploidy status of HR lines. Then, actively growing root tips of about 5-10 mm length were excised in the late afternoon and pretreated overnight with a solution of 0.002 M of 8-hydroquinoline at 4 °C. Pretreated HR tips were fixed in a fresh set of 90% acetic acid solution for 30 min at room temperature and then stored in 70% ethanol at 4 °C until karyologic analysis. After rinsing with distilled water, the root tips were hydrolyzed with HCl (1N) at 60 °C for 9-10 min and then rinsed with distilled water. Excess water was removed by blotting paper and the HRs were immediately incubated in total darkness and at room temperature for a maximum of 2 h in a solution of Feulgen (Jahier et al., 1992). Meristems of HRs tips (2 mm) were then excised and placed on a clean slide and squashed between slide and cover slip in the presence of a drop of 1% acetic carmine. The preparations were observed under light microscope ZEISS AxioScope with a Sony M35 camera. For each line, ten meristems, at least, were analyzed.
2.5. Elicitation of tetraploid hairy roots
8With the aim of increasing the alkaloid content, 20 days old tetraploid and diploid (LDS) lines of HRs, were treated with two elicitors, namely salicylic acid (SA) and acetylsalicylic acid (ASA) at 10-4 M concentration for 24 h. HRs (about 2 cm) were inoculated in conical flasks (250 ml) containing 50 ml of liquid B5 medium, with 30 g·l-1 sucrose and kept on shaker (100 rpm) set at 25 °C under dark conditions. At the end of the elicitation, dry weight and hyoscyamine content were quantified.
2.6. Estimation of biomass
9After elicitation, the HRs were removed from the liquid medium then well wiped with towel paper in order to eliminate the maximum of the traces of the medium. Thereafter, they were directly placed in a drying oven at 65 °C during 24 h and the dry weight was measured. Each treatment was the average of three repetitions.
2.7. Extraction and determination of alkaloids
10For the extraction of hyoscyamine, the protocol given by Amdoun et al. (2009) was adopted. In short, the procedure consists of grinding of about 50 mg of HRs in 30 ml of HCl (0.1N), stirring the mixture for 10 min and then filtering through filter paper. The pH of the filtrate is then adjusted to 10 by ammonia (NH3) and the alkaloids of aqueous phase are exhausted with chloroform (CHCl3) in an equal volume (v: v). In order to absorb the impurities and remove traces of water, a quantity of anhydrous sodium sulfate (Na2SO4) was added to the chloroform solution containing the alkaloids, followed by a filtration, then evaporated in a rotavaporator at 50 °C in order to evaporate the remainder of the chloroform. The dry residue obtained is taken up in 2 ml of dichloromethane (CH2Cl2) and then stirred using a vortex, and the solution obtained is analyzed by gas chromatography (Chrompac, model 9002) equipped with a Flame Ionization Detector FID using N2 as the carrier gas and H2 as the combustion gas. The temperatures of the oven (the colomn), the injector and the detector were set at 250, 260 and 260 °C, respectively. The column used is an apolar type DB5, of an internal diameter of 0.25 µm, an outer diameter of 0.32 µm and 32 m length. The volume injection was 1 µl. The quantification of the hyoscyamine contained in the extract was carried out while being based on the characteristics of the chromatograms obtained after the injection (the time of retention and the surface of the peak) by comparison with the calibration curve obtained by the solutions of the standard of Atropine (Fluka, USA) which is a racemic isomer of the hyoscyamine.
2.8. Statistical analysis
11An analysis of variance (ANOVA) and a Tukey test were applied for analyzing the results using SPSS (10.0) Software.
