- Accueil
- Volume 17 (2013)
- numéro 4
- Plant regeneration via direct shoot organogenesis from cotyledon explants of Bambara groundnut, Vigna subterranea (L.) Verdc.
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Plant regeneration via direct shoot organogenesis from cotyledon explants of Bambara groundnut, Vigna subterranea (L.) Verdc.

Notes de la rédaction
Received on February 28, 2013; accepted on September 17, 2013
Résumé
Régénération de plantes par organogenèse directe à partir des explants cotylédonaires de Voandzou, Vigna subterranea (L.) Verdc. Le voandzou (Vigna subterranea [L.] Verdc.) est principalement cultivé pour la consommation humaine. La présence de facteurs antinutritionnels qui réduisent la qualité des graines et la disponibilité des protéines, mais aussi la sensibilité de la plante aux maladies, nécessitent la mise en place de programmes d’amélioration variétale. Les techniques de culture de tissus sont pratiquement inexistantes chez le voandzou et leur adoption est indispensable pour l’application des méthodes de transformation génétique. Aussi, la présente étude vise à établir un protocole d’induction de bourgeons à partir d’explants cotylédonaires issus de graines matures de voandzou. Différents types et concentrations de phytohormones ont été utilisés pour induire des bourgeons à partir des fragments proximaux, médians et distaux de cotylédons. La benzylaminopurine (3 mg·l-1) seule ou associée à l’acide α-naphtalène acétique (0,05 mg·l-1) a induit la formation de multiples bourgeons. Le potentiel organogène est restreint à la partie proximale du cotylédon. La fréquence d’induction de bourgeons (30 %) et le nombre moyen de bourgeons par explant (12) les plus élevés ont été obtenus avec la face adaxiale du segment proximal en contact avec le milieu de culture. L’efficacité du protocole de régénération établi varie selon le génotype et les écotypes Ci6, Ci2, Ci4 et Ci15 ont exprimé 20 à 30 % de taux d’induction de bourgeons avec en moyenne six à dix bourgeons par explant. L’élongation et l’enracinement des pousses feuillées sont intervenus sur le milieu de base de Murashige et Skoog dépourvu de régulateurs de croissance. Toutes les plantes enracinées ont survécu au transfert en pots puis en serre où la croissance a abouti à la production de graines.
Abstract
Bambara groundnut (Vigna subterranea [L.] Verdc.) is mainly grown for human consumption. However, several factors limit a wider adoption of the crop including the presence of antinutritional factors in the seeds that lower product quality and protein availability but also the plant susceptibility to pests and diseases. Tissue culture techniques are very scanty in Bambara groundnut and should be developed before carrying out genetic transformation for the crop improvement. Therefore, here, an efficient system for in vitro shoot induction from cotyledons derived from mature seeds has been established. Different types and concentrations of plant growth regulators were used to induce buds in embryo-free cotyledon explants. Cotyledons were cut transversally or longitudinally into three segments: proximal, middle and distal part. The influence of explant orientation on the medium, the type of segment and landrace has then been studied. Benzylaminopurine (3 mg·l-1) alone or combined with α-naphthaleneacetic acid (0.05 mg·l-1) induced multiple shoot formations. The organogenic potential was restricted to the proximal segment of cotyledons. Frequency of bud induction (30%) and average number of buds per explant (12) were higher when the adaxial side of the proximal segment was in contact with the medium. Shoot regeneration from cotyledon explants of ten Bambara landraces revealed that the response is genotype-dependent with varieties Ci6, Ci2, Ci4 and Ci15 exhibiting 20 to 30% shoot regeneration and six to ten buds per explant. Regenerated shoot buds excised from explants were elongated and rooted on MS basal medium devoid of plant growth regulators. All rooted plantlets survived to the transfer on a sand soil mixture, and morphologically normal plants were hardened and transferred to greenhouse for further growth to maturity and seed set.
