- Portada
- Volume 19 (2015)
- Numéro 1
- Transgenic crops with an improved resistance to biotic stresses. A review
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Transgenic crops with an improved resistance to biotic stresses. A review

Notes de la rédaction
Received on January 1, 2014; accepted on December 5, 2014
Résumé
Des cultures transgéniques pour une meilleure résistance aux stress biotiques (synthèse bibliographique)
Introduction. Les insectes ravageurs, les maladies et les mauvaises herbes constituent des stress biotiques qui sont des facteurs limitatifs importants pour le rendement des cultures et de la production. Les contraintes liées aux méthodes classiques de sélection rendent indispensable le recours à des méthodes d’amélioration alternatives conférant aux nouvelles variétés une plus grande résistance aux stress biotiques. Les progrès des techniques moléculaires ont conduit à la mise au point de procédés de transformation génétique d'une grande variété de plantes. L’approche via l'ingénierie génétique a démontré sa capacité à fournir d'énormes possibilités de sélection de gènes de résistance provenant d’origines diverses pour assurer une meilleure résistance aux différents stress biotiques.
Littérature. Dans cet article, nous nous concentrons sur les stratégies adoptées pour développer des fonctions insecticides, antifongiques, antibactériennes, antivirales et de détoxification des herbicides dans de nouvelles variétés végétales.
Conclusions. Malgré les préoccupations au sujet de la commercialisation de produits issus de cultures génétiquement modifiées résistantes aux stress biotiques, on constate que la zone de culture de ces lignées est en plein essor. Compte tenu de cette tendance, il faut non seulement s'attendre à un accroissement continu en termes de production et de commercialisation de cultures génétiquement modifiées résistantes aux stress biotiques, mais aussi à ce que cela s’étende aux cultures résistantes aux stress abiotiques (e.g. sècheresse, salinité, etc.) qui sont encore à venir.
Abstract
Introduction. Pests, diseases and weeds (biotic stresses) are significant limiting factors for crop yield and production. However, the limitations associated with conventional breeding methods necessitated the development of alternative methods for improving new varieties with higher resistance to biotic stresses. Molecular techniques have developed applicable methods for genetic transformation of a wide range of plants. Genetic engineering approach has been demonstrated to provide enormous options for the selection of the resistance genes from different sources to introduce them into plants to provide resistance against different biotic stresses.
Literature. In this review, we focus on strategies to achieve the above mentioned objectives including expression of insecticidal, antifungal, antibacterial, antiviral resistance and herbicide detoxification for herbicide resistance.
Conclusions. Regardless of the concerns about commercialization of products from genetically modified (GM) crops resistant to biotic stresses, it is observed that the cultivation area of these crops is growing fast each year. Considering this trend, it is expected that production and commercialization of GM crops resistant to biotic stresses will continue to increase but will also extend to production of crops resistant to abiotic stresses (e.g. drought, salinity, etc.) in a near future.
Tabla de contenidos
1. Introduction
1One way to increase the quantity and quality of food is to reduce damages caused by insects, diseases and weeds to crops. Pathogens cause losses in 10-16% of the global harvest (Chakraborty et al., 2011). This figure for pest damage is about 14-25% of the total agricultural production (DeVilliers et al., 2011). In traditional agriculture, only individuals of the same species (or eventually closely related species) can be crossbred. If in this naturally available gene pool, resistance to biotic stress does not exist, traditional breeders cannot create resistance or introgress this trait into new varieties. Therefore, it is necessary to search for alternative sources of genes in other completely unrelated species of plants or in microbial organisms. Besides, traditional methods are resource- and time-consuming and germplasm dependent (Roy et al., 2011).
2Also, using chemical spray may have adverse effects on human health and the environment, including beneficial organisms and may lead to the development of chemical-resistant insects and weeds (Wahab, 2009).
3Plant genetic engineering has been made possible thanks to the extensive research conducted during the last three decades. This branch of science has enabled researchers to transform plants for enhancing their resistance or tolerance against different biotic stresses.
4Currently, transgenic plants with herbicide, insect pests and virus disease resistance are cultivated in more than 175.2 million hectares in the world (James, 2013) while in 1996, only 1.7 million hectares of land were under transgenic crops. Out of the 27 countries currently contributing to the cultivation of transgenic plants, 19 are developing countries and 8 industrial. During the 1996-2012 period, cumulative economic benefits from transgenic plants were high in developing countries at US$ 47.9 billion compared to US$ 59 billion generated by industrial countries.
5In this review, we mainly discuss on how genetic engineering enables crop improvement for resistance to biotic stresses.
2. Resistance to insects
2.1. Bt crops
6Bacillus thuringiensis is a Gram+ bacterium that produces proteinaceous crystalline (Cry) inclusion bodies during sporulation. It also produces cytotoxins that synergize the activity of Cry toxins. Cry proteins are toxic to insects (mainly against lepidopters), but non-toxic to human and animals (BANR, 2000).