3. Results
3.1. Survival rate
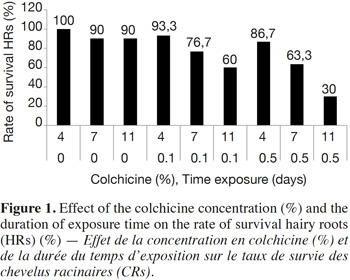
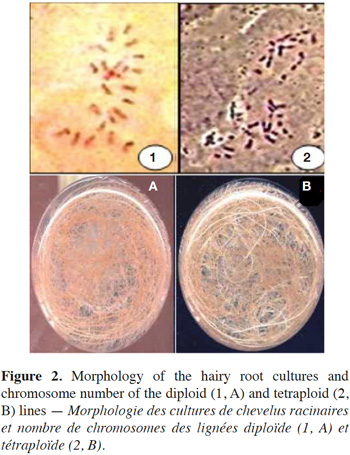
12The results show that the survival rate of treated hairy roots depends on the concentration of colchicine and the duration of exposure time. Indeed, survival decreases relative to the increase in colchicine concentration and the duration of treatment. In the presence of 0.5% of colchicine in the culture medium, the survival rate decreases from 86.6% in 4 days to 63.3% after 7 days and then to 30% after 11 days of exposure. Similarly, for the same duration of treatment, the survival rate of HRs decreases with the increasing colchicine concentration (Figure 1). Moreover, the HRs surviving in the presence of colchicine branch normally and develop new lateral roots. These were excised and then sub-cultured on culture medium to produce new potentially polyploid HRs (Figure 2).
3.2. PCR analysis
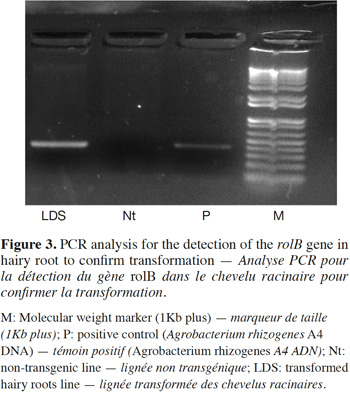
13The transformed nature of D. stramonium hairy root line was confirmed by PCR. For PCR analysis, the hairy roots were subjected to DNA isolation, and amplified using rolB specific primers. The PCR product showed a clear amplification band of rolB gene (400bp) in the hairy root line LDS (Figure 3) similar to that of positive control, while no amplification was observed in nontransformed roots (negative control). These results reveal the insertion of TL-DNA of the Ri plasmid (the presence of rolB gene fragment) into the genome of D. stramonium hairy root line. This gene has been widely used and advocated for confirmation of A. rhizogenes mediated transformation in hairy roots of various plant species.
3.3. Determination of ploidy level
14Because of the small size of the chromosomes and the low frequency of metaphase cells in the HRs tips of D. stramonium, the chromosome count was difficult. Cytological studies carried out on the HR ends of the control (line not treated with colchicine) showed that this line was diploid with 2n = 24 chromosomes. Furthermore, the karyological study of HR lines treated with colchicine showed a variation in their polyploidy level, but the majority presents 2n = 48 chromosomes. Indeed, many lines showed, after chromosome count, a tetraploid level. These have been obtained in particular with the treatment of colchicine (at 0.5% concentration) for 7 and 11 days. The culture of these selected tetraploid lines of HRs showed no morphological differences with diploid lines.
3.4. Effect of elicitation on biomass and hyoscyamine content in tetraploid lines
15The results (Table 1) show that the average dry weight and the content of hyoscyamine vary depending on ploidy level of the line. In general, a significant increase in the dry weight of HRs and their hyoscyamine content were obtained in the tetraploid lines compared with diploid controls. The increased level in the hyoscyamine content reaches 206% in the tetraploid HR line compared with the diploid HR line.
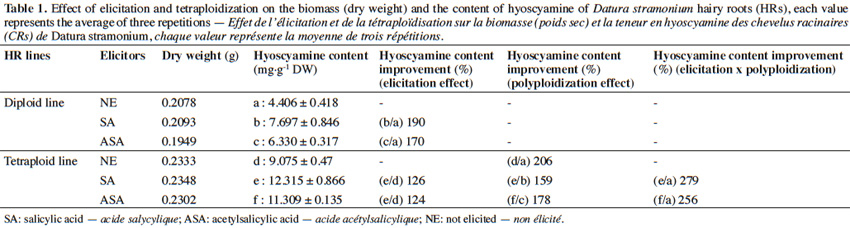
16The results in both the diploid and the two tetraploid HR lines show that ASA exerts an inhibitory effect on growth leading to a slight decrease in biomass compared to the non-elicited lines; while the elicitation by SA causes a slight increase in dry weight. Moreover, the content of hyoscyamine in the elicited HR lines was more important than in those non-elicited ones. The average content in hyoscyamine differed depending on the elicitation and HR line. It ranged from 4.406 mg·g-1 to 12.31 mg·g-1 of dry biomass of diploid HR line respectively in the non-elicited and the elicited with SA.