Table des matières
1. Introduction
1Bambara groundnut (Vigna subterranea [L.] Verdc.) is an African indigenous crop that has been grown for centuries, for both human and animal consumption. The crop is popular in Africa because of its resistance to drought, and its ability to produce reasonable yields when grown on poor soils compared to other more favored species, such as Phaseolus vulgaris (L.) and Arachis hypogaea (L.) (Anchirinah et al., 2001). The annual production is about 330,000 tons, half of which is produced by Africa with Nigeria as being the major producing country (Coudert, 1984). As a leguminous crop, Bambara groundnut is useful in crop rotations as a source of residue nitrogen for the subsequent crop through nitrogen fixation (Ncube et al., 2007).
2Despite its important socio-economic role in semi-arid Africa, the dry seed yields are low because production and improvement of Bambara groundnut have been neglected for many years by researchers (Heller et al., 1997; Massawe et al., 2007).
3Improvement of Bambara groundnut can be achieved by genetic recombination and selection, but reports in this domain are limited (Lacroix et al., 2003). Like other leguminous crops, Bambara groundnut is self-pollinating, and due to the positioning and the morphology of the flowers, natural cross-pollination has never been reported (Adu-Dapaah et al., 2003). Artificial hybridization is possible, but extremely difficult and very low success rates have been reported (< 2% harvested hybrid seeds) (Massawe et al., 2003). Moreover, conventional breeding methods are time consuming and laborious. Consequently, several traits limiting the use of Bambara groundnut remain to be eliminated; in particular the presence in seeds of antinutritional factors, like tannins and oxalates that lower product quality and protein availability (Odumodu, 1992). The crop susceptibility to pests and diseases (Gwekwerere, 1995) and the unpredictable low yield (Massawe et al., 2003) are also limiting factors.
4Recent advances in biotechnology including the direct integration of genes into plants have offered the opportunity to develop improved germplasm by overcoming the difficulties to cross distant taxa. The development of such technologies largely depends on efficient regeneration of fertile mature plants from organs, tissues and protoplasts. However, reports on tissue culture techniques in Bambara groundnut are very scanty. Bambara groundnut plants have been regenerated in vitro from embryo explants (Lacroix et al., 2003; Koné et al., 2009a), epicotyl and hypocotyl explants (Koné et al., 2009b) and cotyledon explants (Koné et al., 2007). In the latter work (Koné et al., 2007), the frequency of shoot regeneration was lower than 10% and the average number of shoots per explant was below one. Moreover, all excised fragments of the cotyledon have been used for bud induction and shoots were only induced from the proximal end of the cotyledon. In Black gram (Vigna mungo L.), Ignacimuthu et al. (1999) reported that cotyledons lacking proximal ends only developed calli while those including the proximal ends were caulogenic. Such induction of shoots from the proximal end of the cotyledon was previously observed by Chandra et al. (1995) in Mungo bean (Vigna radiata L.). Direct organogenesis from explants is a rapid multiplication method of true-to-elite strains and is thus preferred for developing transgenic plants. Since plantlets developed directly without an intervening callus phase, somaclonal variation in the regenerants is likely to be minimized. Organogenesis has been used successfully for genetic modification of plants (Chandra et al., 2003). Considering this challenge, the aim of the present work is to improve regeneration efficiency from cotyledon explants in Bambara groundnut and to study in more detail, the influence of the orientation and type of cotyledon segment on plant regeneration.
5The results from this study can be used for plant regeneration of different Bambara groundnut landraces and should provide a useful base to subsequently exploit transformation technologies, for which the availability of efficient systems of plant regeneration is a prerequisite.
2. Materials and methods
2.1. Plant material
6Mature seeds of Bambara groundnut (V. subterranea) were obtained from Germplasm Bank of Biology and Crop Improvement Laboratory (Unit of Plant Physiology – University Nangui Abrogoua, Côte d’Ivoire). Characteristics of these landraces are indicated in table 1. Cotyledons from mature seeds constitute an excellent and convenient choice of explant for routine plant regeneration due to their year-round availability, easy cultivation and applicability to a wide range of genotypes. Seeds were surface sterilized by immersion in ethanol 70% (v/v) for 1 min, followed by 7% (m/v) Calcium hypochlorite for 30 min. They were then rinsed with sterile distilled water three to four times and soaked in sterile water for 48 h in dark at 25 °C in a culture chamber. The soaked seeds (Figure 1a) were blotted dry on filter paper, decoated (Figure 1b) and the cotyledons were carefully separated and the embryos excised (deembryonated).
