Bioaugmentation-assisted phytoextraction of Co, Pb and Zn: an assessment with a phosphate-solubilizing bacterium isolated from metal-contaminated mines of Boryeong Area in South Korea
Received on June 10, 2014; accepted on January 9, 2015
Résumé
Phytoextraction de Co, PB et Zn par bioaugmentation assistée : une évaluation avec une bactérie solubilisant le phosphate isolée de mines contaminées par les métaux dans la région de Boryeong en Corée du Sud
Description du sujet. Des micro-organismes possédant une tolérance aux métaux et capables de promouvoir la croissance végétale, peuvent jouer un rôle important dans la remédiation de sols contaminés par les métaux.
Objectifs. Le travail avait pour but d'isoler une souche bactérienne solubilisant le phosphate, de vérifier le potentiel de mobilisation du métal de la souche et d'évaluer les effets de la souche sur la croissance et le prélèvement de métaux par Helianthus annuus.
Méthode. Une bactérie solubilisant le phosphate a été isolée de sols contaminés aux métaux sur base d'un halo de grande taille sur un milieu NBRIP. La tolérance aux métaux lourds (Co, Pb, Zn) de la souche a été vérifiée en utilisant une méthode de dilution. L'effet sur la croissance des plantes et sur l'absorption des métaux de la bactérie a été évalué sur H. annuus lors d'une expérimentation en pot. L'étude de l'impact de l'inoculation de la bactérie sur la mobilité des métaux dans le sol a fait l'objet d'autres expérimentations.
Résultats. Sur base d'une analyse séquentielle de 16S rRNA, la souche s'est avérée proche de Klebsielle oxytoca JCM1665. La souche est efficace pour la solubilisation du phosphate à la fois en présence et en l’absence de métaux. Comparé à des plantes non inoculées, la bactérie améliore la croissance de H. annuus (49, 22 et 39 % dans des sols contaminés respectivement au Co, Pb et Zn). L'accumulation et la translocation de Co, Pb et Zn des racines vers les tiges sont aussi favorisées par la souche bactérienne. La fraction soluble de Co, Pb et Zn dans le sol inoculé est augmentée respectivement de 51, 24 et 76 % par rapport à celle des sols non inoculés.
Conclusions. Tenant compte du potentiel de la souche bactérienne sur la croissance des plantes et sur la mobilisation des métaux, on peut recommander son application pratique dans la phytoextraction du Co, du Pb et du Zn de sols contaminés.
Abstract
Description of the subject. Make use of microbes having remarkable metal tolerance and plant growth-promoting abilities to remediate metal-contaminated soils.
Objectives. The objectives were to isolate phosphate solubilizing bacterial strain, assess metal (Co, Pb and Zn) mobilization potential of the strain and to evaluate the effects of the strain on growth and uptake of metals by Helianthus annuus.
Method. A phosphate solubilizing bacterium was isolated from metal-contaminated soils. Heavy metal (Co, Pb and Zn) tolerance of the strain was assessed using the agar dilution method. Bacterial-assisted growth promotion and metal uptake by H. annuus was evaluated in a pot experiment. The impact of bacterial inoculation on the mobility of metals in soil was investigated in a batch experiment.
Results. The strain showed close proximity with Klebsiella oxytoca JCM1665, according to 16S rRNA sequence analysis. The strain was efficient in solubilizing phosphate, both in the presence and absence of metals. Inoculation of the strain enhanced the growth of H. annuus (49, 22 and 39% respectively in Co, Pb and Zn contaminated soils) compared to non-inoculated plants. Accumulation and translocation of Co, Pb and Zn from roots to shoots were also enhanced by the strain. Water soluble fraction of Co, Pb and Zn in soil was increased by 51, 24 and 76% respectively in inoculated soils with regard to those of non-inoculated soils.
Conclusions. Taking the plant growth promotion and metal mobilizing potential of the strain into account, practical application of the strain in enhancing phytoextraction of Co, Pb and Zn from contaminated soils could be recommended.
1. Introduction
1Contamination of soils with metals has become a matter of great concern. Excessive metals in soil could decrease soil fertility and biomass accumulation in crop plants (Whiting et al., 2001; Singh et al., 2006). Furthermore, excessive metals in agricultural soils may threaten food security, and pose health risks to living organisms by metal transfer within the food chain. In addition to the metals with unknown biological functions (Cd, Cr, Pb, Co, Ag, Se, and Hg), essential elements (Fe, Mn, Zn, Cu, Mg, Mo, and Ni) also could accumulate in agricultural soils through wastewater irrigation, animal manures and sewage sludge application, use of fertilizer and agrochemicals (Thomas et al., 2012). Though traces amounts of some essential elements are required by living beings, in excess, they too can be detrimental (Nanda et al., 2013).