17If we compare the results obtained with the non-elicited diploid HR line in the present study, it was found that the levels of improvement in hyoscyamine content are significantly higher with SA in comparison with ASA. Indeed, the improvements are significant, reaching 279% and 256% in the tetraploid HR lines elicited by SA and ASA, respectively. The high values obtained with the tetraploid HR lines elicited with SA and ASA illustrate the favorable combined effect of polyploidization and the elicitation on hyoscyamine content.
4. Discussion
18In the process of polyploid induction, the combination of colchicine concentration and treatment duration are critical factors (Ye et al., 2010). Typically, high concentrations of colchicine coupled with short durations of treatment or, conversely, low concentrations of colchicine coupled with long durations of treatment are preferred. On the other hand, one of the most significant indices for the evaluation of the impact of the treatments of polyploidization is the regeneration rate of the treated material (Stanys et al., 2006). A reverse relation was obtained in our results between the concentration in colchicine and the survival of explants which corroborates with the findings of other studies that feature polyploidy induced by colchicine (Chakraborti et al., 1998; Yang et al., 2006; Sun et al., 2009).
19The diploid number of the untreated line of HR was also previously determined by other authors who have already studied D. stramonium cytogenetics. A few studies have reported that the cytogenetic analysis of HRs lines of D. stramonium had a euoploid number of chromosomes of 2n = 2x = 24 (Aird et al., 1988; Baíza et al., 1999; Berkov et al., 2003).
20The improvement of biomass and secondary metabolite content associated with polyploidy is a desired aim in the biotechnological process in order to increase the target molecule content in medicinal plant species (Liu et al., 2007). It has been reported by Shahriari et al. (2008) that tetraploid hairy roots of Hyoscyamus muticus showed an increase of 17% in dry weight compared with diploid hairy roots. This has also been reported in Salvia miltiorrhiza by Gao et al. (1996), Dracocephalum moldavica (Omidbaigi et al., 2010) and Centella asiatica (Kaensaksiri et al., 2011), where a positive trend in biomass was obtained with the tetraploid plants. This vigorous nature of tetraploid clones was not a surprising observation since larger plant organs are often produced as the result of polyploidy and already observed in other tetraploid plants (Lavania, 2005). This same author reports that polyploidy is also frequently accompanied by remarkable changes in secondary metabolism. Berkov et al. (2002) reported 2.63- and 1.41-fold increase of scopolamine and hyoscyamine content in the leaves of D. stramonium, after induction of artificial tetraploidy by colchicine treatment. The same phenomenon was recorded by Pavlov et al. (2009b) and Jesus-Gonzalez et al. (2003), respectively, in tetraploid hairy root lines of D. stramonium and Artemisia annua. In our study, this increase in hyoscyamine content as a result of tetraploidy could be due to higher activity of enzymes that catalyzes their biosynthesis. Lavania (2005) reported that in autopolyploids, the basic genetic material remains the same, but gene dosage is multiplied and there is potential to modulate gene expression and hence enzyme activity per unit protein. Changing the metabolic profile in autopolyploid plants by simple duplication of the genome was also interpreted as the cause of alterations in the mechanism(s) which regulate the biosynthesis of individual compounds (Dehghan et al., 2012). Yu et al. (2010) have already found in Arabidopsis thaliana that response to polyploidization has a genetic basis and link with DNA methylation.
21Different trials have been used to induce the accumulation of secondary metabolites under in vitro conditions. One of these trials is the induction of the accumulation of these compounds by application of elicitors (Zayed, 2011). Salicylic acid is widely known to elicit a wide range of compounds by inducing the expression of plant genes for various biosynthetic pathways (Baenas et al., 2014). It has also been reported that SA and its exogenic derivatives induce significant effects on the behavior of the vegetable cell at the biological, physiological and biochemical levels. The insignificant increased effect of SA on the biomass obtained in our results confirm those of Kang et al. (2004) in the adventitious roots of Scopolia parviflora. Chaichana et al. (2012) reported that, in general, SA elicitation has a negative effect on growth. In an earlier study, it was reported that SA treatment slightly inhibited the growth of Salvia miltiorrhiza cell cultures (Li et al., 2003). In addition, it was found that increasing the SA concentrations in the medium strongly suppressed the growth of Rubia cordifolia callus cultures (Bulgakov et al., 2002). In recent researches, a negative effect of SA on growth had also been recorded by Ajungla et al. (2009) in root cultures of Datura metel L., (Chotikadachanarong et al., 2011) and Chaichana et al. (2012) in Stemona sp. cultures. The positive effect observed in our case could be explained by the short time of contact. On the other hand, the positive effect of acetylsalicylic acid (ASA) exerted on the biomass of our lines confirm those of Wu et al. (2005) for the hairy root of Panax ginsing treated with (10-4 to 10-3 M) of ASA.
