- Home
- Volume 19 (2015)
- numéro 4
- A survey of bacteria found in Belgian dairy farm products
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
A survey of bacteria found in Belgian dairy farm products

Editor's Notes
Received on January 29, 2015; accepted on June 29, 2015
Résumé
Qualité microbiologique des produits laitiers issus des fermes belges
Description du sujet. Dans le cadre d’un programme public belge de soutien aux fermiers dans la diversification de leurs productions, des échantillons de produits laitiers incluant du yaourt, de la crème glacée, du beurre et du fromage au lait cru ont été prélevés dans 318 fermes en Wallonie, entre les années 2006 et 2014.
Objectifs. Investiguer la qualité microbiologique des produits laitiers fabriqués dans les fermes en Belgique.
Méthode. Selon le Règlement européen EC 2073/2005, des analyses bactériologiques ont été réalisées en vue de la détection et du dénombrement des bactéries telles que les Enterobacteriaceae, Listeria monocytogenes, Salmonella spp., Escherichia coli ainsi que Staphylococcus aureus.
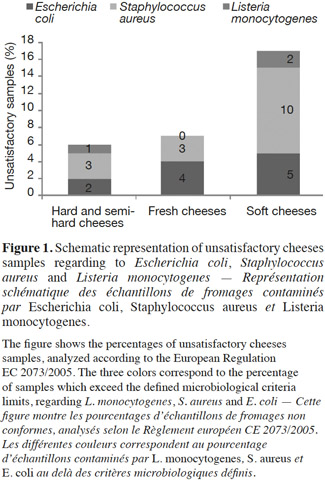
Résultats. Les résultats obtenus sont conformes aux critères microbiologiques définis. Cependant, dans 35 % des échantillons de beurre et 4 % des échantillons de fromages analysés, les nombres de micro-organismes indicateurs d’hygiène des procédés tels qu’E. coli et S. aureus sont au delà des limites microbiologiques fixées. Le nombre d’échantillons non conformes observé parmi les fromages à pâte molle est plus élevé (10 % et 2 % pour S. aureus et L. monocytogenes) que celui observé parmi les fromages à pâte pressée (3 % et 1 %) ainsi que les fromages frais (3 % et 0 %) (P ≥ 0,05). Par ailleurs, le nombre d’échantillons non conformes est significativement élevé pendant l’été pour toutes les bactéries impliquées.
Conclusions. Cette étude montre que la plupart des produits laitiers étudiés est satisfaisant. Cependant, le nombre élevé de micro-organismes indicateurs d’hygiène (e.g., E. coli) dans certains produits comme le beurre, met en évidence l’importance d’appliquer les bonnes pratiques d’hygiène à toutes les étapes de la production afin d’assurer la sécurité des consommateurs.
Abstract
Description of the subject. Due to the potential hazards caused by pathogenic bacteria, farm dairy production remains a challenge from the point of view of food safety. As part of a public program to support farm diversification and short food supply chains, farm dairy product samples including yogurt, ice cream, raw-milk butter and cheese samples were collected from 318 Walloon farm producers between 2006 and 2014.
Objectives. Investigation of the microbiological quality of the Belgian dairy products using the guidelines provided by the European food safety standards.
Method. The samples were collected within the framework of the self-checking regulation. In accordance with the European Regulation EC 2073/2005, microbiological analyses were performed to detect and count Enterobacteriaceae, Listeria monocytogenes, Salmonella spp., Escherichia coli and Staphylococcus aureus.
Results. Even when results met the microbiological safety standards, hygienic indicator microorganisms like E. coli and S. aureus exceeded the defined limits in 35% and 4% of butter and cheese samples, respectively. Unsatisfactory levels observed for soft cheeses remained higher (10% and 2% for S. aureus and L. monocytogenes respectively) than those observed for pressed cheeses (3% and 1%) and fresh cheeses (3% and 0%) (P ≥ 0.05). Furthermore, the percentages of samples outside legal limits were not significantly higher in the summer months than in winter months for all mentioned bacteria.
Conclusions. This survey showed that most farm dairy products investigated were microbiologically safe. However, high levels of hygiene indicators (e.g., E. coli) in some products, like butter, remind us of applying good hygienic practices at every stage of the dairy production process to ensure consumer safety.