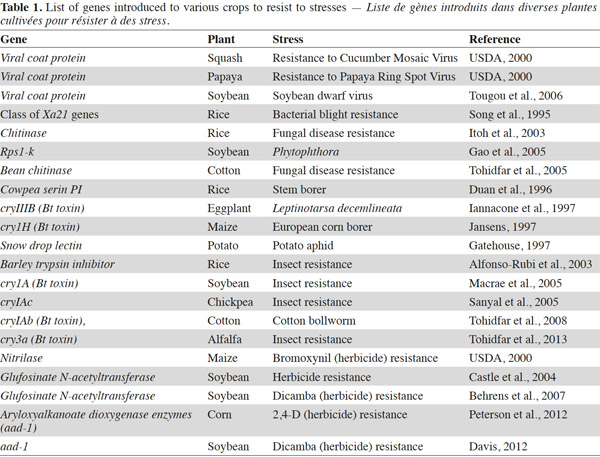
7Bt genes encoding insecticidal Cry proteins have been transferred to relevant crops to confer protection against their most important insect pests (Table 1). Cry proteins once ingested by the insect are solubilized in the mid-gut and are then cleaved there by digestive proteases. Some of the resulting polypetides are able to bind to mid-gut epithelial cell receptors resulting in cell lysis and finally insect death (Gahan et al., 2010). However, this mechanism is not working on all insect species.
8In 2013, the global area of GM crops planted for commercial purposes was 175.2 million hectares, out of which, 23 million hectares were allocated to Bt crops and 47 million hectares to stacked traits (herbicide resistant and Bt crops) (James, 2013).
9Bt maize has been transformed with either cry1Ab, cry1Ac or cry9C to protect it against Ostrinia nubilalis and Sesamia nonagriodes, or with cry1F to protect it against Spodoptera frugiperda, and with cry3Bb, cry34Ab and cry35Ab to protect it against the rootworms of the genus Diabrotica (James, 2012). By the end of the year 2012, more than 18 million hectares were under the cultivation of Bt cotton plants. Most commercially planted Bt cotton contain cry1Ac or a fusion gene of cry1Ac and cry1Ab (James, 2013). Bt potatoes protected against Leptinotarsa decemlineata have also been planted commercially in North America and Europe and contain the cry3Aa gene (Coombs et al., 2002).
10Bt eggplant is another crop which was targeted for control of Leucinodes orbonalis and commercialized in India in 2008. Bt crucifer vegetables are under development and are targeted against Plutella xylostella (James, 2012). Bt rice expressing the Bacillus thuringiensis toxin, is expected to be commercially released in the future (James, 2012). Several genetically-modified (GM) rice varieties have entered and passed field and environmental release trials, and four varieties entered preproduction trials in farmers’ fields in 2001. Also, Bt alfalfa has been produced using cry3a gene against Hypera postica for the first time in Iran (Tohidfar et al., 2013). Finally, the Bt trait has been introduced in soybean through either one or two cry genes among cry1Ab, cry1Ac, cry1F (James, 2013).
2.2. Protease Inhibitors (PI)
11Plant protease inhibitors (PI) are able to protect plants against insect attacks by interfering with the proteolytic activity of insects digestive gut. Among the proteic PIs, serine and cysteine PIs are abundant in plant seeds and storage tissues (Reeck et al., 1997) and may contribute to their natural defense system against insect predation. The first PI gene that was successfully transferred artificially to plant species resulting in enhanced insect resistance was isolated from cowpea and encoded the trypsin/trypsin inhibitor CpTI (Cowpea Trypsin Inhibitor) (Hilder et al., 1987). CpTI + Bt cotton cultivars were commercially released in China in 2000 (Song et al., 2001) and accounted for approximately 15% of the grown cotton in 2005 (He et al., 2008). Oryzacystatin 1 (OC1) is a well-studied cysteine PI from rice seeds which has been successfully introduced into several different crops like rice (Duan et al., 1996), wheat (Altpeter et al., 1999), oilseed rape (Rahbe et al., 2003) and eggplant (Ribeiro et al., 2006). It protects these plant species against beetle attacks and, in some cases, aphids (Sharma et al., 2004). A Bt-corn called Bt-Xtra containing three genes including cry1Ac from B. thuringiensis, bar from Streptomyces higroscopicus and potato proteinase inhibitor (pinII) has been produced (Oksman-Kaldentey et al., 2002).
2.3. Lectins
12Lectins are carbohydrate-binding proteins found in many plant tissues and are abundant in the seeds and storage tissues of some plant species. Plant lectins are particularly effective against the sap sucking Hemiptera (Powell et al., 1995). Therefore, enhancing their presence in some plant tissues may have an insect tolerant effect. Transgenic rice with Galanthus nivalis (snow drop) agglutinin (GNA) has shown resistance to brown plant hopper (BPH) (Nilaparvata lugens) (Li et al., 2005). Allium leaf agglutinin (ASAL) possesses an insecticidal activity in different plants. The ASAL gene was transferred to rice and the transgenic plants showed resistance to hopper insect pests (Saha et al., 2006).
2.4. Alpha-amylase inhibitors
13Alpha-amylase inhibitors are attractive candidates for the control of seed weevils because they are highly dependent on starch as energy source. The bean (Phaseolus vulgaris) amylase inhibitor gene was expressed in seeds of transgenic garden pea (Pisum sativum) and other grain legumes, using a strong seed-specific promoter (Shade et al., 1994). The resulting seeds were resistant to stored product pests such as larvae of bruchid beetles and field pests such as larvae of the pea weevil Bruchus pisorum (Morton et al., 2000). The alpha-amylase inhibitor gene isolated from Phaseolus vulgaris was introduced to chickpea by Agrobacterium-mediated transformation system (Ignacimuthu et al., 2006). Although, the transformation efficiency was low (0.3%), the transformed plants showed a significant resistance to bruchid weevil. Similarly, Coffea arabica plants genetically modified with an alpha-amylase inhibitor gene isolated from Phaseolus vulgaris produced seed extracts capable of inhibiting amylolytic enzyme activity up to 88% (Barbosa et al., 2010).