22The induction of secondary metabolites by an elicitor may cause a decreased cell mass and an increased metabolite production (Heinstein et al., 1994; Goossens et al., 2003). Moreover, our results concerning the hyoscyamine content corroborate also with those of several authors where an improvement in the production of secondary metabolites was recorded after an elicitation phase as was shown by Mehmetoglu et al. (1997), where an improvement of 50% in hyoscyamine content was recorded in Hyoscyamus muticus after elicitation with 4·10-5 M of SA and Kang et al. (2004) where an improvement of 40% of scopolamine was recorded among the adventitious roots of Scopolia parviflora. Similarly, in the results found in the alkaloid accumulation of suspension-cultured Dendrobium huoshanense, it was revealed that 10-4 M SA could enhance the accumulation of alkaloid 1.6 fold when compared with the control (Huang et al., 2010). Furthermore, the elicitation of a hairy root line of Plumbago indica L. with the same concentration (10-4 M) but of acetylsalicylic acid increased the yield of plumbagin to 3.8% DW (Martin et al., 2011). Also, the use of ASA as an elicitor resulted in an increase in total ginseng saponin content of the hairy roots of Panax ginseng at every elicitor dosage (0.1 to 1.0 mM) by about 1.1 times (Jeong et al., 2005) and in scopolamine of transgenic hairy roots of Atropa betica (el Jaber-Vazdekis et al., 2008). The hairy root cultures of Anisodus luridus treated for 24 h with 10-5 and 10-4 M ASA showed much higher accumulation levels of hyoscyamine than the control and 10-3 M ASA treated hairy root cultures (Qin et al., 2014). The positive response of the root cultures to elicitation are possibly associated with the fact that SA and ASA are key endogenous signals involved in the activation of numerous plant defense responses (Baldwin, 1999; Shah et al., 1999) and thus by inducing the expression of plant genes for various biosynthetic pathways (Baenas et al., 2014).
5. Conclusions
23Our results showed that the use of colchicine for induction of polyploidy in the hairy roots of D. stramonium yielded several auto-tetraploid lines. However, the protocol is in need of optimization. Although morphologically similar to the diploid hairy roots, tetraploid hairy roots produce significantly more biomass and hyoscyamine. The elicitation of these tetraploid lines as well as diploids lines, significantly improve the content in hyoscyamine. With the very limited success of the strategy focusing on overexpressing secondary metabolic pathway genes to increase the yield of important secondary metabolites, employing the concept of multiplying the genome number by colchicine or other substances has already shown promising results in a few medicinal plant species. It might be interesting to double the genome by various times to obtain tetra-, hexa- and pentagenome numbers and study their effects on the yield of the hyoscyamine and other tropane alkaloids in Datura species. Further, some genome and epigenome regulators can be applied to study the resulting effect from shifting the genome response based on their involvement in specific epigenetic modifications involved either in specific or the whole regulation mechanisms of tropane alkaloid pathway metabolites.
Bibliographie
Aird E.L.H., Hamill J.D. & Rhodes M.J.C., 1988. Cytogenetic analysis of hairy root cultures from a number of plant species transformed by Agrobacterium rhizogenes. Plant Cell Tissue Organ Cult., 15(1), 47-57.
Ajungla L., Patil P.P., Barmukh R.B. & Nikam T.D., 2009. Influence of biotic and abiotic elicitors on accumulation of hyoscyamine and scopolamine in root cultures of Datura metel L. Indian J. Biotechnol., 8(3), 317-322.
Amdoun R. et al., 2009. Influence of minerals and elicitation on Datura stramonium L. tropane alkaloid production: modelization of the in vitro biochemical response. Plant Sci., 177(2), 81-87.
Amdoun R. et al., 2010. Optimization of the culture medium composition to improve the production of hyoscyamine in elicited Datura stramonium L. hairy roots using the Response Surface Methodology (RSM). Int. J. Mol. Sci., 11(11), 4726-4740.