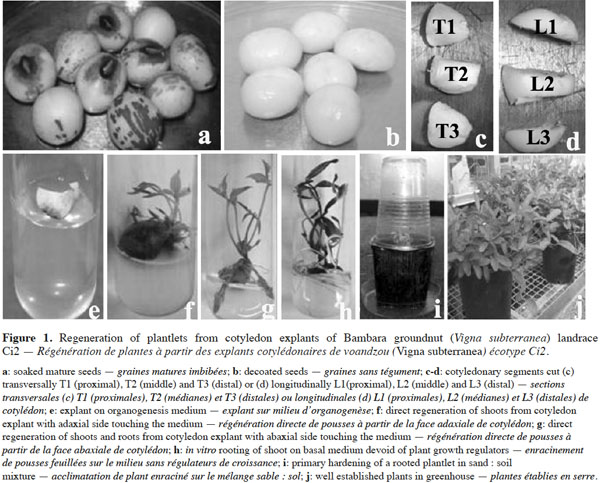
2.2. Media and culture conditions
7Cotyledons were excised transversally in 0.5 cm2 sections and the explants were incubated with their abaxial side in contact with the culture media (Figure 1e). Media consisted of MS basal salt medium (Murashige and Skoog, 1962) supplemented with five different cytokinins (Kinetin [Kn], Benzylaminopurine [BAP], Zeatin [Zea], Thidiazuron [TDZ] and 6-γ-γ-dimethylallylaminopurine [2iP]) at 5 mg·l-1 as previously established by Koné et al. (2007).
8In a second experiment, the basal MS medium was supplemented with 15 different concentrations and combinations of BAP (the cytokinin which has expressed the best response for the shoot induction during the first experiment) and α-naphthaleneacetic acid (NAA) routinely used for in vitro plant regeneration of grain legumes (Ochatt et al., 2000). All media were supplemented with 3% sucrose. The pH was adjusted to 5.5 with NaOH and media were solidified with 0.25% Gelrite (Duchefa) prior to autoclaving at 121 ºC and 1.2 bars for 30 min. All cultures were incubated for four weeks at 25 ± 2 °C under a 16/8-h (light/dark) photoperiod (fluorescent lighting, intensity 100 μEm-2·s-1).
2.3. Explant type and orientation
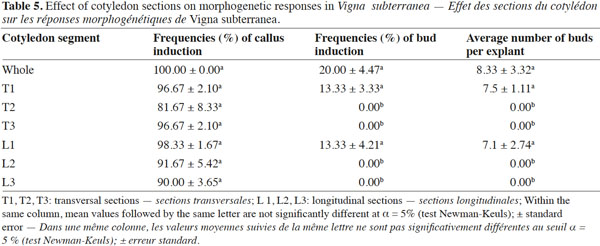
9In order to check the effect of explant type and its orientation, pieces of cotyledon were cultured with their adaxial or abaxial sides in contact with the medium. Each cotyledon was cut transversally (T) or longitudinally (L) into three segments. The segments close to the embryo axis were identified as 1/3 proximal (P), while the other two as 1/3 middle (M) and 1/3 distal (D), respectively (Figure 1c-d). These six explants were cultured on MS basal salts medium supplemented with the optimal combination of growth regulators established in previous experiments.
2.4. Influence of genotypes
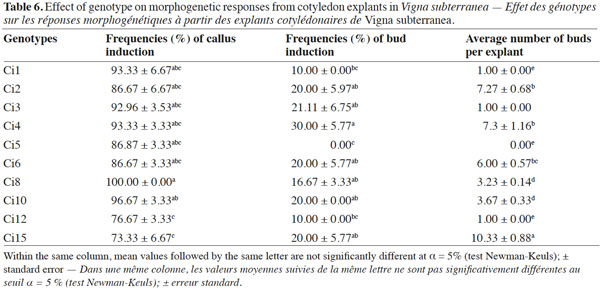
10All the previous experiments were performed with landrace Ci2, which is the most widely cultivated and the most consumed in the northern Côte d’Ivoire (especially in the Department of Korhogo). Thereby, in order to examine the effectiveness of this regeneration system (adaptable to a wide range of genotypes), landraces Ci1, Ci2, Ci3, Ci4, Ci5, Ci6, Ci8, Ci10, Ci12 and Ci15 were included in this study, and the optimal culture conditions (growth regulators combination x explant orientation x explant type) established with landrace Ci2 were applied.