2Due to the detrimental effects of heavy metal contamination, increased attention has been paid on restoration of contaminated soils (Nanda et al., 2013). Depending on the resource availability, severity of the problem, nature of the metals and contaminated soil, different methods have been employed and their effectiveness has been tested (Arunakumara et al., 2013). In this context, phytoremediation, a method which uses plants to extract, sequester and detoxify pollutants has received considerable attention (Arunakumara, 2011). However, the wider application of the technology often encounters challengers due to the limitations such as low soil thickness that can be treated, low translocation rate of metals from roots to shoots, and the slowness of the treatment (Juwarkar et al., 2008; Lebeau et al., 2008).
3The amount of heavy metals uptake in plants varies with the mobility and the concentration of metals in soil (Chen et al., 2010). The interface between soil microbes and plant roots (rhizosphere) is known to have a great influence on the uptake of nutrients as well as on the decrease of metal toxicity (McNear, 2013). Since soil microbes could alter the metal status of the soil (Fazal et al., 2010), exploitation of such microbes to reduce the metal toxicity is worth investigating (Rajkumar et al., 2008a). In this context, some metal resistant bacterial strains were proved to be exceptional at enhancing the growth of the host plant through different mechanisms such as the production of plant growth promoting substances, nitrogen fixation and phosphate solubilization, etc. (Hemambika et al., 2013). As reported by Rajkumar et al. (2008b), heavy metal tolerance of the microbes may be attributed to one or several mechanisms including exclusion, active removal, biosorption, and precipitation or bioaccumulation of metals both in external and intracellular spaces. Therefore, the use of microorganisms in remediation of heavy metal contaminated soils is gaining momentum (Prapagdee et al., 2013). The process of importing microorganisms to the contaminated site is called bioaugmentation, which enhances the metabolic capacities of the indigenous microbiota to boost bioremediation (El Fantroussi et al., 2005). In the present investigation, phosphate solubilizing bacterial strain was isolated from metal-contaminated soils and the mobilization potential of Co, Pb and Zn of the strain assessed. The effects of the isolated strain on plant growth and uptake of Co, Pb and Zn by Helianthus annuus (sunflower) were also evaluated.
2. Materials and methods
2.1. Isolation of phosphate solubilizing bacterial strain
4Heavy metal contaminated soils collected from abandoned mines of Boryeong area in South Korea were used in isolating phosphate solubilizing bacteria. Aliquots of serially diluted soil samples were inoculated on solid NBRIP (National Botanical Research Institute Phosphate) medium containing 10 g glucose, 5 g Ca3(PO4)2, 5 g MgCl2.6H2O, 0.25 g MgSO4.7H2O, 0.2 g KCl, 0.1 g (NH4)2SO4 in 1 l distilled water (Nautiyal, 1999). The pH of the media was adjusted to 7 ± 0.1. The Petri plates were incubated at 30 °C for 7 days. Morphologically distinct colonies with clear halos were purified by repeated subculturing. A strain was screened based on the greater halo size (> 3 mm) and maintained on solid NBRIP agar medium until use.
2.2. Assay of heavy metal tolerance
5Heavy metal tolerance of the isolated bacterial strain was assessed using the agar dilution method (Cervantes et al., 1986). Freshly prepared agar plates were amended with three different heavy metals; CoCl2.6H2O, 2PbCO3.Pb(OH)2, and ZnCl2 at various concentrations ranging from 100-400 mM (Co, Pb and Zn). They were inoculated with isolated strain and heavy metal tolerance was determined by the appearance of the bacterial growth after two days of incubation at 30 °C.
2.3. Strain identification
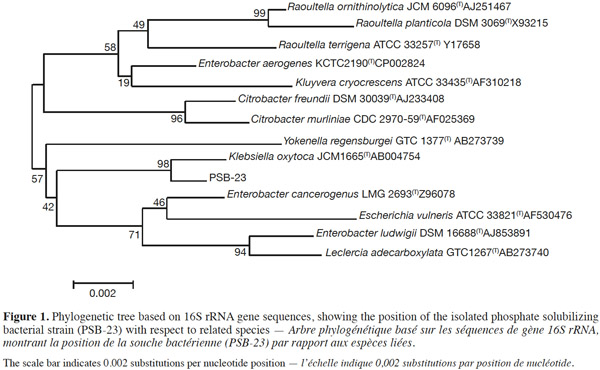
6The partial sequencing of 16S rRNA for the bacterial strain was done with the help of DNA sequencing service, SOLGENT, Daejeon, South Korea using universal primers, 27F (5’-AGAGTTTGATCCTGGCTCAG -3’) and 1492R (5’-GGTTACCTTGTTACGACTT -3’). The online program BLAST was used in identifying the related sequences with known taxonomic information available at the databank of NCBI (http://www.ncbi.nlm.nih.gov/BLAST). A phylogenetic tree was constructed using CLUSTAL X program (Thompson et al., 1997), which involved sequence alignment by neighbor joining method (Saitou et al., 1987) and maximum parsimony using the MEGA4 program (Tamura et al., 2007). Grouping of sequences was based on confidence values obtained by bootstrap analysis of 1,000 replicates. Gaps were edited in the BioEdit program and evolutionary distances were calculated using Kimura-2-parameter model (Kimura, 1980). Reference sequences were retrieved from GenBank under the accession numbers indicated in the trees. The obtained sequences were deposited in the NCBI Genebank under accession number KF836500 (Klebsiella oxytoca).