Baenas N., García-Viguera C. & Moreno D.A., 2014. Elicitation: A tool for enriching the bioactive composition of foods. Molecules, 19(9), 13541-13563.
Baíza A.M., Quiroz-Moreno A., Ruíz J.A. & Loyola-Vargas V.M., 1999. Genetic stability of hairy root cultures of Datura stramonium. Plant Cell Tissue Organ Cult., 59(1), 9-17.
Balbi A.D.V., 2008. Jasmonate signalling network in Arabidopsis thaliana: Crucial regulatory nodes and new physiological scenarios. New Phytol., 177(2), 301-318.
Baldwin I.T., 1999. The jasmonate cascade and the complexity of induced defense against herbivore attack. In: Wink M., ed. Functions of plant secondary metabolites and their exploitation in biotechnology. Kent, UK: Sheffield Academic Press, 155-186.
Benhamou N., 1996. Elicitor-induced plant defence pathways. Trends Plant Sci., 1(7), 233-240.
Berkov S., 2001. Size and alkaloid content of seeds in induced autotetraploids of Datura innoxia, Datura stramonium and Hyoscyamus niger. Pharm. Biol., 39(5), 329-331.
Berkov S. & Philipov S., 2002. Alkaloid production in diploid and autotetraploid plants of Datura stramonium. Pharm. Biol., 40(8), 617-621.
Berkov S. et al., 2003. Alkaloid spectrum in diploid and tetraploid hairy root cultures of Datura stramonium. Z. Naturforsch., C: Biosci., 58(1/2), 42-46.
Bulgakov V.P. et al., 2002. Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J. Biotechnol., 97(3), 213-221.
Chaichana N. & Dheeranupattana S., 2012. Effects of methyl jasmonate and salicylic acid on alkaloid production from in vitro culture of Stemona sp. Int. J. Biosci. Biochem. Bioinf., 2, 146-150.
Chakraborti S.P., Vijayan K., Roy B.N. & Qadri S.M.H., 1998. In vitro induction of tetraploidy in mulberry (Morus alba L.). Plant Cell Rep., 17(10), 799-803.
Chashmi N.A., Sharifi M., Karimi F. & Rahnama H., 2010. Differential production of tropane alkaloids in hairy roots and in vitro cultured two accessions of Atropa belladonna L. under nitrate treatments. Z. Naturforsch., C: Biosci., 65(5-6), 373-379.
Chotikadachanarong K. et al., 2011. Influence of salicylic acid on alkaloid production by root cultures of Stemona curtisii Hook. F. Curr. Res. J. Biol. Sci., 3(4), 322-325.
Dehghan E., Häkkinen S.T., Oksman-Caldentey K.M. & Shahriari Ahmadi F. 2012. Production of tropane alkaloids in diploid and tetraploid plants and in vitro hairy root cultures of Egyptian henbane (Hyoscyamus muticus L.). Plant Cell Tissue Organ Cult., 110(1), 35-44.
Dhawan O.P. & Lavania U.C., 1996. Enhancing the productivity of secondary metabolites via induced polyploidy: a review. Euphytica, 87(2), 81-89.
el Jaber-Vazdekis N., Barres M.L., Ravelo A.G. & Zarate R., 2008. Effects of elicitors on tropane alkaloids and gene expression in Atropa baetica transgenic hairy roots. J. Nat. Prod., 71(12), 2026-2031.
Flores H.E., Vivanco J.M. & Loyola-Vargas V.M., 1999. “Radicle” biochemistry: the biology of root-specific metabolism. Trends Plant Sci., 4(6), 220-226.
Gamborg O.L., Murashige T., Thorpe T.A. & Vasil I.K., 1976. Plant tissue culture media. In Vitro, 12(7), 473-478.
Gao S.L., Zhu D.N., Cai Z.H. & Xu D.R., 1996. Autotetraploid plants from colchicine-treated bud culture of Salvia miltiorrhiza Bge. Plant Cell Tissue Organ Cult., 47(1), 73-77.
Goossens A. et al., 2003. A functional genomics approach toward the understanding of secondary metabolism in plant cells. Proc. Natl. Acad. Sci. U.S.A., 100(14), 8595-8600.
Harfi B. et al., 2011. Effect of culture medium on hyoscyamine production from four Datura sp. hairy roots. Adv. Environ. Biol., 5(5), 1023-1030.