Table of content
1. Introduction
1Milk remains one of the principal products of the dairy sector in Belgium, where the farm cow's milk production reached approximately 3,474.3 millions kg in 2013 (SPF Économie, PME, Classes moyennes et Énergie, 2014). According to the Belgian Dairy Industry Confederation annual report, the sales of dairy products also increased worldwide in 2012 (CBL, 2013). This implies that hygiene concerns, as well as quality control of dairy foods, will continue and the challenges faced by the dairy industry will increase (Hussein et al., 2005). Cheese is a ready-to-eat food easily contaminated on the surface by undesirable microorganisms. Even if some are spoilage microorganisms, which may produce uncharacteristic visual appearance and diminish the commercial value of the cheeses, others are pathogenic such as Listeria monocytogenes, which have been associated with foodborne listeriosis by consumption of cheese (McLauchlin et al., 2004; Pintado et al., 2010). As the basis of dairy products, raw milk has been shown to be a potential source of food pathogens. In addition, bacteria can enter dairy products at many points during their processing. Such points include entry via the starter culture, floor, packaging material and production room air (Cotton et al., 1992; Kousta et al., 2010; Hill et al., 2012). Numerous foodborne diseases have been associated with milk and dairy products in recent years. According to De Buyser et al. (2001), milk and milk products were implicated in 1–5% of total foodborne disease outbreaks between 1988 and 1998. Langer et al. (2012) reviewed the number of dairy-associated outbreaks during 1993-2006 in the United States and found a total of 121 outbreaks resulting in 202 hospitalizations and 2 deaths. Non-pasteurized as well as pasteurized dairy products associated with these outbreaks included fluid milk and cheeses. Among the causative agents, L. monocytogenes, Salmonella spp., Escherichia coli and Staphylococcus aureus were identified.
2Listeria monocytogenes is a ubiquitous pathogen with many possible modes of entry into dairy processing facilities. Parisi et al. (2013) highlighted the wide-spread presence of L. monocytogenes in cheese factories. According to that study, this bacterium can persist for long periods of time, resulting in a continuous contamination of the dairy products. This is of concern because L. monocytogenes has been shown to cause life-threatening disease in foetuses, newborns, immunocompromised people and the elderly (Schuchat et al., 1991).
3Salmonella can cause an illness called salmonellosis in humans. In the European Union (EU), over 100,000 cases are reported each year. Moreover an estimated 1 million salmonellosis cases and more than 400 salmonellosis-associated deaths occur annually in the United States (Galanis et al., 2006; Scallan et al., 2011; EFSA, 2013; Switt et al., 2013). Salmonella species have been isolated from the faeces of healthy dairy cattle, where they may exist as normal members of the gastrointestinal population (Roy et al., 2001; Wells et al., 2001; Callaway et al., 2005). Moreover, Salmonella has been identified as one of the frequent pathogens associated with foodborne diseases implicated in milk and milk products in France and other countries (De Buyser et al., 2001). Escherichia coli is a bacterium that coexists with its human host in the intestines in a mutually beneficial relationship (Tchaptchet et al., 2011). While most strains of E. coli are commensal, some are known to cause severe enteric disease by infection of the epithelial cells or by the production of toxins. Among them, the E. coli strain O157:H7 is the most frequently involved in human diseases (Caro et al., 2011). Food and dairy products are the main vehicles of E. coli O157:H7, as revealed by a study of 90 outbreaks occurring between 1982 and 2006 in several occidental countries (Snedeker et al., 2009).
4The bacterium S. aureus is reported to be one of the most frequent pathogens involved in foodborne diseases associated with dairy products, especially with raw-milk cheese (Techer et al., 2013). The heat-stable enterotoxins produced by this bacterium can cause staphylococcal food poisoning, which ranks as one of the most prevalent causes of gastroenteritis worldwide. Dairy products are frequently involved in food poisoning with enterotoxins levels as low as 0.5 ng·g-1 (Dinges et al., 2000). Moreover, a previous study showed that methicillin-resistant S. aureus was detected in different bovine milks and cheeses marketed in Italy (Normanno et al., 2007; Hennekinne et al., 2012). Listeria monocytogenes and Salmonella spp. are included in the European safety criteria Regulation (EC 2073/2005) for dairy products, where defined limits state these microorganisms should be found in “absence in 25 g” or “less than or equal to 100 CFU·g-1” of product. In the same regulation, E. coli and S. aureus are included in the hygienic criteria. Pathogenic E. coli and S. aureus enterotoxins are also included in the safety criteria regulation.
5In order to develop or to obtain high added value to its productions and to have a consistent contact with the population, it is important for farm producers to diversify their productions.
6In Belgium, the Laboratory of Agro-food Quality and Safety of the University of Liege - Gembloux Agro-Bio Tech, with the support of Public Service of Wallonia (DiversiFerm program), helps and guides the farm producers in their efforts for diversification through hygienic, technological and economic guidance.