2.5. Alternative insecticidal genes
14During vegetative growth, some Bacillus species become the source of insecticidal activities like B. thuringiensis that produces the Vip3A protein against lepidopteran insects such as the black cutworn (Agrotis ipsilon). Unlike the Cry proteins, Vips do not need to be solubilized in the insect gut before they act. They bind to receptors in the insect gut different from those targeted by Cry proteins (Lee et al., 2006). The maize line MIR162 containing the vip3Aa20 gene was authorized for cultivation in USA and Canada in 2010. Vip3Aa20, the modified form of vip3Aa1 gene (CERA, 2010), showed insecticidal effects against a wide host range including the corn earworm, the black cutworm, the fall armyworm and the Western bean cutworm.
3. Genetic engineering of plants for resistance to diseases
3.1. Resistance to fungal diseases
15Chitin constitutes one of the major components of the cell walls of many fungal pathogens such as Rhizoctonia solani and it can be hydrolyzed by chitinase. On the other hand, β-1,3-glucanase is known to degrade glucans which are also present in the fungal cell walls. The synthesis of chitinases and glucanases is known to occur in response to pathogen infection. When both enzymes are simultaneously present, the fungal growth is more effectively inhibited (Neuhaus, 1999).
16In recent years, the possibility of transforming plants with genes encoding β-1,3-glucanase and chitinase (mostly of plant origin) has been explored. Several laboratories have been able to transfer plant or microbial-derived chitinase genes into plants and develop transgenic crops with enhanced resistance to fungal diseases (Table 1). These plants include: grapevine (Yamamoto et al., 2000), peanut (Rohini et al., 2000) and cotton (Tohidfar, 2005; Tohidfar, 2012). The combined expression of chitinase and glucanase in transgenic carrot, tomato and tobacco resulted in a much more effective prevention of fungal disease development (Melchers et al., 2000).
17Polygalacturonase inhibiting proteins (PGIP) are glycoproteins present in the cell wall of many plants and can inhibit the activity of fungal endopolygalacturonases (Oelfose et al., 2006). Fusarium head blight (FHB) is an important disease in wheat that may lead to a contamination of the yielded products with mycotoxins (thrichothecene and deoxynivalenol-DON). Food contamination with DON is a risk for human and animal health. Recently, a L3 gene (N-terminal fragment of yeast ribosomal protein) was transferred to wheat and the transgenic plants showed resistance to Fusarium disease and improved level of DON in transgenic wheat kernel (Di et al., 2010).
18One of the most devastating fungal diseases that threatens the members of Solanacea, especially potatoes, is Phytophthora infestans also known as the late blight. To overcome this infection, several strategies using biotechnology-driven approaches to confer resistance to potato varieties have been proposed. In this regard, several R genes (resistance) have been identified and isolated from various sources (Ballvora et al., 2002; van der Vossen et al., 2005; Pel et al., 2009). The LpiO gene, among the 54 tested effectors, was selected to stimulate innate immunity of Solanum species. Following the hypersensitive responses (HR) caused by LpiO, the source of the R gene Rpi-blb1 was identified. The transient co-expression of LpiO (as effector) and Rpi-blb1 (as resistance gene) in Nicotiana benthamiana led to rapid identification of Rpi-sto1 and Rpi-pta1 as resistant genes to late blight (Vleeshouwers et al., 2008). In another study, a stacking of three broad spectrum potato R genes (Rpi), Rpi-sto1 (Solanum stoloniferum), Rpi-vnt1.1 (Solanum venturii) and Rpi-blb3 (Solanum bulbocastanum) was transformed into susceptible cultivar ‘Desiree’. Near 4% of the transformed plants showed HR against pathogenic effects of Phytophtora (Zhu et al., 2012).
19Another strategy to confer resistance to plants against disease is activating phytoalexins which are parts of defense mechanisms in some plant species. Transformation of rice with stilbene synthase gene (STS) of Vst1, a key enzyme in synthesis of phytoalexin in grape, could improve its resistance to Piricularia orizae (Coutos-Thévenot et al., 2001). Similarly, barley was improved to resist to powdery mildew (Liang et al., 2000). More recently, the role of Mitogen-activated protein kinase (MAPK) cascade, especially OsMKK6, in the regulation of genes responsible for phytoalexin synthesis in rice in response to UV and blast infestation was reported by Wankhede et al. (2013). In their investigation, the expression of phytoalexin in rice was increased specifically under UV radiation. Moreover, the authors reported that the mitogen-activated protein kinase kinase (MAPKK) is a key component of MAPK cascade. They also identified OsMKK6 through studying the expression profile of rice MAPKKs under UV stress and eventually, achieved transgenic rice lines containing OsMKK6 gene showing an over-expression of phytoalexins.
3.2. Resistance to bacterial diseases
20Bacterial blight is a destructive disease of domesticated rice (Oryza sativa) caused by the pathogen Xanthomonas oryzae pv. oryzae. The ethylene responsive (ERF) transcription factors have been demonstrated to have a role in regulating the expression of pathogenesis-related (PR) genes (Grennan, 2008). The expression of cotton ERF in tobacco causes transgenic plants to exhibit greater level of resistance to Xanthomonas (Champion et al., 2009).
