- Accueil
- Volume 18 (2014)
- numéro 2
- Inhibition of Penicillium digitatum by a crude extract from Solanum nigrum leaves
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Inhibition of Penicillium digitatum by a crude extract from Solanum nigrum leaves

Notes de la rédaction
Received on May 24, 2013; accepted on February 13, 2014
Résumé
L'inhibition de Penicillium digitatum par un extrait brut de feuilles de Solanum nigrum. Un extrait aqueux brut de feuilles lyophilisées de Solanum nigrum a été évalué pour sa composition phytochimique et son activité antifongique contre Penicillium digitatum, l'agent causal de la moisissure verte des agrumes. L’analyse phytochimique de l'extrait a révélé la présence de certaines substances bioactives telles que les alcaloïdes, les tanins, les flavonoïdes, les saponines, etc. L'extrait a montré une zone d'inhibition remarquable contre l'agent pathogène dans des essais de diffusion en gélose. Le stockage de l'extrait à 4 °C pendant 60 jours n'a eu aucun effet sur son activité antifongique in vitro. En outre, l'extrait a été testé pour son activité antifongique in vivo (essais préventif et curatif) contre P. digitatum sur citrons inoculés par blessure. Un important effet antifongique (essai préventif) a été observé après 7 jours de stockage (100 % d'inhibition), bien que cette activité ait diminué après 14 et 21 jours (85,71 et 57,14 % d'inhibition, respectivement). Une légère activité antifongique (essai curatif) a été observée seulement après 7 jours de stockage (% d'inhibition : 14,29 ± 1,63). Les résultats préliminaires de cette étude pourraient contribuer au développement de nouveaux agents antifongiques pour protéger les fruits de citron contre les maladies fongiques de post-récolte.
Abstract
An aqueous crude extract from Solanum nigrum lyophilized leaves was evaluated for its phytochemical composition and antifungal activity against Penicillium digitatum, the causative agent of green mold of citrus fruit. Phytochemical analysis of the extract revealed the presence of some bioactive substances such as alkaloids, tannins, flavonoids, saponins, etc. The extract showed a remarkable inhibition zone against the pathogen in agar well diffusion assays carried out in Petri plates. Storage of the extract at 4 °C for 60 days had no effect on its in vitro antifungal activity. Further, the extract was tested for its in vivo (preventive- and curative treatments) antifungal activity on lemons wound-inoculated with P. digitatum. An important preventive antifungal effect was observed after 7 days of storage (100% of inhibition), although this activity decreased after 14 and 21 days (85.71 and 57.14% of inhibition, respectively). A slight curative antifungal activity was observed only after 7 days of storage (14.29% of inhibition). Preliminary findings from this study may contribute to the development of new antifungal agents to protect the lemon fruits from postharvest fungal diseases.
Table des matières
1. Introduction
1Penicillium digitatum is the causal agent of green mold, one of the most common post-harvest disease affecting citrus fruits (Sommer et al., 2002; Plaza et al., 2004). Proper agricultural practices (e.g. minimizing wounds on fruit, proper storage temperature and humidity management, etc.) are recommended to reduce economic losses by this pathogen (Eckert et al., 1998), although chemical control and use of fungicides are the most effective way of preventing the occurrence of this post-harvest disease. However, indiscriminate postharvest use of synthetic fungicides, such as imazalil, thiabendazole, and o-phenylphenol (Poppe et al., 2003), has increased the risk of high-level toxic residues in the products and development of antimicrobial resistance (Holmes et al., 1999).
2Plants can represent an important source to provide alternatives to some of the more overused antimicrobial compounds currently available. Plants, in fact, contain thousands of bioactive substances, which can be divided chemically into a number of groups among which are alkaloids, phenols and phenolic glycosides, resins, oleosins, steroids, tannins and terpenoids (Ferreira et al., 2008). Besides, plant fungicides seem to have less of an impact on the environment, since they are more biodegradable and have short-lasting residual periods (Huang et al., 2005). Therefore, the research on bioactive substances from plant sources to develop environmental-friendly fungicides has been intensified and a number of plant extracts has been tested in vitro and in vivo for antifungal activity against several plant pathogenic fungi (Ojala et al., 2000; Pretorius et al., 2002; Choi et al., 2004).