7According to the European Regulation EC 2073/2005, microbiological analyses should be carried out frequently by the producers to ensure the safety of the food products (self-checking). This is the context in which different data from microbiological analyses have been collected from the farm producers, as part of the self-checking program. The original raw data obtained from the DiversiFerm program include the results from analysis of yogurt, ice cream, raw milk butter and raw milk cheeses. It is important to note that these dairy products were all made in an artisanal way in Walloon farms.
8The present work reports a critical evaluation of the data collected over several years. We first investigated the microbiological quality of the dairy products using the guidelines provided by the European food safety standards. We then studied the seasonal distribution of bacteria in the non-complying dairy products from Walloon farms.
2. Materials and methods
9Yogurt, ice cream, raw-milk butter and raw-milk cheeses including fresh, soft and pressed cheeses were collected from each of 318 separate farms located throughout the Walloon Region between 2006 and 2014. Fresh cheeses were described as unripened cheeses with short shelf-lives (1-3 weeks), while soft cheeses were aged two months or less. Pressed cheeses, rich in flavor and dry in texture, were the most aged cheeses. Analyses were performed at various stages of their shelf life.
10The same sites were sampled at least twice a year, within the self-checking context, and a total of 1,128 dairy product samples were collected. The analyses were conducted by two accredited environmental, toxicology and food control laboratories, in accordance with the European Regulation EC 2073/2005. The detection or enumeration was performed for foodborne pathogens or microorganisms safety indicators like L. monocytogenes and Salmonella spp. and for indicators of hygiene like E. coli and S. aureus. Reference methods were used for the enumeration of Enterobacteriaceae (ISO 21528-2), E. coli (ISO 16649-2) and S. aureus (ISO 6888-2). The S. aureus enterotoxin detection was performed by the method vidas set2 and the kit set-RPLA.
11The analyses of L. monocytogenes and Salmonella spp. were performed using validated alternative methods. Analytical methods used for assessing L. monocytogenes levels are either a detection method in 25 g or the colony-count technique in 1 g. When detected, the colony count technique was applied.
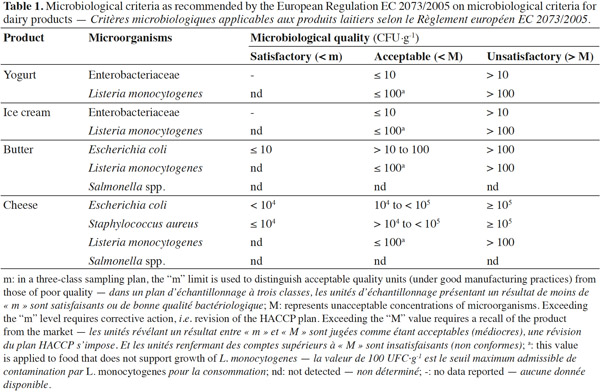
12All the data were compiled in a Microsoft ACCESS 2007 database and exported to Microsoft Excel 2010 for descriptive and statistical analyses. The minimum, maximum, frequencies and arithmetic mean values were calculated for E. coli, L. monocytogenes and S. aureus. The microbiological quality of dairy products involved in this study was assessed using criteria in the European Regulation EC 2073/2005 (Table 1).
3. Results and discussion
3.1. Microbiological quality of cheeses
13For this study, 142 pressed cheese, 267 fresh cheese and 92 soft cheese samples were analyzed for S. aureus, E. coli, L. monocytogenes and Salmonella, since these microorganisms present the greatest concern for cheese makers (Johnson et al., 1990; Hill et al., 2013). In 98% of fresh, pressed-type cheeses tested, the microbiological safety and hygiene criteria were achieved, but about 3% and 4% of samples were not within the defined limits regarding to S. aureus and E. coli respectively (Table 2). The cheeses batch where S. aureus levels exceeded the legal limits (105 CFU·g-1), were tested for S. aureus enterotoxins as required by food safety criteria and withdrawn from sale or recalled from the market, even if none of the samples tested contained enterotoxins.