21The Harpins (hrp) genes encode type III secretory pathways and are required by many phytopathogenic bacteria to elicit a hypersensitive response (HR) on non-host or resistant host plants and for pathogenesis on susceptible hosts. When Harpins (hrp) genes are secreted to the plant cells from bacterial pathogens, localized cell death happens through series of reactions like reactive oxygen species (ROS) accumulation. Exploiting this strategy, transgenic plants resistant to bacterial pathogens have been produced. Harpin NEa (HrpNEa) is encoded by the gene hrpN located on the chromosome of Erwinia causing the fire blight disease of apple. HrpNEa is a known inducer of systemic acquired resistance (SAR) in plants. Several studies have demonstrated that enhanced HrpNEa levels in transgenic plants increase the resistance to bacteria (Malnoy et al., 2005). Recently, a plant ferrodoxin like protein (PFLP) was transferred to Arabidopsis. Expression of PFLP protein enhanced resistance to bacterial disease. PFLP is a photosynthetic type ferredoxin with an N-terminal signal peptide for chloroplast localization. Presence of PFLP in transgenic plants confers resistance against bacterial disease, however, the relationship still remains unclear (Lin et al., 2010).
22Another approach for engineering of plant resistance against bacterial disease is based on the transformation with a gene encoding a toxin-detoxifying enzyme from the pathogen itself. Pseudomonas syringae pv. tabaci produces the toxin called tabtoxin. In plants, tabtoxin is converted to tabtoxinine-β-lactam which inhibits glutamine synthase leading to an accumulation of cytotoxic ammonia. The pathogen protects itself against the toxin by expression of the tabtoxin resistance gene (ttr), which is able to protect P. syringae by acetylating tabtoxin to an inactive form. The transgenic tobacco, expressing ttr gene, displayed a reduction in disease symptoms (Batchvarova et al., 1998).
3.3. Resistance to viral diseases
23To date, over seven hundred plant viruses have been identified which cause various diseases and significant crop losses. Due to the lack of viricides, virus diseases are conventionally controlled using certified virus free planting material, eradicating infected plants and spraying chemicals against virus vectors. Coat protein-mediated resistance to viruses has been one of the successes of plant genetic engineering. Using this approach, several major crop plants have been engineered to resist important viral pathogens and, eventually, the resistant plants have been commercialized, for example potato event HLMT15-15 which is tolerant to PYV (Potato Y Virus) or potato event RBMT21-350 which is resistant to PLRV (Potato Leaf Roll Virus) (James, 2013).
24Papaya ringspot virus (PRSV), causing serious losses in papaya fruit production in several countries, exists in the form of different strains. Resistance to this virus was obtained in a high-yielding papaya hybrid using the viral coat protein sequence as the transgene (Gonsalves, 2004). Attempts have been made to assess the effectiveness of transformation of plants with viral genes other than coat protein gene, to provide protection against viral diseases. Otang Ntui et al. (2014) produced transgenic tobacco expressing defective Cucumber Mosaic Virus (CMV) replicase-derived dsRNA which was highly resistance.
25The ability of the sense and antisense RNA of the replication-associated protein encoded by AC1 (African cassava mosaic virus replication-associated) or C1 gene of geminiviruses to protect plants against virus infection was also assessed (Zhang et al., 2005). There are also reports on resistance to virus in transgenic plants mediated by a defective movement protein (MP) of virus (Hallwass et al., 2014; Peiró et al., 2014).
26Antibody engineering has been developed as a novel approach to produce pathogen-resistant plants by expressing antibodies or rAb fragments that are capable of inactivating pathogens or proteins involved in pathogenesis (Cardoso et al., 2014).
27In addition to the above-mentioned examples, another method for engineering virus resistance has been explored, including the use of pokeweed antiviral protein (PAP) and 2′,5′-oligoadenylate synthetase. PAP has the ability to depurinate highly conserved parts of the ribosome so the protein translation system of virus is inhibited (Thamizhmani et al., 2014).
4. Resistance to herbicides
28The fourteen commercialized herbicide tolerant crops that have been introduced by James (2013) comprise 222 events. However, the glyphosate-tolerant maize, soybean, canola and cotton are the most abundant lines among those crops (Table 1). The introduced crops usually harbor various kinds of genes to become herbicide tolerant, including gat, bxn, surb, dmo, epsps, 2mepsps, etc. Glyphosate is the active component of Roundup®. It is in widespread use as non-selective post-emergence herbicide. Glyphosate works as an analog of enolpuruvate by binding to and inhibiting the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) which is active in the shikimate pathway leading to the synthesis of chorismate-derived metabolites including the aromatic amino acids. It means that inactivating this enzyme by glyphosate kills the plant for complete renewal of proteins becomes impossible (due to the absence of aromatic amino acids). Glyphosate tolerance in genetically modified plants is obtained through the transfer of a mutated gene for EPSPS synthase (Stalker et al., 1985) that will better distinguish its natural substrate enolpyruvate from glyphosate. For example the EPSPS gene isolated from Pseudomonas stutzeri A1501 enhanced resistance to glyphosate up to 200 mM (Aimin et al., 2008). Another plant which is under development for resistance to glyphosate is Amaranthus palmeri (Gaines et al., 2010).