2.4. Effect of heavy metals on bacterial growth
7Growth of bacteria was estimated by the measurement of the absorbance at 660 nm using spectrophotometer (Shimadzu UV-VIS). NBRIP liquid medium supplemented with heavy metals (Co, Pb and Zn) at the concentration of 200 mM was inoculated with bacterial suspension (106 CFU1·ml-1) and incubated with continuous shaking at 30 °C. Samples from cultures grown in NBRIP liquid medium were diluted 1:1 (v/v) using 1 N HCl to dissolve the residual insoluble phosphate and measured against a blank (Rodríguez et al., 2000).
2.5. Assay of inorganic phosphate solubilization
8Bacterial culture having 106 CFU·ml-1 (2 days old) was inoculated in sterilized liquid NBRIP medium (250 ml) supplemented with different heavy metals (Co, Pb and Zn) at the concentration of 200 mM and incubated with continuous shaking at 30 °C. Bacterial culture inoculated in metal-free NBRIP medium was considered as the control. A sample (10 ml) of each cultured and control were taken and centrifuged at 8,000 g for 15 min. The clear supernatant was used in determining the pH and amount of phosphorous released into the medium. The availability of P in the supernatant was measured colorimetrically by the method of Murphy and Riley (1962).
2.6. Effect of bacterial strain on growth and metal uptake by Helianthus annuus
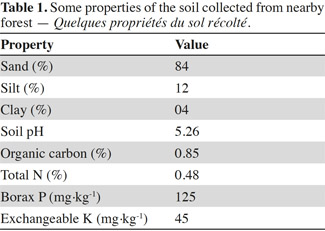
9A pot experiment was conducted under greenhouse conditions at the College of Agriculture, Chungnam National University, Daejeon, South Korea. Soil collected from several locations of a nearby forest was mixed, air dried and sieved (2 mm). Some important characteristics of the soil were given in table 1. Sterilized soil (by steaming at 100 °C for three consecutive days) was amended with aqueous solutions of different heavy metals (Co, Pb and Zn) to achieve the final concentrations of 200 mg·kg-1 soil. They were then kept for two weeks in a greenhouse for metal stabilization and used in filling the plastic pots (25 cm diameter, 35 cm height). Seeds of H. annuus were surface sterilized by immersing in alcohol (70% v/v) for 40 s, NaClO (1.0% w/v) for 15 min followed by rinsing several times with sterile distilled water. Seeds sown in germination trays containing sterilized non-contaminated soil were provided with 14/10 light/dark regime and kept at 25 °C for germination. Bacterial cultures grown under standard conditions for 2 days were harvested by centrifugation at 8,000 g for 15 min. Harvested bacterial cultures were washed twice with sterile distilled water and resuspended in biological saline (0.85% KCl w/v) to be used in inoculation. Three weeks old seedlings were carefully uprooted from the germination bed and their roots were dipped in the bacterial culture (109 CFU·ml-1) for 2 h. They were transplanted into the plastic pots (five plants per pot) containing 300 g of metal contaminated or non-contaminated soil and allowed to grow at 25 °C and 14/10 light/dark regime. The average pH of soil at the time of planting was recorded as 6.65. Three weeks later, the plants were carefully uprooted and the root surfaces were thoroughly cleaned with distilled water. As growth parameters, fresh and dry biomasses were measured. Each treatment had three replicates. Accumulation of metal in plant biomass was quantified as described by Freitas et al. (2004).
2.7. Mobility of the metals in soil
10The impact of bacterial inoculation on the mobility of metals in soil was investigated under laboratory conditions with 50 ml scaled polypropylene centrifuge tubes. The bacterial strain transferred into 100 ml flasks containing LB broth was cultured aerobically on a rotating shaker (150 g) at 30 °C until reaching the final concentration of 106 CFU·ml-1. The bacterial cells were then harvested by centrifugation at 6,000 g for 15 min and washed twice in phosphate buffer (pH 7.0). The bacterial pellet was washed in sterile water, re-centrifuged, and finally re-suspended in 5 ml sterile water. Artificially contaminated soil (1 g) in the centrifuge tubes was inoculated with small aliquots (up to 1 ml) of the final washed bacterial culture. After being weighed, the tubes were wrapped with brown paper and placed on an orbital shaker at 200 g at 25 °C. At the end of the period of 10 d, the weight of the tubes was recorded and 10 ml of sterile water were added to each tube to extract water soluble heavy metals from the soil. The extracts were centrifuged at 7,000 g for 10 min and filtered and the metal contents in the filtrate were determined using an atomic absorption spectrophotometer (Perkinelmer, Analyst 800, USA). Artificially contaminated soil in centrifuge tubes without bacterial inoculation served as the control.
3. Results
3.1. Isolation and identification of phosphate solubilizing bacterial strain
11According to 16S rRNA sequence analysis, the phosphate solubilizing bacterial strain having the highest degree of metal tolerance was shown to display a close proximity with K. oxytoca JCM1665. Phylogenetic tree (Figure 1) depicts the position of the isolated strain with respect to the related species. The strain was found to be positive in ACC disseminate activity, Ammonia, IAA and HCN production (already published, Walpola et al., 2013a).
