- Portada
- Volume 18 (2014)
- Numéro 1
- Hierarchization of factors driving soil macrofauna in North Algeria groves
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Hierarchization of factors driving soil macrofauna in North Algeria groves

Notes de la rédaction
Received on March 14, 2013; accepted on January 6, 2014
Résumé
Hiérarchisation des facteurs déterminant la macrofaune du sol de vergers du nord de l’Algérie. Cette étude a pour but de rassembler des données sur la macrofaune du sol et d’évaluer sa dynamique saisonnière dans les vergers du nord de l’Algérie, peu étudiés sur ce thème. Les invertébrés ont été recueillis dans trois régions de Kabylie sous climat subhumide (Nezla et Guendoul) et semi-aride (Bouira). L’importance est de déterminer les niveaux d'abondance de la macrofaune du sol et de hiérarchiser les facteurs qui contrôlent leur distribution. Les effets de site (climat), de saison et de type de verger (Ficus carica L. et Olea europaea L.) ont été étudiés en particulier. Parmi 24 taxons identifiés, les fourmis et les vers de terre sont dominants et représentent respectivement 70 % et 16 % des individus. Les facteurs « site » et « saison » ainsi que leur interaction influencent l'abondance totale des macro-invertébrés du sol, en particulier les vers de terre qui sont très sensibles aux climats plus secs. Par contre, le facteur « type de verger » influence peu l’abondance des vers de terre, mais bien significativement l'abondance des fourmis. Il apparait que les macro-invertébrés sont principalement influencés par le climat et la saison. Afin de mieux statuer sur nos résultats et nos conclusions, il est nécessaire d’élargir ce type d’étude à différents sols et vergers sous d’autres variantes du climat de l’Afrique du Nord.
Abstract
The current study gathers new data on soil macro-invertebrates in North Algerian orchards in order to evaluate their seasonal dynamics. Invertebrate samples were collected from three sites in Kabylie: two from sub-humid areas (Nezla and Guendoul) and one from a semi-arid area (Bouira). The objectives were to determine levels of soil macrofauna abundance and to rank the factors controlling their distribution in order of importance. We particularly focused on the effects of site (climate), season and type of orchard (Ficus carica L. and Olea europaea L.). We collected 24 taxa, of which 70% were ants and 16% earthworms. Site and seasonal factors as well as the interaction between these two elements were found to significantly influence total soil macro-invertebrate abundance. In particular, earthworms were found to be highly sensitive to aridity. In contrast, the type of grove explained only a small part of earthworm variance, whereas it had a significant influence on ant abundance. In order to be able to say whether these conclusions are typical for other comparable orchards in North Africa, more studies of this kind are needed.
Tabla de contenidos
1. Introduction
1Although soil fauna is known to influence soil function (Lavelle et al., 2001), invertebrate communities in North African soils are still poorly described. In Mediterranean soil ecosystems, climatic conditions have been shown to have great influence on soil macrofauna, which are often particularly sensitive to temperature (Sharon et al., 2001; Antunes et al., 2008), precipitation (Morón-Ríos et al., 2010) and soil humidity (Dodd et al., 1997; El-Sharabasy et al., 2008). The dominant climatic and pedological characteristics of this region favour soil erosion and salinity, both of which may have a negative impact on soil biodiversity (Pueyos et al., 2007; Errouissi et al., 2011). These factors also constrain soil community dynamics (Véla et al., 2007; Fadda et al., 2008). In arid conditions the presence of macrofauna improves nitrogen nutrition and the availability of water for the horticultural plants (Ouédraogo et al., 2006). Apart from providing fruit and bioenergy, orchards in Southern Mediterranean regions also contribute to the mitigation of soil erosion (Martins da Silva et al., 2011; Ramos et al., 2011).
2In Algeria, olive (Olea europaea L.) and fig (Ficus carica L.) orchards are widespread due to their socio-economic importance and their robustness (Ruano et al., 2004; Fayez et al., 2011). However, over the last few decades these orchards have been progressively neglected in favour of more high yielding horticultural systems. Such policies have led to a deterioration of agro-ecosystems in these areas, which has resulted in recent attempts to restore their functionality (Santos et al., 2007).