29The bar gene from Streptomyces hygroscopicus encodes a phosphinothricin acetyl transferase (PAT) that acetylates the free NH2 groups of phosphinothricin (PPT), the component of herbicides, thereby inactivating its herbicide activity. So, a transgenic line encoding PAT becomes resistant like the sweet potato expressing the bar gene (Zhang et al., 2008). Glyphosate oxidoreductase (GOX) from Ochrobactrum anthropi strain LBAA and the pat gene, homolougus to bar, from Streptomyces viridochromogenes which encodes N-acetyltransferases are two other genes that can inactivate glyphosate and glufosinate, respectively (Green et al., 2011).
30A GmGSTU gene from soybean was transferred to tobacco. The GmGSTU4 is an isoenzyme which has catalytic activity for diphenylether herbicide fluorodifen/alachlor (Benekos et al., 2010). Recently, an imidazolinone resistance (IR) XA17 gene was introduced into maize. Transgenic lines showed resistance to imazaquin and nicosulfuron herbicides. In these lines, the yield loss was minimized by a considerable level through weed control (Menkir et al., 2010). Another mechanism that deactivates glyphosate into a non toxic N-acetylglyphosate is by introducing the glyphosate N-acetyltransferase (Gat) from Bacillus licheniformis to plant (Siehl et al., 2007). The soybean and corn plants were tolerant to glyphosate when they were transformed with GAT gene (Castle et al., 2004). In addition to the above mentioned genes, the other genes transformed to plant species are tabulated in table 1.
5. Discussion
31Resistance of transgenic plants to pests, pathogens and herbicides has been achieved in more than 20 different crops including maize, potato, squash, sugar beet, wheat, cotton, soybean, oilseed rape, tomato, tobacco, rice, barley, papaya and alfalfa. The extensively field tested transgenic plants that have met the stringent biosafety guidelines have been released for commercial cultivation (James, 2013). Very high levels of resistance to insect pests and viral diseases have been reached while examples of successful protection to bacterial and fungal diseases are still scarce. In USA, permissions for commercialization of more than 10 transgenic pest resistant crop varieties have been granted: insect resistant potato, maize and cotton expressing Bt toxins, virus resistant papaya and squash expressing viral coat proteins. Several other transgenic crops are also approaching commercialization (James, 2013). In the field of pest and disease resistance, it is likely that more insect resistant crops expressing Bt toxins or virus resistant crops engineered with viral genes will enter the market in the near future. Within some years, varieties with enhanced resistance against fungal and bacterial pathogens may also become available.
32Virus resistance is mostly achieved by introducing gene sequences derived from pathogenic viruses into the crop genome using gene silencing, antisense RNA and RNAi techniques (Ramesh et al., 2007; Yan et al., 2007).
33Strategies applied to achieve fungal resistance make use of plant genes acting on different levels of the plant defense system against pathogens. Chitinase and glucanase genes have been used in several crops and have led to significant protection in some cases (Jongedijk et al., 1995; Tohidfar et al., 2012). The growing understandings on plant defense mechanisms are expected to lead to increased levels of protection in the near future (Swathi et al., 2008; Takakura et al., 2008).
34Investigated methods to obtain resistance to bacteria have not led to high levels of protection yet. Other partially successful strategies make use of genes which encode toxin detoxifying enzymes.
35Despite the present concerns about transgenic plants, it is observed that the cultivation area of transgenic crops is growing fast each year and many of them are commercially released and produced. Considering the production trend of these crops, it is expected that the production and commercialization of GM crops resistant to abiotic stresses (drought, salinity, etc.) happens in a near future.
Acknowledgements
36We wish to give our sincere acknowledgements to Dr Mohammadreza Parvin and Dr Maryam Shahbazi for drafting the abstract in French.
Bibliographie
Aimin L. et al., 2008. A single residue mutation of 5-enoylpyruvylshikimate- 3-phosphate synthase in Pseudomonas stutzeri enhances resistance to the herbicide glyphosate. Biotechnol. Lett., 30, 1397-1401.
Alfonso-Rubi J. et al., 2003. Transgenic expression of trypsin inhibitor CMe from barley in Indica and Japonica rice, confers resistance to the rice weevil Sitophilus oryzae. Transgenic Res., 12, 23-31.
Altpeter F. et al., 1999. Increased insect resistance in transgenic wheat stably expressing trypsin inhibitor CMe. Mol. Breeding, 5, 53-63.
Ballvora A. et al., 2002. The R1 gene for potato resistance to late blight (Phytophthora infestans) belongs to the leucine zipper/NBS/LRR class of plant resistance genes. Plant J., 30, 361-371.
BANR (Board on Agriculture and Natural Resources), 2000. Genetically modified pest protected plant: science and regulation. Washington: National Academy Press.
Barbosa A.E. et al., 2010. Alpha-amylase inhibitor-1 gene from Phaseolus vulgaris expressed in Coffea arabica plants inhibits alpha-amylases from the coffee berry borer pest. BMC Biotechnol., 10, 44, doi: 10.1186/1472-6750-10-44.
Batchvarova R. et al., 1998. Transgenic tobacco cultivars resistant to Pseudomonas syringae pv. tabaci. Theor. Appl. Genet., 97, 981-989.
Behrens M.R. et al., 2007. Dicamba resistance: enlarging and preserving biotechnology-based weed management strategies. Science, 316(5828), 1185-1188.
Benekos K. et al., 2010. Overexpression of a specific soybean GmGSTU4 isoenzyme improves diphenyl ether and chloroacetanilide herbicide tolerance of transgenic tobacco plants. J. Biotechnol., 150, 195-201.