3.2. Effect of heavy metals on bacterial growth
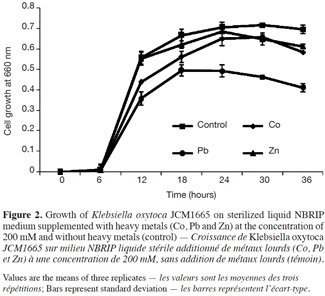
12As depicted in figure 2, none of the metals was found to be highly toxic to the strain during the incubation period of 36 h. However, compared to the metal free culture medium, slight reductions in bacterial growth were observed in metal supplemented media.
3.3. Inorganic phosphate solubilization
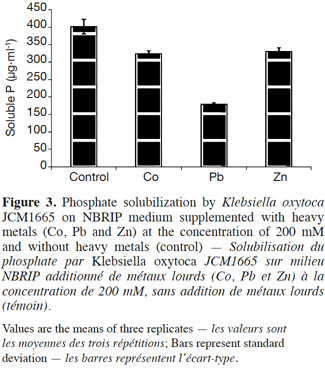
13As indicated by the results (Figure 3), the strain was shown to be capable of utilizing tri-calcium phosphate as the unique source of phosphate. However, the presence of heavy metals in NBRIP medium (200 mM) caused reductions in phosphate solubilization (19, 55, and 18% respectively for Co, Pb and Zn) compared with the control.
3.4. Effect of bacterial strain on growth and metal uptake by Helianthus annuus
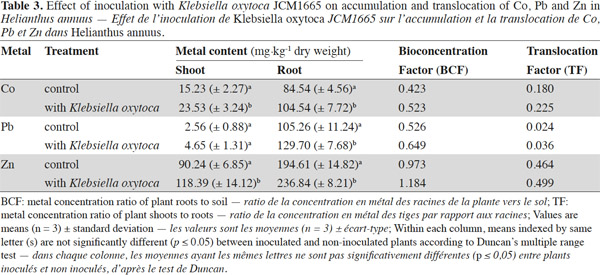
14Accumulation of plant biomass was found to be increased when H. annuus plants were inoculated with the strain. As shown in table 2, the exposure of non-inoculated plants to heavy metals resulted in severe growth inhibition. The reductions in fresh and dry weight of non-inoculated plants exposed to Pb toxicity were 29 and 13%, respectively. Inoculation with the strain however resulted in increased fresh and dry weights of plants in the presence of heavy metals. For example, the fresh weights of the plants exposed to Pb, Co and Zn were respectively 27, 34 and 27% higher than those of non-inoculated plants. The corresponding dry weight increments were recorded as 23, 26 and 25% respectively.
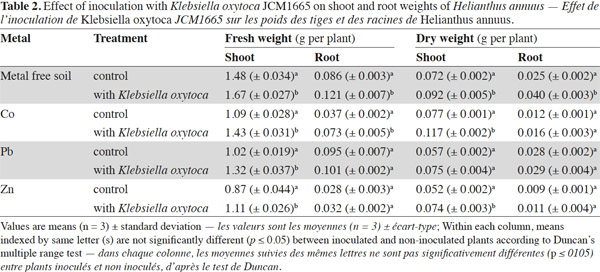
15Inoculation with K. oxytoca JCM1665 resulted in increased accumulation of metals both in the shoots and roots (Table 3). The accumulations of Co, Pb and Zn in shoots were respectively 54, 82 and 31% higher than those of non-inoculated plants. The corresponding accumulations in roots were 24, 23 and 22% (respectively for Co, Pb and Zn) higher than those of non-inoculated plants. Furthermore, metal accumulation in roots was found to be considerably higher than that of in shoots regardless of inoculation or non-inoculation. However the translocation factor (TF) of Zn was found to be significantly higher than that of the other two metals. Inoculation of the bacterial strain led to increase both TF and BCF of the three metals distinctly though low bioconcentration factor (BCF) was recorded from Co and Pb.
3.5. Mobility of the metals in soil
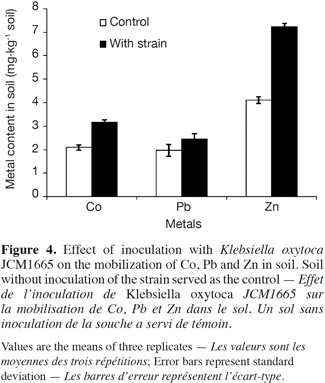
16Figure 4 depicted the results of metal mobilization of the strain assessed in a batch experiment. The inoculation of the strain led to increase the contents of water soluble metals in the soil. The mobilizations of Co, Pb and Zn were respectively 51, 20 and 76% higher than those of the control soil.