3The aim of the present work was to understand the relative importance of three factors recognized as being important for soil invertebrate communities: the climate (sub-humid vs semi-arid), the season (winter vs summer) and the orchard type (fig vs olive groves). We hypothesized that macro-invertebrate abundance would be controlled by interactions between these three factors. This study, carried out in traditional rural fig and olive orchards, beside more intensively cultivated orchards in the same region, should constitute a reference for future assessments of soil biological activity in orchard systems in this geographical area.
2. Materials and methods
2.1. Study sites
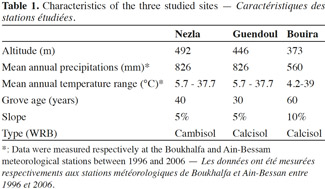
4Three sites were selected on a 52 km North-South transect in Kabylie in northern Algeria: Nezla (36°47’4” N; 4°36’40” E), Guendoul (36°44’20” N; 4°13’5” E) and Bouira (36°23’43” N; 3°53’14” E). Some characteristics of these sites are given in table 1. The Djurdjura Mountains separate Bouira from Nezla and Guendoul. The climate is sub-humid in Nezla and Guendoul and semi-arid in Bouira, with De Martonne indices of 26.6 and 19.3 respectively. Both fig and olive groves were studied at each site. The mean age of trees ranged between 30 and 60 years and mean density was about 100-120 trees per hectare. Also, in both fig and olive groves studied, the composition of the plant cover is similar and composed by same species notably Avena sterilis, Inula viscosa, Oxalis pes-caprae, Daucus carota, Galactites tomentosa, Scolymus hispanicus. The productivity of these groves is irregular and mainly for local consumption. Management is very low-intensity, with no use of phytosanitary products, fertilization or irrigation. Fructification and thinning cuts were made during the harvest, but this is neither a systematic nor an intensive procedure (M.A.D.R., 2009). This kind of agro-ecosystem is currently still the dominant orchard management type in the region.
2.2. Soil sampling and characterisation
5The soils were Calcisols at Guendoul and Bouira and Cambisols at Nezla (WRB, 1998). Soils were sampled in January 2007 with a boring sampler (4 cm in diameter, 20 cm depth). We collected five soil samples randomly from each plot. Soil characteristics were determined on three replicates from the composite samples according to standard methods described in Jackson (1967). Particle size distribution was measured according to the Robinson pipette method (i.e. organic matter oxidation by H2O2, shaking in a sodium hexametaphosphate solution). Soil pH was measured in a 1/5 soil distilled water suspension. The CaCO3 content was determined using the HCl 1 M volumetric method. Cation exchange capacity (CEC) was measured according to the ammonium acetate method. Organic C was determined by sulfochromic oxidation. Bioavailable phosphorus was extracted by shaking soil samples in NaHCO4 0.5 M solution at pH = 8.5 (soil/solution ratio of 1/10) during 1 h (Olsen et al., 1977). The suspension was then filtered and phosphorus concentration in the solution was determined by colorometry. Exchangeable-K was extracted by shaking soil samples in an ammonium acetate 1 M solution at pH 7 (soil/solution ratio of 1/10) for 1 h (Quemener, 1979). The suspension was then filtered and K+ concentration in the solution was determined by flame spectrometry.
2.3. Invertebrate sampling and identification
6Invertebrate sampling was carried out in winter (January) and summer (June) 2007 following the methodology outlined in Coineau (1974). In each selected orchard, six sampling zones of 30 x 30 cm, at least 10 m distance from each other, were set up. Two depth profiles were sampled at each sampling site. Large invertebrates were then hand-sorted in the laboratory. Remaining invertebrates were then extracted from the soil using the Berlese-Tullgren method (Pesson, 1971). After extraction, all invertebrates were preserved in 70° alcohol for later identification. Due to the still currently very incomplete taxonomic catalogue of Algerian soil fauna, invertebrates were only identified to order level in most cases. Taking account of their social behavior and the resulting aggregated distribution, ants were analyzed separately from other macro-invertebrates. Hence, total density referred to the density of all invertebrates but excluding ants.