3Plant extracts are prepared by using different extraction procedures (Vinatoru et al., 2001; Ong, 2004; Ncube et al., 2008; Das et al., 2010), in which solvents diffuse into the vegetal material and solubilize metabolic plant compounds with similar polarity. Leaves, flowers, fruits, barks, roots used as starting vegetal material can be fresh or dried, although in most reported works dried plant parts are used. The dry form is preferred over the fresh one because the differences in water content within different plant tissues may affect solubility of subsequent separation by liquid-liquid extraction, and the metabolic plant compounds to be used as antimicrobials should be relatively stable (Dilika et al., 1996; Baris et al., 2006). However, plant extracts may lose their bioactivity as obtained from dried material. Mbwambo et al. (2009) reported that an extract from fresh freeze-dried leaves of Warburgia ugandensis exhibited both antibacterial and anticandida activities compared to air-dried leaves, which exhibited antibacterial activity only.
4Lyophilisation (also known as freeze-drying) is a more complex form of drying (Ciurzynska et al., 2011), widely applied in several sectors (e.g. pharmaceutical, biotechnology, food industry, etc.) because it gives the opportunity to avoid the damages (e.g. loss of activity/availability, denaturation, etc.) caused by heating the material, by maintaining it frozen throughout drying. Lyophilisation is often used after the extraction of bioactive compounds, allowing a subsequent plant extract rehydration in a desired solvent and to a desired concentration (Al-Fatimi et al., 2007; Pereira et al., 2007; Lin et al., 2011). Conversely, studies in which lyophilisation is used to increase the storage life of starting plant material have been less frequent. In this study, fresh leaves of Solanum nigrum were collected and lyophilized to better preserve their bioactive substances. This plant is a rich source of phytochemicals (Atanu et al., 2011), although in Italy it is considered as weed of arable land and gardens. Solanum nigrum lyophilized leaves were used to obtain an aqueous crude extract, whose phytochemical composition and antifungal activity against P. digitatum were evaluated by using in vitro and in vivo techniques.
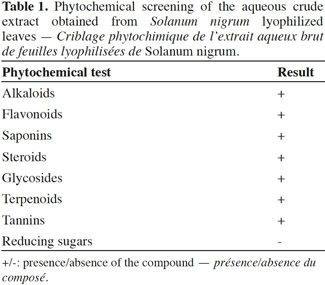
2. Materials and methods
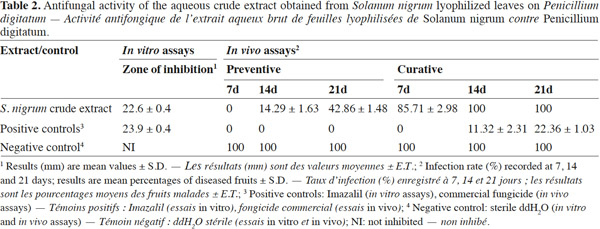
2.1. Plant material
5Healthy S. nigrum leaves used as experimental material were collected from farm lands in Province of Potenza (southern Italy), in October 2012. The collected plant material was placed in a polyethylene bag to prevent loss of moisture during transportation to the laboratory. Solanum nigrum leaves were washed and freeze-dried under vacuum at 0.250 mbar pressure and at -53 °C (Model Freezone 18, Labconco, Kansas City, MI, USA) for 24 h. Lyophilisation reduced leave weight by more than 87%. Lyophilized leaves were then powdered with a laboratory blender (Model 7010S, Waring Laboratory, New Hartford, CT, USA) for 30 s.
2.2. Preparation of a crude extract from S. nigrum lyophilized leaves
6An aqueous crude extract from S. nigrum leaves was prepared by infusion. A quantity of 150 ml of boiling double-distilled water (ddH2O) was added to 10 g of lyophilized powder. The infusion so described was allowed to proceed in the dark for 24 h under agitation using a rotary shaker (150 rpm). Thereafter, the mixture was clarified by centrifugation (at 20 000 rpm for 30 min) and the supernatant filtered through Whatman No. 4 paper. The obtained extract was finally sterilized through a filter membrane (0.22 μm) before use.
2.3. Phytochemical screening
7The aqueous crude extract from S. nigrum lyophilized leaves was screened for the presence of several compounds by modifying the phytochemical methods reported in literature (Trease et al., 1989; Pradhan et al., 2010).
8Alkaloids. 5 ml of the extract was treated first with 2 ml of HCl, and then with 1 ml of Dragendroff’s reagent. An orange or red precipitate indicated the presence of alkaloids.