2.5. Rooting and acclimatization of the regenerated plantlets
11The adventitious shoots obtained and measuring 2-3 cm long in this study were excised and placed on a rooting medium consisting of basal MS without growth regulators, as previously stated by Koné et al. (2007; 2009a). Rooted plantlets, measuring 3-4 cm in height and with six to eight roots and four to five leaves were washed free of gelrite before being transferred to small pots filled with an autoclaved and cooled mixture of soil and sand (1:1; v/v). Plants were watered once a week with half-strength MS liquid medium and the pots were covered with transparent plastic bags for the first ten days to maintain high humidity. These bags were gradually perforated then completely removed after four weeks. All pots were maintained in a controlled growth chamber at 25 ± 2 ºC, 40–50% relative humidity, and with a 16/8 h (light/dark) photoperiod. Once uncovered, plants were transferred to the greenhouse.
12The frequencies of induction of callus and buds and the average number of buds per explant were recorded. All experiments were conducted as a completely randomized design with three replicates per treatment and five explants per replicate. Each independent experiment was repeated three times (45 observations). Data were subjected to statistical analysis after transforming the percentage values using arcsin angular transformation. Variation between treatment means was analyzed using Newman-Keuls test at P = 0.05 (Brunning et al., 1977).
3. Results and discussion
3.1. Morphogenetic responses on media supplemented with different cytokinins
13Cotyledon segments have been used in the development of in vitro regeneration protocols in numerous legume species including Vigna radiata (Chandra et al., 1995), Dalbergia sissoo (Roxb.) (Chand et al., 2002) and Lens culinaris (Medik.) (Khawar et al., 2004).
14In the present experience, swelling occurred on cotyledon explants within three days on MS basal medium supplemented with the different tested cytokinins. The initially white cotyledon gradually turned green (Figure 1e) within seven days. After two weeks of culture, calli have been induced at the wounded ends of the cotyledon explants. Gradually, these calli have covered the entire surface of the explant. Subsequently, adventitious buds were induced from the cotyledon explants without any intervening callus production. These buds appeared firstly as nodules, which developed shoots (Figure 1f) after four weeks of culture.
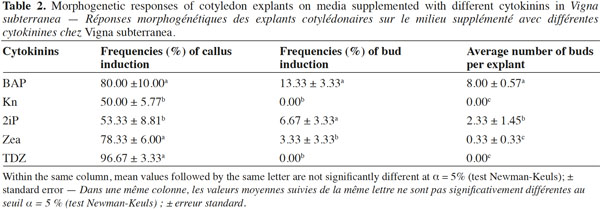
15Table 2 details the morphogenetic responses expressed by the cotyledon explants on media supplemented with different cytokinins. The frequency of callus ranged from 50 to 97% and was highest (96.67%) on a medium supplemented with TDZ. In all treatments, callus increased slightly in size without developing into shoots. Cotyledon explants incubated on media with Kn and TDZ yielded no buds, while the frequency of bud regeneration with BAP, 2ip and Zea varied from 3 to 14%. The treatment containing BAP showed the highest frequency of bud induction (13.33%) and the maximum number of buds per explant (8). The effectiveness of BAP may lie on its ability to enhance the production of natural endogenous hormones inducing shoot organogenesis. BAP is the most widely used and effective cytokinin for various legumes including Vigna spp. (Saini et al., 2002). Optimal shoot regeneration from the cotyledons required relatively high concentrations of BAP. This variation in the action of different cytokinins may be a consequence of their differential uptake and translocation rates (Blakesey, 1991).
3.2. Effect of different concentrations of BAP and NAA
16In table 3, different concentrations of BAP (0, 1, 3 and 5 mg·l-1) were assessed in combination with different concentrations of NAA (0, 0.01, 0.05, 0.1 and 0.5 mg·l-1). No morphogenic responses were observed on cotyledons cultured on MS medium devoid of growth regulators after four weeks of culture. In legume trees, including Pongamia pinnata (L.), neither swelling nor morphogenesis was induced in cotyledons cultured on hormone-free MS medium (Sujatha et al., 2008).