2.4. Statistical analyses
7The Kruskal-Wallis rank sum test was used for looking at soil differences between sites and orchards. Afterwards, multiple comparisons were done to identify groups that differed (Siegel et al., 1988). We also tested for the influence of the factors “site”, “season” and “orchard” and their interactions on both the overall population density and the diversity of invertebrates. For this, analyses of variance were performed on log-transformed data. Tukey's “Honest Significant Difference” method was used to test for multiple comparisons of means.
3. Results
3.1. Soil characteristics
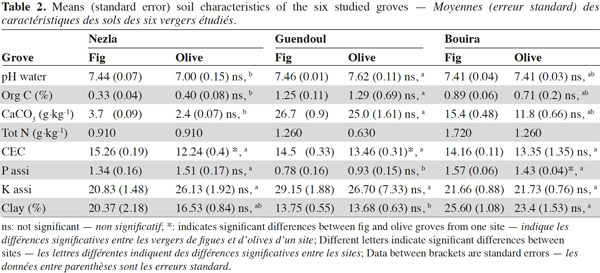
8Soil characteristics are detailed in table 2. No differences in pH and organic C, CaCO3, exchangeable K and clay contents were observed between orchards from any one of the three sites. At Nezla and Guendoul, the CEC was higher in fig than in olive orchard soils. In Bouira soils, the soil available phosphorus content was slightly higher in fig than in olive groves. Regarding differences between the three sites, it appeared that Guendoul presented higher pH, organic C and CaCO3 contents and lesser available P content than Nezla, with intermediate values in Bouira. The available phosphorus content separated Bouira and Nezla from Guendoul. Clay content was twice as high at Bouira than at Guendoul, with 25.6% and 13.7% respectively. Finally, no differences in exchangeable K content and CEC were recorded between sites.
3.2. Macro-invertebrate density and diversity
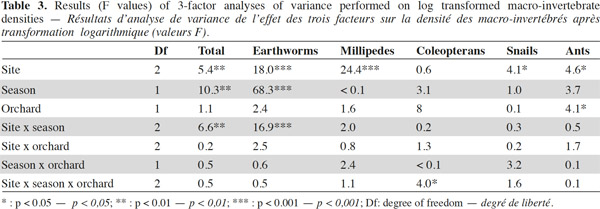
9A total of 2,620 individuals were collected and 24 taxa identified. Ants and earthworms dominated the communities, representing 70% and 16% of total individuals, respectively. Total macro-invertebrate density was highly influenced by the factors “site”, “season” and their interaction but not by the type of grove (Table 3). Maximum population values were recorded during winter in Nezla and Guendoul (sub-humid climate) with values ranging between 130 and 380 ind·m-2. The lowest values were mainly found during winter in Bouira (semi-arid climate) and during summer in Nezla with more or less than 40 ind·m-2.
10This pattern was mainly driven by earthworm densities that reached high values (59 to 324 ind·m-2) during winter in the sub-humid climate regardless of the orchard type (Table 3). In these orchards, earthworms dominated, accounting for 42% to 86% of soil macro-invertebrate density. In other conditions, communities were characterized by scarce or null density of earthworms (0 to 22 ind·m-2). The population levels for ants depended significantly on the site and the type of groves (Table 3). This effect is highlighted by a difference between high densities in fig orchards at Bouira (about 750 to 1,800 ind·m-2 depending on the season), and low densities in olive orchards at Nezla whatever the season and in fig orchards at Bouira during winter (less than 10 ind·m-2).
11Invertebrates other than earthworms and ants were found at lower densities, i.e. always less than 40 ind·m-2. Nevertheless, some groups represented up to 60% of the community. For instance, millipedes at Guendoul exhibited densities ranging from 9 to 33 ind·m-2, representing 13% to 24% of the total population. Their density was almost stable between season and grove type. In all other conditions, millipedes were rare, except in Bouira olive groves during summer (11 ind·m-2). Adult coleopterans also reached up to 30 ind·m-2 particularly during summer in all conditions (7 to 28 ind·m-2) and at Guendoul during winter (30 ind·m-2). Finally, snails were present only in Nezla although at very low densities (up to 6 ind·m-2 and to 9% of density).