9Flavonoids. 1 ml of the extract was treated with few drops of 2% NaOH to produce an intense yellow color. After adding few drops of dilute HCl, the extract became colorless if it contained flavonoids.
10Glycosides. 1 ml of the extract was treated first with 2 ml of CH3COOH mixed with few drops of FeCl3, and then with 1 ml of H2SO4. Formation of a reddish brown color at the junction of two layers and the bluish green color in the upper layer indicated the presence of glycosides.
11Saponins. 1 ml extract was first diluted with 5 ml of ddH2O, and then agitated for 15’. The formation of at least 1 cm layer of foam indicated the presence of saponins.
12Steroids. 1 ml of the extracts was treated first with 10 ml of CHCl3, and then with 10 ml of H2SO4. A red color in the upper layer and a yellow color in H2SO4 layer indicated the presence of steroids.
13Tannins. 1 ml of the extract was first diluted with 4 ml of ddH2O, and then treated with few drops of 10% FeCl3. Formation of a blue/green color indicated the presence of tannins.
14Terpenoids. 5 ml of the extract was treated first with 2 ml of (CH3CO)2O, and then with 2 ml of CHCl3; finally, 2 ml of H2SO4 were added. Formation of reddish violet color indicated their presence in the extract.
15Reducing sugars. 1 ml of the extract was first treated with 5-8 drops of Fehling’s solutions (A and B), and then heated in a water-bath. Formation of a red precipitate indicated the presence of sugars.
2.4. Antifungal activity evaluation
16Plant pathogen. Fungal spores of P. digitatum were harvested by flooding the surface of 10 days-old cultures with sterile 0.85% saline containing 0.1% Tween 80 and scraping the surface of colonies with glass rods. The resulting suspension was agitated, filtered through double-layered sterile cheesecloth to remove hyphal fragments and then diluted with water to an optical density (OD) of 0.1 at 425 nm, equivalent to roughly 106 spores·ml-1 (Smilanick et al., 1992).
17In vitro antifungal activity assays. The antifungal activity of the S. nigrum crude extract was determined by using a modified agar well diffusion assay method. Briefly, about 20 ml of the Saboraud Dextrose Agar (SDA) was poured into Petri plates (9 cm) and allowed to solidify. A quantity of 0.1 ml of P. digitatum spore suspension (104 spores·ml-1) were then spread on a SDA plate. Thereafter, wells of 6 mm diameter were aseptically punched into the agar medium and then filled with 100 μl of the crude extract. Imazalil (1-[2-(2, 4-dichlorophenyl)-2-(2-propenyloxy) ethyl]-1H-imidazole; technical, 97.5% a.i.) at a concentration of 1 g·l-1 was used as positive control, whereas sterile ddH2O as negative control. The plates were incubated for 72 h at 25 °C. The antifungal activity of the extract was evaluated by measuring the zone of inhibition. To assess the effect of storage on the antifungal activity in S. nigrum crude extract, an aliquot was stored in the dark at 4 °C for 60 days. Afterwards, the antifungal activity was retested by agar well diffusion assay as described above. The experiments were carried out with three replicates per treatment and 10 plates were used in each replicate.
18In vivo antifungal activity assays. Lemon fruits cv ‘Primofiore’ were obtained from a local supermarket. The fruits were selected according to their appearance (uniform size and maturity, without wounds) to minimize interference from natural infections. Lemon fruits were washed in running water, dipped in 70% ethanol for 2 min, rinsed twice with sterile ddH2O for 10 min and finally air-dried. One wound was made in the middle of each fruit by a stainless steel cutter with a 2 mm long by 1 mm wide tip. Wounded fruits were then used for the following assays. The antifungal activity of the S. nigrum crude extract was evaluated on P. digitatum post infected or pre-infected fruits. In the first case (preventive treatment by the application of the extract prior to the inoculation of P. digitatum), lemon fruits were immersed in 5 l of the extract for 10 min, left to dry and then immersed in 5 l of spore suspension (containing 106 spores·ml-1) for 10 min. In the second case (curative treatment by inoculation of the P. digitatum followed by the treatment with the extract), each fruit was first immersed in the spore suspension and then in the crude extract. A commercial fungicide and sterile ddH2O were used as positive and negative controls, respectively. After the above assays, all fruits were incubated at 25 °C and 95% RH in sterilized plastic bags. Fruit disease was recorded after 7, 14 and 21 days. A fruit was considered as diseased if decay was visible at the inoculation point regardless of lesion diameter. Disease was scored (0 or 1 for healthy and diseased fruit, respectively) and data per treatment were expressed as infection rates. The experiments were carried out with three replicates per treatment and 10 fruits were used in each replicate.