17All the concentrations of BAP alone or in association with NAA induced callus formation at a frequency ranging from 70 to 94%. Such callus formation has also been reported from cotyledon explants in Acacia sinuata (Lour.) (Vengadesan et al., 2003) and Vigna radiata (Amutha et al., 2003).
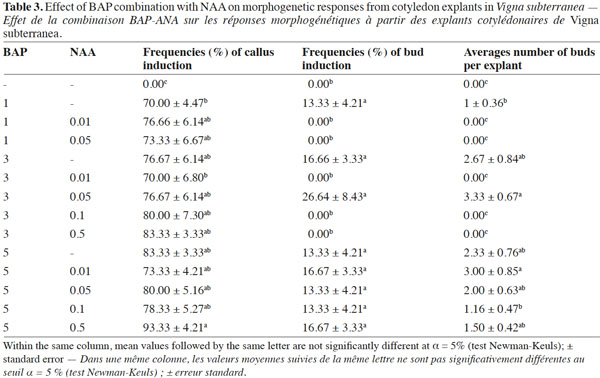
18The highest frequency of buds (26.64%) and the maximum number of buds per explant (3.33) were obtained with a combination of 3 mg·l-1 BAP and 0.05 mg·l-1 NAA. On the other hand, no bud induction occurred from cotyledons cultured on control medium (devoid of growth regulators) nor on medium supplemented with BAP (1 mg·l-1) x NAA (0.01 and 0.05 mg·l-1) or BAP (3 mg·l-1) x NAA (0.01, 0.1 and 0.5 mg·l-1). However, buds appeared on cotyledon explants cultured on the other media tested. A similar hormonal combination (3 mg·l-1 BAP + 0.03 mg·l-1 ANA) also allowed multiple bud formation in Vigna mungo (Ignacimuthu et al., 1999). In many legume species, the synergistic effect of a high concentration of cytokinin with a low concentration of auxin induced better shoot organogenic responses than a medium supplemented with cytokinin alone (Susan et al., 1998).
3.3. Effect of explant orientation and segment type
19The importance of explant orientation to obtain optimum morphogenic responses has been reported in species of the genus Vigna (Tivarekar et al., 2001) and in Prunus dulcis (Mill.) (Ainsley et al., 2001), among others. After four weeks of culture, callus formation and bud induction were observed from both sides of the cotyledons (Table 4). The formation of buds was initiated from the embryonic axis region and no statistical difference was noted between the two orientations in terms of the frequency of either callus or bud induction from explants. Shoots that originated from the adaxial surface penetrated into the medium. The average number of buds per explant obtained from the abaxial side (12) was significantly higher than the number produced from the adaxial side (3.33). In V. subterannea cotyledon culture, buds appeared from both adaxial (Figure 1f) and abaxial (Figure 1g) sides, but the average number of buds per explant was higher in those explants cultured with the abaxial side in contact with the medium. The same response was also obtained from cotyledons of Glycine max (L.) (Hartweck et al., 1988) while, contrary to our results, best organogenic responses in Cicer arietinum (L.) were obtained from the adaxial side of cotyledon explants (Shri et al., 1992). A morphogenetic effect of the position in the organ from which explants are taken has been observed in Citrullus lanatus (Thunb.) (Compton, 2000) and in Anthurium andraeanum (Hort.) (Martin et al., 2003). In this study, callus formation was observed on whole cotyledons and on all six segments (proximal, median and distal), both excised transversally or longitudinally (Table 5), at a frequency ranging from 81 to 100% and without any significant differences between these treatments. Conversely, in terms of caulogenesis, both the longitudinal and transversal segments of middle and distal regions failed to induce buds. Indeed, shoots were induced from the whole cotyledon and the proximal sections (T1 and L1), and bud formation from whole cotyledons was initiated from the embryonic axis region. The frequency of bud induction (20, 13.33 and 13.33%) and the average number of shoots per explant (8.33, 7.5 and 7.1) obtained with the whole cotyledon and the sections T1 and L1 (proximal), respectively, were statistically equal. The proximal segment of the cotyledon (the section near the nodal region) produced more shoots than the middle and distal segments, thereby demonstrating a strong polarity effect. This difference in regenerative capacity between segments may be the result of varying endogenous auxin concentrations in these regions. Thus, the potential regeneration ability from cotyledons is greatly influenced by the distance to the cotyledonary node. Earlier studies in Helianthus annuus (L.) (Abdoli et al., 2003; Vega et al., 2006) have unequivocally established the existence of explant polarity and showed that the proximal cotyledon sections were more organogenic than the distal sections. Murthy et al. (1995) suggested that the proximal region of the cotyledons might be the source of highly regenerative cells.