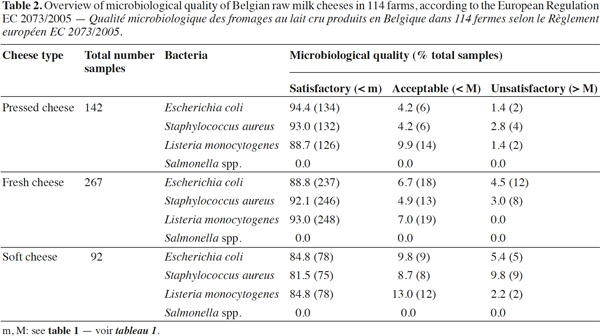
14Listeria monocytogenes was present in about 10% of fresh and pressed cheese samples and in 13% of soft cheese samples. The limit of 100 CFU·g-1 was exceeded in 1% pressed cheeses and 2% soft cheeses. Although L. monocytogenes counts exceeding the level of 100 CFU·g-1 were found in relatively few cheese samples (2% and 1% in soft and pressed cheese samples respectively), this microorganism still remains present in all cheese types (Table 3). The significance of L. monocytogenes in cheese and in samples from dairy plants has been previously reported (Makino et al., 2005; Harakeh et al., 2009; Cagri-Mehmetoglu et al., 2011). Even though large prevention methods exist, more continuous monitoring of hygiene measures is needed to avoid spreading in the production facilities when L. monocytogenes is detected.
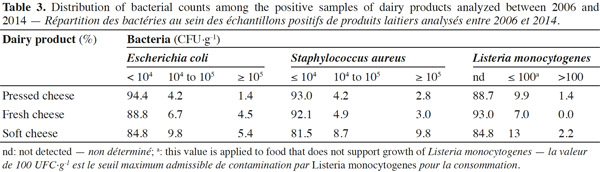
15Staphylococcus aureus criteria levels exceeded limits in 10% of soft cheese samples examined. The counts of this bacterium in about 96% of the cheese samples (all types mingled) analyzed was under the maximum tolerable value M (105 CFU·g-1). The frequent contamination of cheeses or dairy products by S. aureus has been reported by several authors (André et al., 2008; Little et al., 2008; Ostyn et al., 2010; Gücükoüglu et al., 2012). An interesting example of contamination is the one that provoked a food poisoning in Japan in 2000 and that affected 13,420 people due to the ingestion of low-fat milk, contaminated by the enterotoxin (0,08-0,38 ng·ml-1) of S. aureus (Asao et al., 2003). In the present study, no enterotoxins were found in non-complying samples tested. This is in line with the Kousta et al. (2010) study that found that 96% of the cheese samples (n = 351) collected from different cheese producers conformed to the EU criteria for S. aureus. Furthermore, the levels of unsatisfactory samples observed for fresh cheeses remain lower than those observed for the soft cheeses, considering S. aureus and L. monocytogenes (Figure 1) (P ≥ 0.05). Indeed bacterial growth is higher during the cheese ripening process, and it also depends on the specific conditions of cheese varieties such as heat, acid and salt tolerance, initial numbers and individual characteristics of the species or strains in question (Beuvier et al., 2004; Rosengren et al., 2010; Cagri-Mehmetoglu et al., 2011). Lactic fermentation used for fresh cheese production contributes to the reduction of this cheese pH (< 4.5), which prevents the development of pathogens. On the other hand, the enzymatic technology contributes to increasing the pH ranges from 4.5 to 4.8 for soft cheeses and from 4.8 to 5.2 for pressed cheeses (24 h after salting). In addition, soft cheese with higher moisture content (aw 0.97-0.99) provides a more favorable environment for microbial growth than pressed cheese, which presents a moisture content of 0.94-0.97 (Belleflamme et al., 2006; Callon et al., 2011).
16Although soft, fresh and pressed cheese samples were examined for the detection of Salmonella spp., none of them were positive for this pathogen. This absence could be explained by maturation conditions, Salmonella characteristics and the adherence to food management regulations (prerequisites and HACCP; Beuvier et al., 2004). Little et al. (2008) also reported no Salmonella in 1,819 cheese samples investigated in UK. This is in contrast with the literature that tells us that a large variety of dairy products, including cheeses, have been linked to human salmonellosis cases (Callaway et al., 2005; Switt et al., 2013).
3.2. Microbiological quality of butter
17Applying the criteria in the European Regulation EC 2073/2005, 42% of the 363 butter samples examined were satisfactory in their levels of microorganism hygiene indicator E. coli, while 23% of butter samples were acceptable, but 35% exceeded the European Regulation EC 2073/2005 limit for E. coli (ranging from < 1 to 105 CFU·g-1; table 4). Several reasons could explain these unsatisfactory samples; for instance, the raw milk used in the butter-making process may not have been subjected to heat treatment, and any viable microorganisms, which may have originated from a mastitis infection for instance, could have further contaminated the butter.