12The taxonomic richness ranged between 1 and 11. Among the three factors investigated, only the factor “site” significantly influenced the taxonomic richness of macro-invertebrates. The highest values were observed at Guendoul and Bouira, with diversity values of 2.5 and 4, respectively.
4. Discussion
4.1. Hierarchy of factors
13Our results showed that olive and fig trees have similar effects on soil characteristics – consistent with existing literature reports (Dodd et al., 1997; Ricarte et al., 2011). Significant differences were observed with respect to available phosphorus amounts, which were low in all orchards. The low available phosphorus content probably related to the nature of the calcareous parent material, which enhanced its precipitation (Darrah et al., 1994). The type of grove within a single site did not influence total density of soil invertebrates, which is to a large extent ascribed to the similarity of soil characteristics.
14The main result of this work lies on the ranking of factors by importance driving invertebrate communities in Kabylien soils. It clearly emerged from the results that the factors “site” and “season” profoundly affected the total density of soil invertebrates. These results agreed with Sharon et al. (2001). These authors showed that climate was the main factor influencing abundance and diversity of soil macrofauna in Middle East Mediterranean forests. Similar results have been reported for woodlice by Warburg et al. (1984) and Huerta et al. (2012). However, Santos et al. (2007) showed ant density to be controlled by site characteristics and orchard type. To our knowledge, the present work provides the first results from soil invertebrate communities under fig trees in Mediterranean soils. Otherwise, fig tree impacts on soil communities have been described by Merlim et al. (2005) for a tropical climate. Although we did not observe any effects of orchard type (except for ants, table 3), the type and age of vegetation have been reported to drive both abundance and diversity of soil fauna (Neirynck et al., 2000; Woodcock et al., 2005). However, the simplified structure of vegetation in orchards has been found to act negatively on abundance and diversity of soil arthropods (Van der Wal et al., 2009; Martins da Silva et al., 2011). The tree species can also influence soil biota through the interactions between their roots and the soil, such effects being apparently more important when soil organic matter content is low (Eo et al., 2008; Ivask et al., 2008). Furthermore, it has also been found elsewhere that the type of orchard influenced e.g. organic matter dynamics and pH (Cesarz et al., 2007; Carrillo et al., 2011). Some studies have also mentioned that soil fauna composition can interact with vegetation to modify soil characteristics (Jones et al., 1997 ; Jacob et al., 2009).
15Our results also showed that there were clear differences in the densities of soil invertebrates between Nezla and Guendoul on the one hand, and Bouira on the other hand, during the winter. Differences could be due to climate rather than to soil type. Indeed, Nezla and Guendoul, both situated in a similar sub-humid climatic zone, differ from each other for soil characteristics in terms of pH, organic C, CaCO3 and available P contents. During the summer, densities were lower, with the highest values recorded in Nezla (109 ind·m-2) but most of them ranged between 15 and 74 ind·m-2. Surprisingly, Nezla soils hosted the highest densities during winter (> 250 ind·m-2) and the lowest during summer (< 40 ind·m-2). Soil characteristics probably explain this result (Lavelle et al., 2001), particularly in terms of the fact that organic C content is three times less in Nezla soils compared to those at Guendoul. Finally, in addition to the warm summers of Kabylie, soil invertebrates in Bouira were also exposed to cold winters with, for instance, 25 days of frost during winter 2007.
4.2. Effects on macrofaunal groups
16Among the studied communities, taxa exhibited different responses to various environmental factors. For instance, earthworms were significantly numerous in Nezla and Guendoul soils during the winter, while millipede densities were high at Guendoul whatever the season. In the following section, responses to such factors are discussed taxon by taxon.