Cardoso F.M. et al., 2014. Single domain antibodies targeting neuraminidase protect against an H5N1 influenza virus challenge. J. Virol., 88(15), 8278-8296.
Castle L.A. et al., 2004. Discovery and directed evolution of a glyphosate tolerance gene. Science, 304, 1151-1154.
CERA (Center for Environmental Risk Assessment), 2010. GM Crop Database, www.cera-gmc.org/index.php?action =gm_crop_database, (18.10.2010).
Chakraborty S. & Newton A.C., 2011. Climate change, plant diseases and food security: an overview. Plant Pathol., 60(1), 1-14.
Champion A. et al., 2009. Molecular diversity and gene expression of cotton ERF transcription factors reveal that group IXa members are responsive to jasmonate, ethylene and Xanthomonas. Mol. Plant Pathol., 10(4), 471-485.
Coombs J.J. et al., 2002. Combining engineered (Bt-cry3A) and natural resistance mechanisms in potato for control of Colorado potato beetle. J. Am. Soc. Hortic. Sci., 127, 62-68.
Coutos-Thévenot P. et al., 2001. In vitro tolerance to Botrytis cinerea of grapevine 41B rootstock in transgenic plants expressing the stilbene synthase Vst1 gene under the control of a pathogen-inducible PR 10 promoter. J. Exp. Bot., 52(358), 901-910.
Davis V.M., 2012. Integrating 2,4-d and dicamba resistant soybean into Wisconsin cropping systems. Vol. 51. In: Proceedings of the Wisconsin Crop Management Conference, 10-12 January 2012, Exposition Hall, Alliant Energy Center, Madison, Wisconsin, USA. Madison, WI, USA: University of Wisconsin-Extension, College of Agricultural and Life Sciences, 36-37.
DeVilliers S.M. & Hoisington D.A, 2011. The trends and future of biotechnology crops for insect pest control. Afr. J. Biotechnol., 10, 4677-4681.
Di R. et al., 2010. Expression of a truncated form of yeast ribosomal protein L3 in transgenic wheat improves resistance to Fusarium head blight. Plant Sci., 178, 374-380.
Duan X. et al., 1996. Transgenic rice plants harboring an introduced potato proteinase inhibitor II gene are insect resistant. Nat. Biotechnol., 14(4), 494-498.
Gahan L.J., Pauchet Y., Vogel H. & Hecke D.G., 2010. An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet., 6(12), 1-11.
Gaines T.A. et al., 2010. Gene amplification confers glyphosate resistance in Amaranthus palmeri. Proc. Natl. Acad. Sci. U.S.A., 107(3), 1029-1034.
Gao H., Narayanan N.N., Ellison L. & Bhattacharyya M.K., 2005. Two classes of highly similar coiled coil-nucleotide binding-leucine rich repeat genes isolated from the Rps1-k locus encode Phytophthora resistance in soybean. Mol. Plant-Microbe Interact., 18, 1035-1045.
Gatehouse A.M.R. et al., 1997. Transgenic potato plants with enhanced resistance to the tomato moth, Lacanobia oleracea: growth room trials. Mol. Breed., 3(1), 49-63.
Gonsalves D., 2004. Transgenic papaya in Hawaii and beyond. AgBioForum, 7, 36-40.
Green J.M. & Owen M.D.K., 2011. Herbicide resistant crops: utilities and limitations for herbicide resistant weed management. J. Agric. Food Chem., 59, 5819-5829.
Grennan A.K., 2008. Ethylene response factors in jasmonate signaling and defense response. Plant Physiol., 146(4), 1457-1458.
Hallwass M. et al., 2014. The Tomato spotted wilt virus cell-to-cell movement protein (NSM) triggers a hypersensitive response in Sw-5-containing resistant tomato lines and in Nicotiana benthamiana transformed with the functional Sw-5b resistance gene copy. Mol. Plant Pathol., 15(9), 871-880.
He H., Wang Z. & Zhang Y., 2008. Monitoring Bt resistance in the field; China as a case study. In: Ferry N. & Gatehouse A.M.R., eds. Environmental Impact of Genetically Modified/Novel Crops. Wallingford, UK: CAB International.
Hilder V.A. et al., 1987. A novel mechanism of insect resistance engineered into tobacco. Nature, 333, 160-163.
Iannacone R., Grieco D. & Cellini F., 1997. Specific sequence modifications of a cry3B endotoxin gene result in high levels of expression and insect resistance. Plant Mol. Biol., 34, 485-496.
Ignacimuthu S. & Prakash S., 2006. Agrobacterium-mediated transformation of chickpea with alpha-amylase inhibitor gene for insect resistance. J. Biosci., 31(3), 339-345.
Itoh Y. et al., 2003. Family 19 chitinase of Streptomyces griseus HUT6037 increases plant resistance to the fungal disease. Biosci. Biotechnol. Biochem., 67, 847-855.
James C., 2012. Global status of commercialized biotech/GM crops. Brief No. 44. Ithaca, NY, USA: ISAAA.
James C., 2013. Global status of commercialized biotech/GM crops. Brief No. 46. Ithaca, NY, USA: ISAAA.
Jansens S. et al., 1997. Transgenic corn expressing a Cry9C insecticidal protein from Bacillus thuringiensis protected from European corn borer damage. Crop Sci., 37(5), 1616-1624.