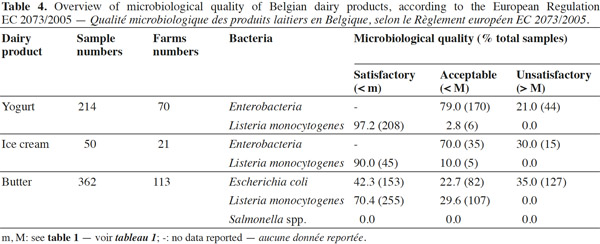
18More frequently, the inadequate cleaning of skimmers could result in a strong growth of E. coli during the maturation step (unpublished results). Another source of contamination by E. coli could be the butter handlers. To our knowledge, there is less information available on the levels of E. coli in raw-milk butter than there is on other dairy products. Considering that pathogenic E. coli have been isolated from dairy products (Hussein et al., 2005), the presence of high levels (> 104 CFU·g-1) of E. coli in some samples tested in our study is a source of concern. Adequate hygienic practices at every stage of the process could help to reduce the health risks linked to these microorganisms, in particular a proper mastitis control program and proper sanitation practices before the maturation of cream starts (milking, skimming and maturation material). Salmonella were absent in all butter samples examined and L. monocytogenes was present at low levels (ranging from 10 to 40 CFU·g-1) and in only 30% of the samples analyzed. The above results are in accordance with other studies that reported little or no butter samples contaminated by L. monocytogenes (Kozak et al., 1996; Aygun et al., 2006). Moreover the L. monocytogenes presence in butter is consistent with environmental contaminations, when cleaning and sanitation of material are ineffective.
3.3. Microbiological quality of yogurt
19A total of 214 yogurt samples were analyzed for the presence of L. monocytogenes and Enterobacteriaceae. As shown in table 4, all the samples tested were at satisfactory or acceptable levels for L. monocytogenes, while 21% were above the legal limits for Enterobacteriaceae. The Enterobacteriaceae counts in unsatisfactory samples ranged from < 102 to 105 CFU·g-1, and 4 out of 44 samples contained about 104–105 CFU·g-1. These levels may be due to contamination after pasteurization. According to European Regulation EC 2073/2005, Enterobacteriaceae are registered as process hygiene indicators in pasteurized milk and other pasteurized dairy products. Therefore, the specific bacteria of this family such as Salmonella and E. coli were enumerated and researched in the unsatisfactory samples before the yogurt sale.
20Normally, yogurt presents a reduced risk of pathogenic bacteria due to the heat treatments applied and the product’s acidic pH of 4-4.5 (Morgan et al., 1993; Hill et al., 2013). However, post-pasteurization contamination and the conditions of fermentation such as temperature, fermentation time and final pH are important factors that may affect the presence and survival of unwanted bacteria. Furthermore, the most pathogenic bacterium of the Enterobacteriaceae family, E. coli O157:H7, could grow in and survive the acidic conditions of yogurt preparation and thus lead to bacterial enteric infections in consumers (Massa et al., 1997; Gulmez et al., 2003; Cirone et al., 2013). The lack of L. monocytogenes in yogurt agrees with Aygun et al. (2006), who found no L. monocytogenes in yogurt samples after they examined 157 dairy products. These results confirmed the effort made by the farmers to ensure compliance with the standard, but also and above all an underestimation of the impact of fungi.
3.4. Microbiological quality of ice cream
21Among the 50 ice cream samples examined in this study, 30% were above the legal criteria for Enterobacteriaceae (Table 4). The levels of Enterobacteriaceae in ice cream were highly variable, ranging from < 10 to 103 CFU·g-1. These Enterobacteriaceae in ice cream could be due to microorganisms introduced originally by milk, by other ingredients or by the food handlers at the location where these products are prepared. An interesting example of the origin of the ice cream contamination is reported by Fetsch et al. (2014). Indeed, after the food poisoning outbreak caused by staphylococcal enterotoxins in ice cream in Germany, Fetsch and collaborators have identified the equipment used for the production of the ice cream or a contaminated ingredient as the most likely introduction sources of S. aureus.
22In this study, the ice cream batches where Enterobacteriaceae levels exceed the legal limits (> 10) were used for the identification of the various members of Enterobacteriaceae at genus and species level. The non-complying batches were withdrawn from sale. In none of the ice cream samples tested, the levels of L. monocytogenes exceeded the 100 CFU·g-1 legal limits, indicating that the microbiological safety criteria were achieved in both cases (Domenech et al., 2013).