Heinstein P.F. & Chang C.J., 1994. Taxol. Annu. Rev. Plant Physiol. Plant Mol. Biol., 45(1), 663-674.
Huang B. et al., 2010. Effects of SNP, PA and SA on cell growth and physiological activities of suspension-cultured protocorn-like bodies of Dendrobium huoshanense C. Plant Physiol. Commun., 46, 423-426.
Jahier J., ed., 1992. Techniques de cytogénétique végétale. Versailles, France : Quæ.
Jeong G.-T. et al., 2005. Production of antioxidant compounds by culture of Panax ginseng C.A. Meyer hairy roots. In: Davison B.H. et al., eds. Twenty-sixth symposium on biotechnology for fuels and chemicals. ABAB Symposium. New York, NY, USA: Humana Press, 1147-1157.
Jesus-Gonzalez L.D. & Weathers P.J., 2003. Tetraploid Artemisia annua hairy roots produce more artemisinin than diploids. Plant Cell Rep., 21(8), 809-813.
Kaensaksiri T., Soontornchainaksaeng P., Soonthornchareonnon N. & Prathanturarug S., 2011. In vitro induction of polyploidy in Centella asiatica (L.) Urban. Plant Cell Tissue Organ Cult., 107(2), 187-194.
Kang S.-M. et al., 2004. Effects of methyl jasmonate and salicylic acid on the production of tropane alkaloids and the expression of PMT and H6H in adventitious root cultures of Scopolia parviflora. Plant Sci., 166(3), 745-751.
Lavania U.C., 2005. Genomic and ploidy manipulation for enhanced production of phyto-pharmaceuticals. Plant Genet. Resour., 3(02), 170-177.
Li G.-J., Wang S.C., Xia K. & Zhou X., 2003. Effect of yeast elicitor and salicylic acid on the fluctuation of phytohormone contents in Ti-transformed Salvia miltiorrhiza cell cultures. Plant Growth Regul., 39(1), 27-32.
Liu Z. & Gao S., 2007. Micropropagation and induction of autotetraploid plants of Chrysanthemum cinerariifolium (Trev.) Vis. Vitro Cell. Dev. Biol. - Plant, 43(5), 404-408.
Makhzoum A., Petit-Paly G., St Pierre B. & Bernards M.A., 2011. Functional analysis of the DAT gene promoter using transient Catharanthus roseus and stable Nicotiana tabacum transformation systems. Plant Cell Rep., 30, 1173-1182.
Makhzoum A., Sharma P., Bernards M.A. & Trémouillaux-Guiller J., 2013. Hairy roots: An ideal platform for transgenic plant production and other promising applications. In: Gang D.R., ed. Phytochemicals, plant growth, and the environment. New York, NY, USA: Springer New York, 95-142.
Makhzoum A., Benyammi R., Moustafa K. & Trémouillaux-Guiller J., 2014. Recent advances on host plants and expression cassettes’ structure and function in plant molecular pharming. BioDrugs Clin. Immunother. Biopharm. Gene Ther., 28(2), 145-159.
Malik S., Bhushan S., Sharma M. & Singh Ahuja P., 2011. Physico-chemical factors influencing the shikonin derivatives production in cell suspension cultures of Arnebia euchroma (Royle) Johnston, a medicinally important plant species. Cell Biol. Int., 35(2), 153-158.
Malik S. et al., 2013. Root-zone temperature alters alkaloid synthesis and accumulation in Catharanthus roseus and Nicotiana tabacum. Ind. Crops Prod., 49, 318-325.
Martin K.P., Sabovljevic A. & Madassery J., 2011. High-frequency transgenic plant regeneration and plumbagin production through methyl jasmonate elicitation from hairy roots of Plumbago indica L. J. Crop Sci. Biotechnol., 14(3), 205-212.
Mehmetoglu Ü. & Curtis W.R., 1997. Effects of abiotic inducers on sesquiterpene synthesis in hairy root and cell-suspension cultures of Hyoscyomus muticus. Appl. Biochem. Biotechnol., 67(1-2), 71-77.
Mira F.R., 2009. The effect of arbuscular mycorrhiza on micropropagated olive (Olea europaea L.) plant growth. PhD thesis: Università Degli Studi Della Tuscia, Viterbo (Italy).
Murashige T. & Skoog F., 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant., 15(3), 473-497.