Jongedijk E. et al., 1995. Synergistic activity of chitinases and β-1,3-glucanases enhances fungal resistance in transgenic tomatoplants. Euphytica, 85, 173-180.
Lee M.K., Miles P. & Chen J.S., 2006. Brush border membrane binding properties of Bacillus thuringiensis Vip3A toxin to Heliothis virescens and Helicoverpa zea midguts. Biochem. Biophys. Res. Commun., 339, 1043-1047.
Li G.Y. et al., 2005. Insect resistance to Nilaparvata Lugens and Cnaphalocrocis medinalis in transgenic indica rice and the inheritance of gna+sbti transgenes. Pest Manage. Sci., 61, 390-396.
Liang H. et al., 2000. A transgenic wheat with a stilbene synthase gene resistant to powdery mildew obtained by biolistic method. Chin. Sci. Bull., 45, 634-638.
Lin Y.H. et al., 2010. Plant ferredoxin-like protein (PFLP) outside chloroplast in Arabidopsis enhances disease resistance against bacterial pathogens. Plant Sci., 179, 450-458.
Macrae T.C. et al., 2005. Laboratory and field evaluations of transgenic soybean exhibiting highdose expression of a synthetic Bacillus thuringiensis cry1A gene for control of Lepidoptera. J. Econ. Entomol., 98, 577-587.
Malnoy M., Venisse J.S. & Chevreau E., 2005. Expression of a bacterial effector hairpin N causes increased resistance to fire blight in Pyrus communis. Tree Genet. Genomes, 1, 41-49.
Melchers L.S. & Stuiver M.H., 2000. Novel genes for disease resistance breeding. Curr. Opin. Plant Biol., 3, 147-152.
Menkir A., Chikoye D. & Lum F., 2010. Incorporating an herbicide resistance gene into tropical maize with inherent polygenic resistance to control Striga hermonthica (Del.) Benth. Plant Breeding, 129, 385-392.
Morton R.L. et al., 2000. Bean alpha-amylase inhibitor 1 in transgenic peas (Pisum sativum) provides complete protection from pea weevil (Bruchus pisorum) under field conditions. Proc. Natl. Acad. Sci. U.S.A., 97, 3820-3825.
Neuhaus J.M., 1999. Plant chitinases (PR-3, PR-4, PR-8, PR-11). In: Datta S.K. & Muthukrishnan S., eds. Pathogenesis-related proteins in plants. Boca Raton, FL,USA: CRC Press, 77-105.
Oelfose D. et al., 2006. Apple polygalacturonase inhibition potential expressed in transgenic tobacco inhibits polygalacturonases from fungal pathogens of apple and anthracnose of lupins. Phytochemistry, 67, 255-263.
Oksman-Kaldentey K.M. & Barz W.H., 2002. Plant biotechnology and transgenic plants. New York, USA: Marcel Dekker, Inc.
Otang Ntui V. et al., 2014. Transgenic tobacco lines expressing defective CMV replicase-derived dsRNA are resistant to CMV-O and CMV-Y. Mol. Biotechnol., 56, 50-63.
Peiró A. et al., 2014. The movement protein (NSm) of Tomato spotted wilt virus is the avirulence determinant in the tomato Sw-5 gene-based resistance. Mol. Plant Pathol., 15(8), 802-813.
Pel M.A. et al., 2009. Mapping and cloning of late blight resistance genes from Solanum venturii using an interspecific candidate gene approach. MPMI, 22, 601-615.
Peterson M.A., Simpson D.M., Bing J.W. & Cui C.C., 2012. Characterization of protein expression and agronomics of enlist corn. Indianapolis, IN, USA: Dow AgroSciences.
Powell K.S, Gatehouse A.M.R., Hilder V.A. & Gatehouse J.A., 1995. Antifeedant effects of plant lectins and an enzyme on the adult stage of the rice brown plant hopper, Nilaparvata lugens. Entomol. Exp. Appl., 75, 51-59.
Rahbe Y. et al., 2003. Effects of the cysteine protease inhibitor oryzacystatin (OC-I) on different aphids and reduced performance of Myzus persicae on OC-I expressing transgenic oilseed rape. Plant Sci., 164, 441-450.
Ramesh S.V., Mishra A.K. & Praveen S., 2007. Hairpin RNA-mediated strategies for silencing of tomato leaf curl virus AC1 and AC4 genes for effective resistance in plants. Oligonucleotides, 17, 251-257.
Reeck G.R. et al., 1997. Proteinase inhibitors and resistance of transgenic plants to insects. In: Carozzi N. & Koziel M., eds. Advance in insect control: the role of transgenic plants. London: Taylor and Francis Press, 157-183.
Ribeiro A.P.O. et al., 2006. Effect of eggplant transformed with oryzacystatin gene on Myzus persicae and Macrosiphon euphorbiae. J. Appl. Entomol., 130, 84-90.
Rohini V., Sankara K. & Rao K., 2000. Transformation of peanut (Arachis hypogaea L.) with tobacco chitinase gene: variable response of transformants to leaf spot disease. Plant Sci., 160, 889-898.
Roy B.S., Noren K., Mandal A.B. & Basu A.K., 2011. Genetic engineering for abiotic stress tolerance in agricultural crops. Biotechnology, 10, 1-22.
Saha P. et al., 2006. Transgenic rice expressing Allium sativum leaf lectin with enhanced resistance against sap-sucking insect pests. Planta, 23, 1329-1343.