4. Seasonal distribution of bacteria in dairy products
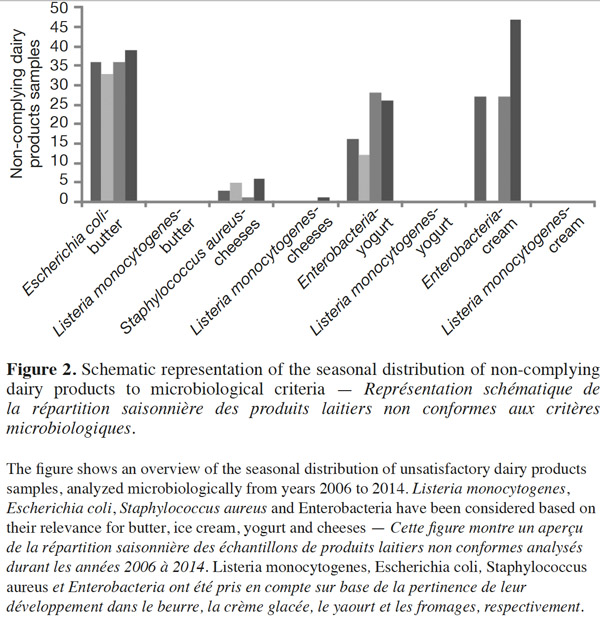
23Figure 2 shows the seasonal distribution of unsatisfactory dairy samples analyzed microbiologically from 2006 to 2014. Listeria monocytogenes, E. coli, S. aureus and Enterobacteriaceae have been considered based on their prevalent presence in butter, ice cream, yogurt and cheeses. Even if there are some differences between the percentages of the non-complying dairy products over the seasons, the proportion of these products is not significantly higher (P ≥ 0.05) in the summer months than in winter with regards to the mentioned bacteria, by the XLSTAT 2014 test. The proportion of samples contaminated by L. monocytogenes beyond the legal limits of 100 CFU·g-1 remained at almost zero across all four seasons. However, statistically significant (P < 0.01) seasonal differences in the incidence of L. monocytogenes have been found in samples of raw caprine milk (Gaya et al., 1996). According to these authors, the highest incidence occurred in autumn. Several authors have indicated that Shiga-toxin producing E. coli excretion by healthy cattle in raw milk varies according to season, peaking in warmer months (Heuvelink et al., 1998; Berry et al., 2010; Farrokh et al., 2013). In the same vein, Caro et al. (2011) have reported that summer is the season with the highest frequency of positive samples for E. coli O157.
5. Conclusions
24All analytical results were compared to the safety and hygiene criteria defined by the European Regulation EC 2073/2005. All samples analyzed were free of Salmonella spp. As regards L. monocytogenes, their levels rarely exceeded the legal limit of 100 CFU·g-1. Nevertheless, there is a concern about the presence of L. monocytogenes in raw-milk butter, raw-milk cheeses and ice cream samples tested. In order to assess good hygiene practices, hygiene indicators (E. coli, S. aureus) were counted in this study. Although the detected levels of bacteria were reasonable in most of the samples tested, our study highlighted important hygiene issues. The levels of E. coli exceeded defined limits in 35% of butter samples and the levels of S. aureus exceeded limits in 4% of cheese samples tested. These microorganisms can present a hazard in foods and can affect the health of the consumer. To avoid any health hazards in these artisanal food products, adequate hygienic practices are needed at every stage of the process to assess and control the growth of the bacteria. This survey emphasized the microbiological quality of farm dairy products and provided indications to ensure the safety of the farm food products.
25Acknowledgements
26This work was supported by DiversiFerm, a grant from the Public Service of Wallonia, Directorate-General for Agriculture, Natural Resources and the Environment, Belgium. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We also thank the producers and the personnel of laboratories who collected samples for this study.
Bibliographie
André M.C.D.P.B. et al., 2008. Comparison of Staphylococcus aureus isolates from food handlers, raw bovine milk, and Minas Frescal cheese by antibiogram and pulsed-field gel electrophoresis following SmaI digestion. Food Control, 19, 200-207.
Asao T. et al., 2003. An extensive outbreak of staphylococcal food poisoning due to low-fat milk in Japan: estimation of enterotoxin A in the incriminated milk and powdered skim milk. Epidemiol. Infect., 130, 33-40.
Aygun O. & Pehlivanlar S., 2006. Listeria spp. in the raw milk and dairy products in Antakya, Turkey. Food Control, 17, 676-679.
Belleflamme C., Di Tanna S. & Sindic M., 2006. Le risque Listeria monocytogenes pour la transformation laitière fermière. Filière Ovine Caprine, 18.
Berry E.D. & Wells J.E., 2010. Escherichia coli 0157:H7: recent advances in research on occurrence, transmission and control in cattle and the production environment. Adv. Food Nutr. Res., 60, 67-117.