3. Results and discussion
19The phytochemical screening results of the aqueous extract of S. nigrum lyophilized leaves are reported in table1. Standard qualitative methods showed the presence of alkaloids, flavonoids, saponins, steroids, glycosides, terpenoids and tannins. These compounds are generally synthesized in the secondary metabolism of the plant for several reasons (e.g. defense against predation by harmful organisms, adaptation to environmental changes, attraction of beneficial organisms, etc.) and are deposited in specific parts or in all parts of the plant. These compounds have become of great interest owing to the potential exploitation of their biological activity (Ncube at al., 2008; Das et al., 2010).
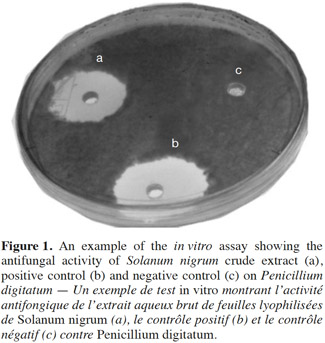
20The results from the in vitro antifungal assays are reported in table 2. The aqueous crude extract of the lyophilized leaves of S. nigrum was effective against P. digitatum in the agar well diffusion assay. The inhibition zone of the extract (22.6 ± 0.4 mm) was similar to that of the positive control (~24 mm), whereas no inhibition was observed for negative control (Figure 1). These results are in agreement with those of Kanan et al. (2008), who found an important antifungal activity of various crude extracts of S. nigrum against four P. digitatum isolates. According to these authors, the antifungal activity of S. nigrum leaves and fruits could be related to the presence of some steroidal alkaloids, such as solamargine, solasonine, solanine and saponin (Mohamed et al., 1996; Kambizi et al., 2001; Zhou et al., 2006). This agrees with previous studies (Muto et al., 2006; Zhou et al., 2006; Al-Fatimi et al., 2007) indicating that steroidal alkaloids have an antifungal activity against several agronomical important fungi, such as Aspergillus spp., Rhizopus spp., Fusarium spp., Mucor mucedo, Bipolaris oryzae, Rhizoctonia solani, etc. It has been reported (Lin et al., 2011) that an ethanol extract of S. nigrum inhibited spore germination of Alternaria brassicicola, the causative agent of cabbage black leaf spot disease. Bioassay-guided fractionation of the ethanol extract led to the identification of the antifungal principle responsible of complete inhibition of spore germination, a steroidal saponin known as degalactotigonin. However, the fractionation process of a plant extract may reduce its antifungal activity. Kanan et al. (2008) reported that Trigonella foenum-graecum L. fractions, although rich in alkaloids, showed a reduced antifungal activity against Penicillium isolates compared to the crude extracts. This result indicates that the antifungal activity of some plant extracts may be due to the synergistic interaction of their compounds.
21According to our results, storage at 4 °C of the S. nigrum crude extract had no effect on its antifungal activity. The mean inhibition zones of fresh and stored samples were not significantly different (22.6 ± 0.4 vs 22.5 ± 0.4, t = 0.72, d.f. = 58, p-value < 0.05), indicating that after 60 days of storage the extract still retained its original inhibitory potential.