17As previously described by Rouabah et al. (2001), the warm and dry summer of Algeria leads to a widespread cessation of earthworm activity (in this study, except for the Guendoul olive orchards). Soil dryness leads directly to a drop in worm numbers. In Bouira however, worm density was always very low, probably also since in this semi-arid zone, winters are characterized by cold temperatures and low soil humidity. Comparing densities recorded in the present study with literature on Maghrebian soils is rather difficult considering the small number of studies on earthworms, most of which have been taxonomic studies (Omodeo et al., 2003). Moreover, even from this small sample size, some data are not considered to be reliable. For instance, Errouissi et al. (2011) reported densities of earthworms in agricultural plots, indicating that Lumbricus terrestris L. (1798) reached densities up to 50 ind·m-2. However, an exhaustive review, Omodeo et al. (2003) showed that L. terrestris is not found in the earthworm fauna of Maghreb. This confusion can probably be explained by the paucity of taxonomic expertise for soil invertebrates in North Africa.
18During the winter, which corresponds to the rainy season in Kabylie, earthworms were numerous in the topsoil at Guendoul and Nezla (59-141 and 202-324 ind·m-2, respectively) (Table 4). These densities were rather higher than densities reported for other Mediterranean orchards. For instance, Morón-Ríos et al. (2010) found densities of 50 and 170 ind·m-2 during autumn 2002 and spring 2003, respectively. Kherbouche et al. (2012) recorded 150 ind·m-2 during springtime. However, in contrast to these groves, the Kabylien groves studied were not ploughed. The well-known negative impact of cultivation on earthworms probably explains this difference (Kherbouche et al., 2012).
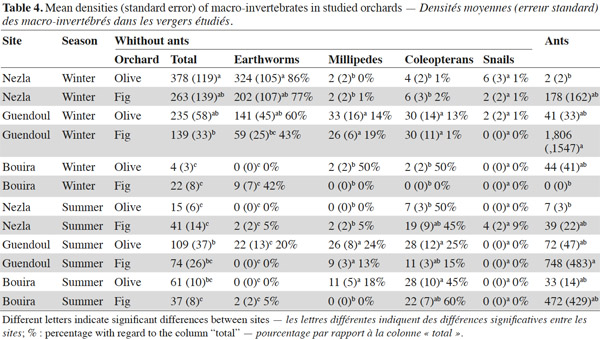
19In the orchards studied here, coleopteran densities were consistently low (2-30 ind·m-2), especially in Bouira and Guendoul during winter (< 10 ind·m-2). In comparison, Santos et al. (2007) found 250-909 ind·m-2 under olive groves in April and July, respectively, in Iberian Mediterranean orchards. Coleopterans in groves have been demonstrated to be influenced by year-round climate variations and management regimes (Ruano et al., 2004) or by soils (Martins da Silva et al., 2011). Coleopterans play important roles in orchard ecosystems such as these: in particular, they are recognized as regulators since some soil larvae are rhizophagous and other species are predators either at larval or imaginal stage (Ruano et al., 2004). Therefore, they are well documented and established as bio-indicators of soil quality (Shanas et al., 2011).
20In contrast to the other groups, ant populations varied significantly according to site and grove type but not with season (Table 3). Aridity does not seem to reduce their abundance (Delsinne et al., 2010). Ants generally had very high densities on the sites studied here (Table 4), but were less abundant at Nezla during the two seasons, particularly under olive trees and they disappeared at Bouira in the wintertime. They were abundant in Guendoul (1806-748 ind·m-2) respectively in winter and summer under fig trees. The abundance of ants under olive trees was low and did not vary. This low density is striking compared to the results of Santos et al. (2007) who reported 1,182-3,120 ind·m-2. Other studies have shown that ants play important ecological role in such groves (Castro et al., 1996; Garrido-Jurado et al., 2011). Several authors have suggested their importance as bio-indicators for agro-ecosystems (Lobry de Bruyn, 1999), under fig trees (Merlim et al., 2005) and under olive trees with a very heterogeneous distribution and variable (Pereira et al., 2004; Santos et al., 2007). Despite this apparent ecological importance, ants are still inadequately studied in Mediterranean ecosystems (Ottonetti et al., 2008).
21Snails were collected in very small numbers at Nezla, but nowhere else. These invertebrates are very sensitive to fluctuations in soil humidity (Pokryszko et al., 2011). Moisture has been shown to be the key determining factor of variation in their abundance and diversity in soils (Pennings et al., 2005; Cameron et al., 2006). This taxonomic group is also influenced by other soil properties and the nature of the vegetation (Omodeo et al., 2003; Ondina et al., 2004; Cameron et al., 2010).