4. Discussion
17Microorganisms isolated from polluted soils possess the ability to withstand against multiple pollutants as they have adapted to such environments (Pal et al., 2005; Abou-Shanab et al., 2007). The growth response of the present strain under metal contamination conditions is in line with the results of Rajkumar et al. (2008b) and Braud et al. (2009), who observed Co, Pb and Zn resistance in Bacillus weihenstephanensis and Cr and Pb resistance in Pseudomonas aeruginosa respectively. Similarly, Raja et al. (2009) identified Proteus vulgaris, Acinetobacter radioresistens and Pseudomonas aeruginosa as Cd, Ni and Pb resistant bacteria. Ahemad et al. (2011) reported three Pseudomonas isolates with high degree of Zn resistance. Prapagdee et al. (2013) identified Klebsiella sp. BAM1 as Cd resistance bacteria and Bhadra et al. (2006) screened Ni resistant isolate Acinetobacter junii. The adaptation of bacteria to metal stress environments is associated with various resistance mechanisms (Tak et al., 2013). These mechanisms could be utilized in detoxification and removal of heavy metals from contaminated soils (Ahmed et al., 2005).
18Plant growth promoting characteristics of the present strain were previously studied and the strain was found to be positive in ACC disseminate activity, Ammonia, IAA and HCN production (already published, Walpola et al., 2013b). Helianthus annuus, a species known to have the ability to accumulate biomass rapidly while taking up substantial amounts of metals (Turgut et al., 2004) was employed in the present study to assess the effectiveness of the strain as a plant growth-promoter. Our results are in agreement with Prapagdee et al. (2013), who reported that growth of H. annuus could be enhanced by the inoculation of Micrococcus sp. MU1 and Klebsiella sp. BAM1 under Cd contaminated conditions. Furthermore, Jiang et al. (2008) observed growth enhancement of corn and tomato plants when inoculated with Burkholderia sp. J62. Similar findings were also reported by Egamberdiyeva et al. (2002) for corn inoculated with Pseudomonas fluorescens PsIA12. According to Belimov et al. (2004), inoculation with rhizobacteria resulted in 42% increase in growth of the barley plant compared to the control. Belimov et al. (2001) also observed bacterial-assisted growth enhancement in Brassica napus grown in a soil contaminated with Cd. The plant growth-promoting potential of the present strain could be attributed at least partly to the phosphate solubilization ability of the strain under metal stress conditions. In this regards, Rajkumar et al. (2005) also reported that phosphate solubilization ability of Pseudomonas sp. could contribute to the growth enhancement of the inoculated plants. Inoculation of phosphate solubilizing Bacillus subtilis SJ-101 resulted in higher shoot and root length and biomass with or without Ni (Zaidi et al., 2006). Bacteria is reported to promote the growth of plants indirectly through producing antibiotics to inhibit soil pathogens, and directly through increasing nutrient and water uptake and thereby the plant biomass (Belimov et al., 2004). Production of siderophores, specific enzymes, and organic acids involved in phosphorus solubilization, and fixation of atmospheric N2, bacteria could assist plants to withstand against metal toxicity (Kloepper, 2003). In this regards, Borgmann (2000) reported that Kluyvera ascorbata SUD165 protected Brassica juncea and Brassica campestris against Ni, Pb and Zn toxicity through the production of enzyme ACC deaminase.
19The amount of metals accumulated in root systems was generally found to be higher than that in shoots, which could primarily be attributed to the poor translocation of heavy metals from roots to shoots (Rajkumar et al., 2006). However, as shown in table 3, translocation factor of each metal was increased with the inoculation of the strain, which was of enormous practical significance. Furthermore, metal accumulations in both shoots and roots were found to be higher in inoculated plants than in non-inoculated plants. Similar observations were made by Rajkumar et al. (2008b) for Zn accumulation in H. annuus inoculated with Bacillus weihenstephanensis. However, according to Wani et al. (2007), inoculation of Bradyrhizobium sp. on surface sterilized seeds of Vigna radiate reduced the concentration of Ni in roots, shoots and grains by 15, 19 and 22%, respectively, compared to non-inoculated plants.
20Acidification of the growth medium, basically through the production of low molecular weight organic acids could enhance microbial mediated inorganic phosphate solubilization (Walpola et al., 2013b). Analogous to their findings, an inverse relationship between pH and soluble phosphorus concentration was observed in the present study. The inter-relationships among soil pH, solubility and speciation of metals have been intensively investigated (Gadd, 2004). Bacteria such as Azotobacter chroococcum (N-fixing bacteria), Bacillus megaterium (P-solubilizer) and Bacillus mucilaginosus (K-solubilizer) (Wu et al., 2006) and Bacillus sp. RJ16 (Sheng et al., 2006) were reported to decrease the pH, enhancing the bioavailability of Cd, Pb and Zn (Chen et al., 2005). As stated by Zaidi et al. (2006), reduction in pH from 7.5 to 4.8 with the inoculation of phosphate solubilizing Bacillus subtilis SJ-101 possibly created favorable conditions for the solubilization of metals and their subsequent uptake by the plants. The increased accumulation of metals in the presence of bacterial strain might be due to the increased uptake of metals under acidic soil conditions created by the phosphate solubilization (Rajkumar et al., 2008b). Compared to the non-inoculated controls, inoculation of Cd-resistant bacterial strains significantly increased the uptake of Cd by Brassica napus, as a result of pH reduction (Sheng et al., 2006). However, on the contrary, inoculation of some microorganisms such as Glomus caledonium (Chen et al., 2004) and Glomus mosseae (Citterio et al., 2005) were reported to have no effects on the speciation of Cd and Zn, and Cr and Ni, resulting in no marked impacts on the rate of phytoextraction.