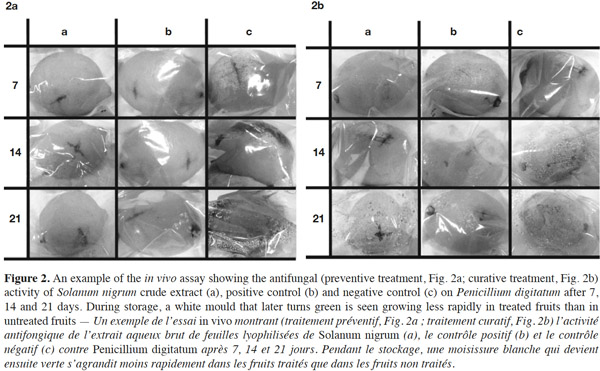
22With regard to in vivo assays (Table 2; Figure 2a), after 7 days of storage none of the lemon fruits treated with the crude extract before inoculation (i.e. preventive treatment) showed any visible sign of infection (100% of growth inhibition) and the same result was obtained for positive controls. However, the green mold incidence among treated fruits increased during storage: after 14 and 21 days, the infection rate (%) was 14.29 (± 1.63) and 42.86 (± 1.48), respectively. When the extract was applied after inoculation (i.e. curative treatment; Table 2; Figure 2b), 85.71% of lemons showed signs of infection after 7 days of storage. The infection rate among positive controls was 0%, although this rate increased with time. Fourteen days after inoculation, all treated fruits were infected. Lemons treated with ddH2O (negative controls) were all infected (0% of growth inhibition) in both assays (preventive and curative treatments) after 7 days of storage. Overall, these results are consistent with our earlier observations (data not shown), and suggest that the extract produced unfavorable conditions for fungal growth, probably due to an interaction with fruit tissues. However, under identical conditions of fungal exposure, this interaction is more likely to occur before inoculation, as lemons were less susceptible to show signs of infection.
23Nevertheless, the marked discrepancy between the effect of the extract on growth inhibition in preventive and curative treatments may be attenuated by changing some aspects. Studies such as that conducted by Tayel et al. (2009) have shown that the growth of P. digitatum on lemon fruits can be inhibited by immersion in a pomegranate peel extract. The authors reported that this antifungal activity is concentration and immersion time dependent, and the required concentration of the extract is lower in the preventive than in curative treatments. Therefore, the growth inhibition percentage of our crude extract in preventing and controlling citrus green mold may be improved by increasing the extract concentration and the immersion time of fruits.
4. Conclusion
24Solanum nigrum contains alkaloids, flavonoids, saponins, steroids and other important compounds with pharmacological prospects (Atanu et al., 2011). These compounds indicate that this agriculturally neglected plant can be a potential source of precursors in the development of natural drugs. In this study, we detected these compounds in a crude extract obtained from lyophilized leaves of S. nigrum, thus indicating that lyophilisation can be a suitable dehydration process to preserve the bioactive compounds in plant material. Furthermore, lyophilisation has revealed to be an excellent pre-treatment method for plant materials because this process yields a fine, dry sample. Even though lyophilisation has some inherent disadvantages (e.g. high cost of equipment, high energy costs, long process time), it can give the opportunity to increase plant material storability, thus making plant material available for a longer period. The presence of bioactive compounds in the extract could be responsible for its antifungal activity against P. digitatum, the causative agent of green mold of citrus fruits. This result is particularly important due to the fact that the extract was prepared by using a very simple procedure (i.e. infusion) and a non-toxic solvent, which does not interfere with the bioassays (i.e. water). The extract was found as effective as commercial antifungal imazalil when tested in vitro against P. digitatum. Moreover, the extract could be stored at 4 °C as our results showed that there was no significant loss of activity at this temperature over 60 days. An important in vivo antifungal activity was also observed; the extract, in particular, was found to be more effective for prevention rather than control of P. digitatum. However, further studies are needed to determine more reliably the concentrations required for efficacy and to better understand the exact antifungal mechanism of action of the compound(s) detected in the extract.
Bibliographie
Al-Fatimi M., Wurster M., Schroder G. & Lindequist U., 2007. Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J. Ethnopharmacol., 111, 657-666.
Atanu F.O., Ebiloma U.G. & Ajayi E.I., 2011. A review of the pharmacological aspects of Solanum nigrum Linn. Biotechnol. Mol. Biol. Rev., 6, 1-7.
Baris O. et al., 2006. Biological activities of the essential oil and methanol extract of Achillea Biebersteinii Afan. (Asteraceae). Turk. J. Biol., 30, 65-73.
Choi G.J. et al., 2004. In vivo antifungal activities of 57 plant extracts against six plant pathogenic fungi. Plant Pathol. J., 20, 184-191.
Ciurzynska A. & Lenart A., 2011. Freeze-drying – application in food processing and biotechnology – a review. Pol. J. Food Nutr. Sci., 61, 165-171.
Das K., Tiwari R.K.S. & Shrivastava D.K., 2010. Techniques for evaluation of medicinal plant products as antimicrobial agent: current methods and future trends. J. Med. Plants Res., 4, 104-111.
Dilika F., Afolayan A.J. & Meyer J.J.M., 1996. Comparative antibacterial activity of two Helichrysum species used in male circumcision in South Africa. S. Afr. J. Bot., 63, 158-159.