22Millipedes were mostly observed at Guendoul during both seasons, where they reached densities up to 33 ind·m-2 (Table 4). Millipedes also represented up to 50% of community density in Bouira demonstrating their characteristic abundance in semi-arid climates (Galanes et al., 2011).
5. Conclusion
23This study allowed us to rank three factors (soil type, climate and fruit tree) influencing the abundance of macro-invertebrates in North African olive and fig groves. It appears for most of the macro-invertebrates studied that they are favorably influenced by humid climate and site. Moreover, it provides valuable data on the varying abundances and diversity of soil macro-invertebrates under traditional olive and fig groves in three regions of Kabylie. However, the results presented in this study may only be valid for this region. To make more general statements about the abundance of macro-invertebrates in traditionally maintained groves (in terms of soil management regime), this study should be extended to sites encompassing a wider range of different soils, orchard types, and climatic situations across North Africa. However, this study, reporting on conditions in traditionally managed fig and olive groves, which exist side-by-side with more intensively managed groves throughout the region, could constitute a valuable reference for soil biological activities in horticulture for this area.
24Acknowledgements
25We would like to thank Folkert van Oort for his very useful comments (UR251 PESSAC INRA Versailles). We are grateful to the owners of the orchards for having let us make our study in their groves. We thank all the students who took part in soil sampling.
Bibliographie
Antunes S.C. et al., 2008. Spatial and temporal distribution of litter arthropods in different vegetation covers of Porto Santo Island (Madeira Archipelago, Portugal). Eur. J. Soil Biol., 44, 45-56.
Cameron R.A.D., Pokryszko B.M. & Long D.C., 2006. Snail faunas in Southern English calcareous woodlands: rich and uniform, but geographically differentiated. J. Conchology, 39, 1-13.
Cameron R.A.D., Pokryszko B.M. & Horsak M., 2010. Land snail faunas in Polish forests: patterns of richness and composition in a post-glacial landscape. Malacologia, 53, 77-134.
Carrillo Y. et al., 2011. Soil fauna alter the effects of litter composition on nitrogen cycling in a mineral soil. Soil Biol. Biochem., 43, 1440-1449.
Castro J., Campos P. & Pastor M., 1996. Soil arthropods under the influence of soil management systems in olive orchards and sunflower. Boletin Sanidad Vegetal, 22, 557-570.
Cesarz S. et al., 2007. Earthworm communities in relation to tree diversity in a deciduous forest. Eur. J. Soil Biol., 43, 61-67.
Coineau Y., 1974. Introduction à l’étude des microarthropodes du sol et de ses annexes. Paris : Doin Éditions.
Darrah P.R. & Jones D.L., 1994. Role of derived organic acids in the immobilisation of nutriment from the rhizosphere. Plant Soil, 166, 247-257.
Delsinne T., Roisin Y., Herbauts J. & Leponce M., 2010. Ant diversity along a wide rainfall gradient in the Paraguayan dry Chaco. J. Arid Environ., 74, 1149-1155.
Dodd M.B. & Lauenroth W.K., 1997. The influence of soil texture on the soil water dynamics and vegetation structure of a shortgrass steppe ecosystem. Plant Ecol., 133, 13-28.
El-Sharabasy H.M., Hassan M.F. & Mohamed A.I., 2008. Occurrence of soil mites at Al-Maghara Region, Sinai Peninsula. J. Egypt. Soc. Acarology, 2, 31-35.
Eo J. & Nakamoto T., 2008. Spatial relationships between roots and soil organisms under different tillage systems. Eur. J. Soil Biol., 44, 277-282.
Errouissi F., Ben Moussa-Machraoui S., Ben-Hammouda M. & Nouira S., 2011. Soil invertebrates in durum wheat (Triticum durum L.) cropping system under Mediterranean semi arid conditions: a comparison between conventional and no-tillage management. Soil Tillage Res., 112, 122-132.
Fadda S. et al., 2008. Conservation of grassland patches failed to enhance colonization of ground-active beetles on formerly cultivated plots. Environ. Conserv., 35, 109-116.