21Generally, the low amount of metals extracted by plants from a soil is attributed mainly to the low availability of metals. As reported by several authors, the available metal content in a soil is less than 1% of the total metal content (Whiting et al., 2001; Braud et al., 2006). Metal availability is influenced by the nature of the metal and soil characteristics such as pH, CEC and organic matter (Kayser et al., 2001; Lebeau et al., 2008). Bioaugmentation could enhance metal bioavailability by increasing the concentration of the available fractions. As revealed by the present results, the release of heavy metals from the non-soluble phases to soluble phases could be facilitated by the bacterial strain. Therefore, increased accumulation of metals, in particular Zn in both the shoots and roots of H. annuus could be attributed to the higher water soluble metal contents in soil inoculated with bacterial strain. The present findings of metal mobilization are in agreement with Wu et al. (2006) and Prapagdee et al. (2012), who also reported bacteria-assisted increase in heavy metal mobilization. As reported by the results of previous studies, H. annuus is capable of accumulating high amounts of Pb, Cd, Cu, Zn and Co, in both the shoots and the roots (Boonyapookana et al., 2005; Marchiol et al., 2007). According to Braud et al. (2006), inoculation of Pseudomonas aeruginosa and Pseudomonas fluorescens has resulted in 113% increment of Pb content in the exchangeable fraction of the soil. However, the Pb concentration bound to free Mn oxides, organic matter and in the residual fraction remained stable. Abou-Shanab et al. (2006) observed an increase in extractable Ni with Microbacterium arabinogalactanolyticum by a factor up to 15. As reported by Baum et al. (2006), the concentrations in NH4NO3-extractable Cd, Cu, Pb and Zn in a soil bioaugmented with ectomycorrhizal fungus Paxillus involutus, were 1.22-, 1.11-, 1.33- and 1.33-fold higher than those of non-bioaugmented soil, depending on the soil composition. Assessments of the bioavailable fractions of metals have been performed under varied conditions with different extractants (Di Gregorio et al., 2006; Wu et al., 2006), MgCl2 (Braud et al., 2006), NH4NO3 (Baum et al., 2006), NH4O-Ac (Wu et al., 2006), DTPA (Di Gregorio et al., 2006; Wu et al., 2006), KNO3 (Di Gregorio et al., 2006) and HCl (Wang et al., 2007), thus contrasting the results of bioaugmentation studies is hard to perform.
5. Conclusions
22Inoculation of K. oxytoca enhanced dry matter accumulation in H. annuus plants. Furthermore, inoculation promoted Co, Pb and Zn uptake and their translocation from roots to shoots. Taking the higher metal mobilization potential of the strain also into account, K. oxytoca JCM1665 could be recommended as an ideal candidate for bioaugmentation studies.
Bibliographie
Abou-Shanab R.A.I., Angle J.S. & Chaney R.L., 2006. Bacterial inoculants affecting nickel uptake by Alyssum murale from low, moderate and high Ni soils. Soil Biol. Biochem., 38, 2882-2889.
Abou-Shanab R.A.I., Van Berkum P. & Angle J.S., 2007. Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere, 68, 360-367.
Ahemad M. & Malik A., 2011. Bioaccumulation of heavy metals by zinc resistant bacteria isolated from agricultural soils irrigated with wastewater. Bacteriol. J., 2, 12-21.
Ahmed N., Nawaz A. & Badar U., 2005. Screening of copper tolerant bacterial species and their potential to remove copper from the environment. Bull. Environ. Contam. Toxicol., 74, 219-226.
Arunakumara K.K.I.U., 2011. Use of crop plants for removal of toxic metals. In: Khan M.S., Zaidi A., Goel R. & Mussarrat J., eds. Bio-management of metal contaminated soils. Springer, 439-457.
Arunakumara K.K.I.U., Walpola B.C. & Yoon M.H., 2013. Agricultural methods for toxicity alleviation in metal contaminated soils. Korean J. Soil Sci. Fert., 46, 73-80.
Baum C., Hrynkiewicz K., Leinweber P. & Meissner R., 2006. Heavy-metal mobilization and uptake by mycorrhizal and nonmycorrhizal willows (Salix dasyclados). J. Plant Nutr. Soil Sci., 169, 516-522.
Belimov A.A. et al., 2001. Characterisation of plant growth-promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol., 47, 642-652.
Belimov A.A. et al., 2004. Employment of rhizobacteria for the inoculation of barley plants cultivated in soil contaminated with lead and cadmium. Microbiology, 73, 99-106.
Bhadra B., Nanda A.K. & Chakraborty R., 2006. Inducible nickel resistance in a river isolate of India phylogenetically ascertained as a novel strain of Acinetobacter junii. World J. Microbiol. Biotechnol., 22, 225-232.