Beuvier E. & Buchin S., 2004. Raw milk cheeses. In: Fox F.P., McSweeney P.L.H., Cogan T.M. & Guinee T.P., eds. Cheeses: chemistry, physics and microbiology. Vol. 1: general aspects. Elsevier Ltd, 319-344.
Cagri-Mehmetoglu A. et al., 2011. Incidence of Listeria monocytogenes and Escherichia coli O157:H7 in two Kasar cheese processing environments. Food Control, 22, 762-766.
Callaway T.R. et al., 2005. Fecal prevalence and diversity of Salmonella species in lactating dairy cattle in four states. J. Dairy Sci., 88, 3603-3608.
Callon C. et al., 2011. Simplification of a complex microbial antilisterial consortium to evaluate the contribution of its flora in uncooked pressed cheese. Int. J. Food Microbiol., 145, 379-389.
Caro I., Mateo J., Rúa J. & Del Rosario García-Armesto M., 2011. Occurrence of Escherichia coli O157, O111 and O26 in raw ewe's milk and performance of two enrichment broths and two plating media used for its assessment. Int. J. Food Microbiol., 146, 84-87.
Cirone K. et al., 2013. Growth of Mycobacterium avium subsp. paratuberculosis, Escherichia coli and Salmonella enteritidis during preparation and storage of yogurt. ISRN Microbiol., 2013:247018.
CBL (Confédération Belge de l’Industrie Laitière), 2013. Rapport annuel. Année d’activités 2012. Leuven, Belgique : CBL.
Cotton L.N. & White C.H., 1992. Listeria monocytogenes, Yersinia enterocolitica, and Salmonella in dairy plant environments. J. Dairy Sci., 75, 51-57.
De Buyser M.L., Dufour B., Maire M. & Lafarge V., 2001. Implication of milk and milk products in food-borne diseases in France and in different industrialized countries. Int. J. Food Microbiol., 67, 1-17.
Dinges M.M., Orwin P.M. & Schlievert P.M., 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev., 13, 16-34.
Domenech E., Amorós J.A. & Escriche I., 2013. Effectiveness of prerequisites and the HACCP plan in the control of microbial contamination in ice cream and cheese companies. Foodborne Pathog. Dis., 10, 222-228.
European Commission, 2005. Commission Regulation (EC) No. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. (L-338), 1-26.
EFSA (European Food Safety Authority), 2013. Analysis of the baseline survey on the prevalence of Listeria monocytogenes in certain ready-to-eat foods in the EU, 2010-2011. Part A: Listeria monocytogenes prevalence estimates. EFSA J., 11, 6.
Farrokh C. et al., 2013. Review of Shiga-toxin-producing Escherichia coli (STEC) and their significance in dairy production. Int. J. Food Microbiol., 162, 190-212.
Fetsch A. et al., 2014. Staphylococcus aureus food-poisonning outbreak associated with the consumption of ice-cream. Int. J. Food Microbiol., 187, 1-6.
Galanis E. et al., 2006. Web-based surveillance and global Salmonella distribution, 2000-2002. Emerging Infect. Dis., 12, 381-388.
Gaya P., Saralegui C., Medina M. & Nuñez M., 1996. Occurrence of Listeria monocytogenes and other Listeria spp. in raw caprine milk. J. Dairy Sci., 79, 1936-1941.
Gücükoglu A. et al., 2012. Detection of enterotoxigenic Staphylococcus aureus in raw milk and dairy products by multiplex PCR. J. Food Sci., 77, 11.
Gulmez M. & Guven A., 2003. Survival of Escherichia coli O157:H7, Listeria monocytogenes 4b and Yersinia enterocolitica O3 in different yogurt and kefir combinations as prefermentation contaminant. J. Appl. Microbiol., 95, 631-636.
Harakeh S. et al., 2009. Antimicrobial resistance of Listeria monocytogenes isolated from dairy-based food. Sci. Total Environ., 407, 4022-4027.
Hennekinne J.A., De Buyser M.L. & Dragacci S., 2012. Staphylococcus aureus and its food poisoning toxins: characterization and outbreak investigation. FEMS Microbiol. Rev., 36, 815-836.
Heuvelink A.E. et al., 1998. Isolation and characterization of verocytotoxin-producing Escherichia coli o157 strains from Dutch cattle and sheep. J. Clin. Microbiol., 36, 878-882.
Hill A.R. & Kethireddipalli P., 2013. Dairy products: cheese and yogurt. Biochem. Foods, 3, 319-362.