Sanyal I., Singh A.K., Kaushik M. & Amla D.V., 2005. Agrobacterium-mediated transformation of chickpea (Cicer arietinum L.) with Bacillus thuringiensis cry1Ac gene for resistance against pod borer insect Helicoverpa armigera. Plant Sci., 168(4), 1135-1146.
Shade R.E. et al., 1994. Transgenic pea-seeds expressing the alpha-amylase inhibitor of the common bean are resistant to bruchid beetles. Nat. Biotechnol., 12, 793-796.
Sharma H.C., Sharma K.K. & Crouch J.H., 2004. Genetic transformation of crops for insect resistance: potential and limitations. Crit. Rev. Plant Sci., 23, 47-72.
Siehl D.L., Castle L.A., Gorton R. & Keenan R.J., 2007. The molecular basis of glyphosate resistance by an optimized microbial acetyltransferase. J. Biol. Chem., 282, 11446-11455.
Song W.Y. et al., 1995. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science, 270, 1804-1806.
Song X.X. & Wang S.M., 2001. Status and evaluation on the expression of cotton varieties in the production in China in the past 20 years. Cotton Sci., 13, 315-320.
Stalker D.M., Hiatt W.R. & Comai L., 1985. A single amino acid substitution in the enzyme 5-enolpyruvylshikimate-3-phosphate synthase confers resistance to the herbicide glyphosate. J. Biol. Chem., 260, 4724-4728.
Swathi A.T., Divya K., Jami S.K. & Kirti P.B., 2008. Transgenic tobacco and peanut plants expressing a mustard defensin show resistance to fungal pathogens. Plant Cell Rep., 27, 1777-1786.
Takakura Y. et al., 2008. Expression of a bacterial flagellin gene triggers plant immune responses and confers disease resistance in transgenic rice plants. Mol. Plant Pathol., 9, 525-529.
Thamizhmani R. & Vijayachari P., 2014. Association of dengue virus infection susceptibility with polymorphisms of 2′-5′-oligoadenylate synthetase genes: a case–control study. Braz. J. Infect. Dis., 18(5), 548-550.
Tohidfar M., Ghareyazie B., Mohammadi M. & Abdmishani C., 2005. Agrobacterium-mediated transformation of cotton using a chitinase gene. Plant Cell Tissue Organ Culture, 83, 83-96.
Tohidfar M. et al., 2008. Agrobacterium-mediated transformation of cotton (Gossypium hirsutum) using a synthetic cry1Ab gene for enhanced resistance against Heliothis armigera. Iran. J. Biotechnol., 6(3), 164-173.
Tohidfar M., Hossaini R., Shokhandan Bashir N. & Tabatabaei M., 2012. Enhanced resistance to Verticillium dahliae in transgenic cotton expressing an endochitinase gene from Phaseolus vulgaris. Czech J. Genet. Plant Breeding, 4, 345-355.
Tohidfar M., Zare N., Salhi G. & Eftghari M., 2013. Agrobacterium-mediated transformation of alfalfa (Medicago sativa) using a synthetic cry3a gene to enhance resistance against alfalfa weevil. Plant Cell Tissue Organ Culture, 113, 227-235.
Tougou M. et al., 2006. Development of resistant transgenic soybeans with inverted repeat-coat protein genes of soybean dwarf virus. Plant Cell Rep., 25(11), 1213-1218.
USDA, 2000. APHIS-PPQ-PRA Biotechnology Authorizations. Permits notifications and determinations of non-regulated status (as of 6/30/2000), http://www.aphis.usda.gov (Biotechnology section), (December 2000).
Van der Vossen E. et al., 2005. The Rpi-blb2 gene from Solanum bulbocastanum is a Mi-1 gene homolog conferring broad spectrum late blight resistance in potato. Plant J., 44, 208-222.
Vleeshouwers V.G.A.A. et al., 2008. Effector genomics accelerates discovery and functional profiling of potato disease resistance and Phytophthora infestans avirulence genes. PLoS ONE, 3(8), e2875.
Wahab S., 2009. Biotechnological approach in the management of plant pests, diseases and weeds for sustainable agricultural. J. Biopesticides, 2, 115-134.
Wankhede D.P., Kumar K., Singh P. & Sinha A.K., 2013. Involvement of mitogen activated protein kinase kinase 6 in UV induced transcripts accumulation of genes in phytoalexin biosynthesis in rice. Rice, 6(35), 1-8.
Yamamoto T. et al., 2000. Transgenic grapevine plants expressing a rice chitinase with enhanced resistance to fungal pathogens. Plant Cell Rep., 19, 639-646.
Yan F. et al., 2007. Transgenic wheat expressing virus-derived hairpin RNA is resistant to Barley yellow dwarf virus. Yi Chuan, 29, 97-102 (in Chinese).
Zhang P., Vanderschuren H., Fütterer J. & Gruissem W., 2005. Resistance to cassava mosaic disease in transgenic cassava expressing antisense RNAs targeting virus replication genes. Plant Biotechnol. J., 3(4), 385-397.
Zhang W., Chen J., Zhang H. & Song F., 2008. Overexpression of a rice diacylglycerol kinase gene OsBIDK1 enhances disease resistance in transgenic tobacco. Mol. Cells, 26, 258-264.
Zhu S. et al., 2012. Functional stacking of three resistance genes against Phytophthora infestans in potato. Transgenic Res., 21, 89-99.