Boonyapookana B. et al., 2005. Phytoaccumulation of lead by sunflower (Helianthus annuus), tobacco (Nicotiana tabacum), and vetiver (Vetiveria zizanioides). J. Environ. Sci. Health Part A, 40, 117-137.
Borgmann U., 2000. Methods for assessing the toxicological significance of metals in aquatic ecosystems: bio-accumulation-toxicity relationships, water concentrations and sediment spiking approaches. Aquat. Ecosyst. Health, 3, 277-289.
Braud A. et al., 2006. Changes in extractability of Cr and Pb in a polycontaminated soil after bioaugmentation with microbial producers of biosurfactants, organic acids and siderophores. Water Air Soil Pollut., 6, 261-279.
Braud A., Jézéquel K., Bazot S. & Lebeau T., 2009. Enhanced phytoextraction of an agricultural Cr-, Hg- and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere, 74, 280-286.
Cervantes C. et al., 1986. Resistance to metal by Pseudomonas aeruginosa clinical isolates. Microbios, 48, 159-163.
Chen B. et al., 2004. Effects of EDTA application and arbuscular mycorrhizal colonization on growth and zinc uptake by maize (Zea mays L.) in soil experimentally contaminated with zinc. Plant Soil, 261, 219-229.
Chen Y.E., Yuan S., Su Y.Q. & Wang L., 2010. Comparison of heavy metal accumulation capacity of some indigenous mosses in Southwest China cities: a case study in Chengdu city. Plant Soil Environ., 56, 60-66.
Chen Y.X., Wang Y.P., Lin Q. & Luo Y.M., 2005. Effect of copper-tolerant rhizosphere bacteria on mobility of copper in soil and copper accumulation by Elsholtzia splendens. Environ. Int., 31, 861-866.
Citterio S. et al., 2005. The arbuscular mycorrhizal fungus Glomus mosseae induces growth and metal accumulation changes in Cannabis sativa L. Chemosphere, 59, 21-29.
Di Gregorio S. et al., 2006. Combined application of Triton X-100 and Sinorhizobium sp. Pb002 inoculum for the improvement of lead phytoextraction by Brassica juncea in EDTA amended soil. Chemosphere, 63, 293-299.
Egamberdiyeva D., Juraeva D., Gafurova L. & Höflich G., 2002. Promotion of plant growth of maize by plant growth promoting bacteria in different temperature and soils. In: van Santen E., ed. Making conservation tillage conventional: building a future on 25 years of research. Proceedings of the 25th annual southern conservation tillage conference for sustainable agriculture, 24-26 June 2002, Alabama Agricultural Experiment Station and Auburn University, Auburn, USA. Special Report No. 1. Auburn, AL, USA: Alabama Agricultural Experiment Station and Auburn University.
El Fantroussi S. & Agathos S.N., 2005. Is bioaugmentation a feasible strategy for pollutant removal and site remediation? Curr. Opin. Microbiol., 8, 268-275.
Fazal H. & Bano A., 2010. The effect of diazotrophs (rhizobium and azatobactor) on growth and biomass of maize in lead (Pb) polluted soil, and accumulation of the lead in different parts of plant. Pak. J. Bot., 42, 4363-4370.
Freitas H., Prasad M.N.V. & Pratas J., 2004. Analysis of serpentinophytes from north-east of Portugal for trace metal accumulation-relevance to the management of mine environment. Chemosphere, 54, 1625-1642.
Gadd G.M., 2004. Microbial influence on metal mobility and application for bioremediation. Geoderma, 122, 109-119.
Hemambika B., Balasubramanian V., Kannan V.R. & James R.A., 2013. Screening of chromium-resistant bacteria for plant growth-promoting activities. Soil Sediment Contam., 22, 717-736.
Jiang C.Y., Sheng X.F., Qian M. & Wang Q.Y., 2008. Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere, 72, 157-164.
Juwarkar A.A. & Jambhulkar H.P., 2008. Phytoremediation of coal mine spoil dump through integrated biotechnological approach. Bioresour. Technol., 99, 4732-4741.
Kayser G., Korckritz T. & Markert B., 2001. Bioleaching for the decontamination of heavy metals. Wasser Boden, 53, 54-58.
Kimura M., 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol., 16, 111-120.
Kloepper J.W., 2003. A review of mechanisms for plant growth promotion by PGPR. In: Sixth international PGPR Workshop, 5-10 October 2003, Calicut, India, 81-92.
Lebeau T., Braud A. & Jézéquel K., 2008. Performance of bioaugmentation-assisted phytoextraction applied to metal contaminated soils: a review. Environ. Pollut., 153, 497-522.
Marchiol L., Fellet G., Perosa D. & Zerbi G., 2007. Removal of trace metals by Sorghum bicolor and Helianthus annuus in a site polluted by industrial wastes: a field experience. Plant Physiol. Biochem., 45, 379-387.
McNear Jr. D.H., 2013. The rhizosphere - roots, soil and everything in between. Nat. Educ. Knowl., 4, 1.