Fayez K.A. & Mahmoud S.Y., 2011. Detection and partial characterization of a putative closterovirus affecting Ficus carica: molecular, ultrastructural and physiological aspects of infected leaves. Acta Physiol. Plant, 33, 2187-2198.
Galanes I.T. & Thomlinson J.R., 2011. Soil millipede diversity in tropical forest patches and its relation to landscape structure in northeastern Puerto Rico. Biodivers. Conserv., 20, 2967-2980.
Garrido-Jurado I., Ruano F., Campos M. & Quesada-Moraga E., 2011. Effects of soil treatments with entomopathogenic fungi on soil dwelling non-target arthropods at a commercial olive orchard. Biol. Control, 59, 239-244.
Huerta E. & van der Wal H., 2012. Soil macroinvertebrates’ abundance and diversity in home gardens in Tabasco, Mexico, vary with soil texture, organic matter and vegetation cover. Eur. J. Soil Biol., 50, 68-75.
Ivask M. et al., 2008. Invertebrate communities (Annelida and epigeic fauna) in three types of Estonian cultivated soils. Eur. J. Soil Biol., 44, 532-540.
Jackson M.L., 1967. Soil chemical analysis. Englewood Cliff, N.J., USA: Prentice Hall.
Jacob M. et al., 2009. Nutrient release from decomposing leaf litter of temperate deciduous forest trees along a gradient of increasing tree species diversity. Soil Biol. Biochem., 4, 2122-2130.
Jones C.G., Lawton J.H. & Shachak M., 1997. Positive and negative effects of organisms as physical ecosystem engineers. Ecology, 78, 1946-1957.
Kherbouche D., Bernhard-Reversat F., Moali A. & Lavelle P., 2012. The effect of crops and farming practices on earthworm communities in Soummam valley, Algeria. Eur. J. Soil Biol., 48, 17-23.
Lavelle P. & Spain A.V., 2001. Soil ecology. Dordrecht, The Netherlands: Kluwer Academic Publishers.
Lobry de Bruyn L.A., 1999. Ants as bioindicators of soil function in rural environments. Agric. Ecosyst. Environ., 74, 425-441.
M.A.D.R. (Ministère de l’Agriculture et du Développement Rural), 2009. Statistiques agricoles, superficies et produits. Alger : Ministère de l’Agriculture et du Développement Rural.
Martins da Silva P.M., Aguiar C.A.S., de Faria e Silva I. & Serrano A.R.M., 2011. Orchard and riparian habitats enhance ground dwelling beetle diversity in Mediterranean agro-forestry systems. Biodivers. Conserv., 20, 861-872.
Merlim A.O., Guerra J.G.M., Junqueira R.M. & de Aquino A.M., 2005. Soil macrofauna in cover crops of figs grown under organic management. Sci. Agricola, 62, 57-61.
Morón-Ríos A., Rodríguez Miguel Á., Pérez-Camacho L. & Rebollo S., 2010. Effects of seasonal grazing and precipitation regime on the soil macroinvertebrates of a Mediterranean old-field. Eur. J. Soil Biol., 46, 91-96.
Neirynck J., Mirtcheva S., Sioen G. & Lust N., 2000. Impact of Tilia platyphyllos Scop., Fraxinus excelsior L., Acer pseudoplatanus L., Quercus robur L. and Fagus sylvatica L. on earthworm biomass and physico-chemical properties of a loamy topsoil. For. Ecol. Manage., 133, 275-286.
Olsen S.R., Bowman R.A. et Watanabe F.J., 1977. Comportement du phosphore dans le sol et interactions avec les autres éléments nutritifs. Phosphore Agric., 70, 35-52.
Omodeo P., Rota E. & Baha M., 2003. The megadrile fauna (Annelida: Oligochaeta) of Maghreb: a biogeographical and ecological characterization. Pedobiologia, 47, 458-465.
Ondina P., Hermida J., Outeira A. & Mato S., 2004. Relationship between terrestrial gastropod distribution and soil properties in Galicia (NW Spain). Appl. Soil Ecol., 26, 1-9.