Hill B., Smythe B., Lindsay D. & Shepherd J., 2012. Microbiology of raw milk in New Zealand. Int. J. Food Microbiol., 157, 305-308.
Hussein H.S. & Sakuma T., 2005. Prevalence of Shiga toxin-producing Escherichia coli in dairy cattle and their products. J. Dairy Sci., 88, 450-465.
Johnson E.A., Nelson J.H. & Johnson M., 1990. Microbiological safety of cheese made from heat-treated milk, II. Microbiology. J. Food Protein, 53, 519-540.
Kousta M., Mataragas M., Skandamis P. & Drosinos E.H., 2010. Prevalence and sources of cheese contamination with pathogens at farm and processing levels. Food Control, 21, 805-845.
Kozak J., Balmer T., Byrne R. & Fisher K., 1996. Prevalence of Listeria monocytogenes in foods: incidence in dairy products. Food Control, 7, 215-221.
Langer A.J. et al., 2012. Nonpasteurized dairy products, disease outbreaks, and state laws United States, 1993–2006. Emerging Infect. Dis., 18, 3.
Little C.L. et al., 2008. Microbiological quality of retail cheeses made from raw, thermized or pasteurized milk in the UK. J. Food Microbiol., 25(2), 304-312.
Makino S.I. et al., 2005. An outbreak of food-borne listeriosis due to cheese in Japan, during 2001. Int. J. Food Microbiol., 104, 189-196.
Massa S., Altieri C., Quaranta V. & De Pace R., 1997. Survival of Escherichia coli O157:H7 in yoghurt during preparation and storage at 4 °C. Lett. Appl. Microbiol., 24, 347-350.
McLauchlin J., Mitchell R.T., Smerdon W.J. & Jewell K., 2004. Listeria monocytogenes and listeriosis: a review of hazard characterisation for use in microbiological risk assessment of foods. Int. J. Food Microbiol., 92, 15-33.
Morgan D. et al., 1993. Verotoxin-producing Escherichia coli O157:H7 infections associated with the consumption of yoghurt. Epidemiol. Infect., 111, 181-187.
Normanno G. et al., 2007. Occurrence, characterization and antimicrobial resistance of enterotoxigenic Staphylococcus aureus isolated from meat and dairy products. Int. J. Food Microbiol., 115, 290-296.
Ostyn A. et al., 2010. First evidence of a food poisoning outbreak due to staphylococcal enterotoxin type E, France, 2009. Eurosurveillance, 15(3).
Parisi A. et al., 2013. Occurrence of Listeria spp. in dairy plants in southern Italy and molecular subtyping of isolates using AFLP. Food Control, 29, 91-97.
Pintado C.M.B.S., Ferreira M.A.S.S. & Sousa I., 2010. Control of pathogenic and spoilage microorganisms from cheese surface by whey protein films containing malic acid, nisin and natamycin. Food Control, 21, 240-246.
Rosengren Å. et al., 2010. Occurrence of foodborne pathogens and characterization of Staphylococcus aureus in cheese produced on farm-dairies. Int. J. Food Microbiol., 144, 263-269.
Roy H. et al., 2001. Pathogenicity of different serogroups of avain salmonellae in specific- pathogen free chickens. Avian Dis., 45, 922-937.
Scallan E. et al., 2011. Foodborne illness acquired in the United States-major pathogens. Emerging Infect. Dis., 17, 7-15.
Schuchat A., Swaminathan B. & Broome C.V., 1991. Epidemiology of human listeriosis. J. Clin. Microbiol. Rev., 4, 169-183.
Snedeker K.G., Shaw D.J., Locking M.E. & Prescott R.J., 2009. Primary and secondary cases in Escherichia coli O157 outbreaks: a statistical analysis. BMC Infect. Dis., 9, 144.
SPF Économie, P.M.E., Classes moyennes et Énergie, 2013. Chiffres clés de l’agriculture. Bruxelles : Direction Genérale de Statistique.
Switt A.I.M. et al., 2013. Salmonella bacteriophage diversity reflects host diversity on dairy farms. Food Microbiol., 36, 275-285.
Tchaptchet S. & Hansen J.J., 2011. The Yin and Yang of host-commensal mutualism. Gut Microbes, 2, 347-352.
Techer C. et al., 2013. Detection and quantification of staphylococcal enterotoxin A in foods with specific and sensitive polyclonal antibodies. J. Food Control, 32, 255-261.
Wells S.J. et al., 2001. Fecalshedding of Salmonella spp. by dairy cows on farm and at cull cow markets. J. Food Prot., 64, 3-11.