Murphy J. & Riley J.P., 1962. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta, 27, 31-36.
Nanda S. & Abraham J., 2013. Remediation of heavy metal contaminated soil. Afr. J. Biotechnol., 12, 3099-3109.
Nautiyal C.S., 1999. An efficient microbiological growth medium for screening of phosphate solubilizing microorganisms. FEMS Microbiol. Lett., 170, 265-270.
Pal A., Dutta S., Mukherjee P.K. & Paul A.K., 2005. Occurrence of heavy metal resistance in microflora from serpentine soil of Andaman. J. Basic Microbiol., 45, 207-218.
Prapagdee B., Chumphonwong N., Khonsue N. & Mongkolsuk S., 2012. Influence of cadmium resistant bacteria on promoting plant root elongation and increasing cadmium mobilization in contaminated soil. Fresenius Environ. Bull., 21, 1186-1191.
Prapagdee B., Chumphonwong N. & Mongkolsuk S., 2013. Bioaugmentation with cadmium-resistant plant growth-promoting rhizobacteria to assist cadmium phytoextraction by Helianthus annuus. Chemosphere, 92, 659-666.
Raja C.E., Edward C., Selvam G.S. & Kiyoshi O., 2009. Isolation, identification and characterization of heavy metal resistant bacteria from sewage. In: Proceedings of the International joint symposium on geodisaster and geoenvironment in Asia, JS Fukuoka, Japan, 205-211.
Rajkumar M., Nagendran R., Lee K.J. & Lee W.H., 2005. Characterization of a novel Cr6+ reducing Pseudomonas sp. with plant growth-promoting potential. Curr. Microbiol., 50, 266-271.
Rajkumar M., Nagendran R., Lee K.J. & Lee W.H., 2006. Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere, 62, 741-748.
Rajkumar M. & Freitas H., 2008a. Effects of inoculation of plant-growth promoting bacteria on Ni uptake by Indian mustard. Bioresour. Technol., 99, 3491-3498.
Rajkumar M., Ma Y. & Freitas H., 2008b. Characterization of metal-resistant plant-growth promoting Bacillus weihenstephanensis isolated from serpentine soil in Portugal. J. Basic Microbiol., 48, 500-508.
Rodríguez H., Gonzalez T. & Selman G., 2000. Expression of a mineral phosphate solubilizing gene from Erwinia herbicola in two rhizobacterial strains. J. Biotechnol., 84, 155-161.
Saitou N. & Nei M., 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol., 4, 406-425.
Sheng X.F. & Xia J.J., 2006. Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere, 64, 1036-1042.
Singh S. & Aggarwal P.K., 2006. Effect of heavy metals on biomass and yield of different crop species. Indian J. Agric. Sci., 76, 688-691.
Tak H.I., Ahmad F. & Babalola O.O., 2013. Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. Rev. Environ. Contam. Toxicol., 223, 33-52.
Tamura K., Dudley J., Nei M. & Kumar S., 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol., 24, 1596-1599.
Thomas E.Y., Omueti J.A.I. & Ogundayomi O., 2012. The effect of phosphate fertilizer on heavy metal in soils and Amaranthus caudatu. Agric. Biol. J. N. Am., 3, 145-149.
Thompson J.D. et al., 1997. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res., 25, 4876-4882.
Turgut C., Pepe M.K. & Cutright T.K., 2004. The effect of EDTA and citric acid on phytoremediation of Cd, Cr, and Ni from soil using Helianthus annuus. Environ. Pollut., 131, 147-154.
Walpola B.C. et al., 2013a. Optimization of indole-3-acetic production by phosphate solubilization bacteria isolated from waste mushroom bed of Agaricus bisporus. J. Mushrooms, 11, 53-62.
Walpola B.C. & Yoon M.H., 2013b. Isolation and characterization of phosphate solubilizing bacteria and their co-inoculation efficiency on tomato plant growth and phosphorous uptake. Afr. J. Microbiol. Res., 7, 266-275.
Wang F.Y., Lin X.G. & Yin R., 2007. Role of microbial inoculation and chitosan in phytoextraction of Cu, Zn, Pb and Cd by Elsholtzia splendense, a field case. Environ. Pollut., 147, 248-255.
Wani P.A., Khan M.S. & Zaidi A., 2007. Effect of metal tolerant plant growth promoting Bradyrhizobium sp. (vigna) on growth, symbiosis, seed yield and metal uptake by green gram plants. Chemosphere, 70, 36-45.
Whiting S.N., Souza M.P. & Terry N., 2001. Rhizosphere bacteria mobilize Zn for hyperaccumulation by Thlaspi caerulescens. Environ. Sci. Technol., 35, 3144-3150.
Wu Q.T. et al., 2006. Selection of appropriate organic additives for enhancing Zn and Cd phytoextraction by hyperaccumulators. J. Environ. Sci., 18, 1113-1118.
Zaidi S., Usmani S., Singh B.R. & Musarrat J., 2006. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere, 64, 991-997.
Notes
1 CFU: Colony Forming Unit