Namdeo A.G., 2007. Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn. Rev., 1(1), 69-79.
Ollagnier S., Kervio E. & Rètey J., 1998. The role and source of 5P-deoxyadenosyl radical in a carbon skeleton rearrangement catalyzed by a plant enzyme. FEBS Lett., 437, 309-312.
Omidbaigi R., Yavari S., Hassani M.E. & Yavari S., 2010. Induction of autotetraploidy in dragonhead (Dracocephalum moldavica L.) by colchicine treatment. J. Fruit Ornam. Plant Res., 1(18), 25-35.
Otto S.P. & Whitton J., 2000. Polyploid incidence and evolution. Annu. Rev. Genet., 34, 401-437.
Pavlov A., Berkov S., Weber J. & Bley T., 2009a. Hyoscyamine biosynthesis in Datura stramonium hairy root in vitro systems with different ploidy levels. Appl. Biochem. Biotechnol., 157(2), 210-225.
Pavlov A., Georgiev V.G., Marchev A.S. & Berkov S.H., 2009b. Nutrient medium optimization for hyoscyamine production in diploid and tetraploid Datura stramonium L. hairy root cultures. World J. Microbiol. Biotechnol., 25(12), 2239-2245.
Pitta-Alvarez S.I., Spollansky T.C. & Giulietti A.M., 2000. The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enzyme Microb. Technol., 26(2-4), 252-258.
Qin B. et al., 2014. Effects of acetylsalicylic acid and UV-B on gene expression and tropane alkaloid biosynthesis in hairy root cultures of Anisodus luridus. Plant Cell Tissue Organ Cult., 117(3), 483-490.
Shah J., 2003. The salicylic acid loop in plant defense. Curr. Opin. Plant Biol., 6(4), 365-371.
Shah J., Kachroo P. & Klessig D.F., 1999. The Arabidopsis ssi1 mutation restores pathogenesis-related gene expression in npr1 plants and renders defens in gene expression salicylic acid dependent. Plant Cell, 11(2), 191-206.
Shahriari F.A., Dehghan E., Farsi M. & Azizi M., 2008. Tetraploid induction of Hyoscyamus muticus L. using colchicine treatment. Pak. J. Biol. Sci., 11(24), 2653-2659.
Srivastava S. & Srivastava A.K., 2007. Hairy root culture for mass-production of high-value secondary metabolites. Crit. Rev. Biotechnol., 27(1), 29-43.
Stanys V., Weckman A., Staniene G. & Duchovskis P., 2006. In vitro induction of polyploidy in japanese quince (Chaenomeles japonica). Plant Cell Tissue Organ Cult., 84(3), 263-268.
Sun Q., Sun H., Li L. & Bell R.L., 2009. In vitro colchicine-induced polyploid plantlet production and regeneration from leaf explants of the diploid pear (Pyrus communis L.) cultivar, “Fertility”. J. Hortic. Sci. Biotechnol., 84(5), 548-552.
Wang K.L.-C., Li H. & Ecker J.R., 2002. Ethylene biosynthesis and signaling networks. Plant Cell, 14(1), 131-151.
Weber J., Georgiev V., Pavlov A. & Bley T., 2008. Flow cytometric investigations of diploid and tetraploid plants and in vitro cultures of Datura stramonium and Hyoscyamus niger. Cytometry A, 73A(10), 931-939.
Wu J.Y., Wong K., Ho K.P. & Zhou L.G., 2005. Enhancement of saponin production in Panax ginseng cell culture by osmotic stress and nutrient feeding. Enzyme Microb. Technol., 36(1), 133-138.
Yang X.M. et al., 2006. In vitro tetraploid induction via colchicine treatment from diploid somatic embryos in grapevine (Vitis vinifera L.). Euphytica, 152(2), 217-224.
Ye Y.M. et al., 2010. Morphological and cytological studies of diploid and colchicine-induced tetraploid lines of crape myrtle (Lagerstroemia indica L.). Sci. Hortic., 124(1), 95-101.
YuZ. et al., 2010. Impact of natural genetic variation on the transcriptome of autotetraploid Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A., 107(41), 17809-17814.
Zayed R., 2011. Efficient in vitro elicitation of β-carboline alkaloids in transformed root cultures of Peganum harmala. Bull. Fac. Pharm. Cairo Univ., 49(1), 7-11.