Eckert J.W. & Eaks I.L., 1998. Postharvest disorders and diseases of citrus fruits. In: Reuther W., Calavan E.C. & Carman G.E., eds. The citrus industry. Berkeley, CA, USA: University of California Press, 179-260.
Ferreira D. et al., 2008. Tannins and related polyphenols: perspectives on their chemistry, biology, ecological effects, and human health protection. Phytochemistry, 69, 3006-3008.
Holmes G.J. & Eckert J.W., 1999. Sensitivity of Penicillium digitatum and P. italicum to postharvest citrus fungicides in California. Phytopathology, 89, 716-721.
Huang H.C. & Chou C.H., 2005. Impact of plant disease biocontrol and allelopathy on biodiversity and agricultural sustainability. Plant Pathol. Bull., 14, 1-12.
Kambizi L. & Afolayan A.J., 2001. An ethnobotanical study of plants for the treatment of sexually transmitted diseases (Njovhera) in Guruve District, Zimbabwe. J. Ethnopharmacol., 77, 5-9.
Kanan G.J. & Al-Najar R.A., 2008. In vitro antifungal activities of various plant crude extracts and fractions against citrus post-harvest disease agent Penicillium digitatum. Jordan. J. Biol. Sci., 1, 89-99.
Lin T.C., Fan M.C., Wang S.Y. & Huang J.W., 2011. Identification of the Solanum nigrum extract component involved in controlling cabbage black leaf spot disease. J. Agric. Food Chem., 59, 1667-1672.
Mbwambo Z.H., Erasto P., Innocent E. & Masimba P.J., 2009. Antimicrobial and cytotoxic activities of fresh leaf extracts of Warburgia ugandensis. Tanzan. J. Health Res., 11, 75-78.
Mohamed S. et al., 1996. Antimycotic screening of 58 Malaysian plants against plant pathogens. Pestic. Sci., 47, 259-264.
Muto M. et al., 2006. Toxicity of black nightshade (Solanum nigrum) extracts on Alternaria brassicicola, causal agent of black leaf spot of Chinese cabbage (Brassica pekinensis). J. Phytopathol., 154, 45-50.
Ncube N., Afolayan S.A.J. & Okoh A.I., 2008. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. Afr. J. Biotechnol., 7, 1797-1806.
Ojala T. et al., 2000. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol., 73, 299-305.
Ong E.S., 2004. Extraction methods and chemical standardization of botanicals and herbal preparations. J. Chromatogr. B, 812, 23-33.
Pereira A.P. et al., 2007. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. cv. Cobrançosa) leaves. Molecules, 12, 1153-1162.
Plaza P., Usall J., Teixidó N. & Viñas I., 2004. Effect of water activity and temperature on competing abilities of common postharvest citrus fungi. Int. J. Food Microbiol., 90, 75-82.
Poppe L., Vanhoutte S. & Hofte M., 2003. Modes of action of Pantoea agglomerans CPA-2, an antagonist of postharvest pathogens on fruit. Eur. J. Plant Pathol., 109, 963-973.
Pradhan P. et al., 2010. Pharmacognostic, phytochemical and quantitative investigation of Saraca asoca leaves. J. Pharm. Res., 3, 776-780.
Pretorius J.C., Zeitsman P.C. & Eksteen D., 2002. Fungitoxic properties of selected South African plant species against plant pathogens of economic importance in agriculture. Ann. Appl. Biol., 141, 117-124.
Smilanick J.L. & Denis-Arrue R., 1992. Control of green mold of lemons with Pseudomonas species. Plant Dis., 76, 481-485.
Sommer N.F., Fortlage R.J. & Edwards D.C., 2002. Postharvest diseases of selected commodities. In: Kader A.A., ed. Postharvest technology of horticultural crops (Publication 3311). Berkeley, CA, USA: University of California, 197-249.
Tayel A.A., El-Baz A.F., Salem M.F. & El-Hadary M.H., 2009. Potential applications of pomegranate peel extract for the control of citrus green mould. J. Plant Dis. Prot., 116, 252-256.
Trease E.G. & Evans W.C., 1989. Pharmacognosy. 13th ed. London: Balliere Tindall, 167-235.
Vinatoru M., 2001. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem., 8, 303-313.
Zhou X. et al., 2006. Steroidal saponins from Solanum nigrum. J. Nat. Prod., 69, 1158-1163.