Ottonetti L., Tucci L., Chelazzi G. & Santini G., 2008. Stable isotopes analysis to assess the trophic role of ants in a Mediterranean agroecosystem. Agric. For. Entomol., 10, 29-36.
Ouédraogo E., Mando A. & Brussaard L., 2006. Soil macrofauna affect crop nitrogen and water use efficiencies in semi-arid West Africa. Eur. J. Soil Biol., 42, 275-277.
Pennings S.C. & Silliman B.R., 2005. Linking biogeography and community ecology: latitudinal variation in plant-herbivore interaction strength. Ecology, 86, 2310-2319.
Pereira J.A. et al., 2004. Ants as predators of the egg parasitoid Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae) applied for biological control of the olive moth, Prays oleae (Lepidoptera: Plutellidae) in Portugal. Biocontrol Sci. Technol., 14, 653-664.
Pesson P., 1971. La vie dans les sols : aspects nouveaux, études expérimentales. Paris : Éd. Gauthiers-Villars.
Pokryszko B.M., Cameron R.A.D., Mumladze L. & Tarkhnishvili D., 2011. Forest snail fauna from Georgian Transcaucasia: patterns of diversity in a Pleistocene refugium. Biol. J. Linn. Soc., 102, 239-250.
Pueyos Y. & Alados C.L., 2007. Abiotic factors determining vegetation patterns in a semi-arid Mediterranean landscape: different responses on gypsum and non-gypsum substrates. J. Arid Environ., 69, 490-505.
Quemener J., 1979. The measurement of soil potassium. Research topics No 4/1978. Bern: International Potash Institute.
Ramos M.E., Robles A.B., Sanchez-Navarro A. & Gonzalez-Rebollar J.L., 2011. Soil responses to different management practices in rainfed orchards in semi arid environments. Soil Tillage Res., 112, 85-91.
Ricarte A., Marcos-Garcıa M.A. & Moreno C.E., 2011. Assessing the effects of vegetation type on hoverfly (Diptera: Syrphidae) diversity in a Mediterranean landscape: implications for conservation. J. Insect Conserv., 15, 865-877.
Rouabah L. & Descamps M., 2001. Biologie des Oligochètes Lumbricus terrestris, Allolobophora et Dendrobaena pygmea dans le Constantinois (Est Algérien). Bull. Soc. Zool. Fr., 126, 49-58.
Ruano F. et al., 2004. Use of arthropods for the evaluation of the olive-orchard management regimes. Agric. For. Entomol., 6, 111-120.
Santos S.A.P., Cabanas J.E. & Pereira J.A., 2007. Abundance and diversity of soil arthropods in olive grove ecosystem (Portugal): effect of pitfall trap type. Eur. J. Soil Biol., 43, 77-83.
Shanas U. et al., 2011. Landscape and a political border determine desert arthropods distribution. J. Arid Environ., 75, 284-289.
Sharon R., Degan G. & Warburg M., 2001. Comparing the soil macro-fauna in two oak-wood forests: does community structure differ under similar ambient conditions? Pedobiologia, 45, 355-366.
Siegel S. & Castellan N.J., 1988. Nonparametric statistics for the behavioural sciences. New York, NY, USA: MacGraw Hill Int.
Van der Wal A. et al., 2009. Dissimilar response of plant and soil biota communities to long-term nutrient addition in grasslands. Biol. Fertil. Soils, 45, 663-667.
Véla E. & Benhouhou S., 2007. Évaluation d’un nouveau point chaud de biodiversité végétale dans le bassin méditerranéen (Afrique du Nord). C.R. Biol., 330, 589-605.
Warburg M.R., Linsenmair K.E. & Bercovitz K., 1984. The effect of climate on the distribution and abundance of isopods. In: Sutton S.L. The biology of terrestrial isopods. Oxford: Clarendon Pr., 339-367.
Woodcock B.A. et al., 2005. Grazing management of calcareous grasslands and its implications for the conservation of beetle communities. Biol. Conserv., 125, 193-202.
WRB (World Reference Base for Soil Resources), 1998. World Reference Base for Soil Resources. Roma: AISS, IRSIC, FAO.