3.4. Genotype effect
20The in vitro regeneration competence can vary considerably among various species of a genus or among cultivars within the same species (Henry et al., 1994). The tissue culture protocol described in the current study promoted regeneration from cotyledons of all genotypes tested, although the frequencies varied in terms of shoot regeneration and average number of shoots per explant (Table 6). Ci12 (76.67%) and Ci15 (73.33%) exhibited the lowest frequency of callus formation. The frequency of bud induction varied from 10 to 30% with maximum responses exhibited by landrace Ci4, while the highest average number of buds per explant was obtained with landrace Ci15 (10.33) followed by Ci4 (7.3), Ci2 (7.27) and Ci6 (6). Variability in organogenic responses among genotypes was commonplace in numerous grain legumes including Vigna unguiculata (L.) (Brar et al., 1999), Lathyrus sativus (L.) (Ochatt et al., 2007) and also in our previous work with V. subterranea (Koné et al., 2007).
3.5. Rooting of shoots buds and plant hardening
21The adventitious shoots induced during this study continued to proliferate, showing no signs of senescence or necrosis. Furthermore, they did not require additional transfer to shoot multiplication or elongation medium, which is a standard procedure in tissue culture techniques (Quintero-Jiménez et al., 2010). All green healthy shoot buds transferred on basal medium MS devoid of plant growth regulators rooted well within three weeks (Figure 1h). Similar observations have been reported in Cajanus cajan (L.) (Mohan et al., 2000). Rooted plantlets were transferred to a soil and sand (1:1, v/v) mixture for four weeks and then successfully acclimatized in a growth chamber (Figure 1i). Of the plantlets transferred to greenhouse, more than 70% healthy plants survived and set seed (Figure 1j).
4. Conclusion
22This study demonstrates that BAP (3 mg·l-1) associated with NAA (0.05 mg·l-1) triggers meristematic activity of cotyledonary explant cells in V. subterranea. The proximal segment of the cotyledon with abaxial side in contact of medium has the highest morphogenic potential. Compared to the previous work on tissue culture of Bambara groundnut, the use of the proximal end of cotyledon as explant has widely enhanced the frequency of bud induction (10 to 30%) and the average number of buds per explant (1 to 10). Shoot rooting was obtained in MS basal medium without any plant growth regulators. This protocol may find application in development of transgenesis in Bambara groundnut. The present work was performed with mature seeds and the organogenic potential was only restricted to the proximal end of cotyledon. In order to improve the shoot regeneration frequency, investigations should be carried out with cotyledons derived from immature seeds, which could have the capability to induce buds from all over their surface.
23Acknowledgements
24Koné Mongomaké is grateful to the Ministry of Higher Education and Scientific Research for its financial support.
Bibliographie
Abdoli M., Moieni A. & Dehghani H., 2003. Effects of genotype and cotyledon section on organogenesis in sunflower. Iran. J. Biotechnol., 1, 234-238.
Adu-Dapaah H.K. & Sangwan R.S., 2003. Agronomic and biotechnological approaches to Bambara groundnut improvement. In: Proceedings of the International Bambara groundnut Symposium, 8th–12th August, 2003, Botswana College of Agriculture, Gaborone, Botswana, 245-254.
Ainsley P.J. et al., 2001. Regeneration of almond from immature seed cotyledons. Plant Cell Tissue Organ Culture, 67, 221-226.
Amutha S., Ganapathi A. & Muruganantham M., 2003. In vitro organogenesis and plant formation in Vigna radiata (L.) Wilczek. Plant Cell Tissue Organ Culture, 72, 203-207.
Anchirinah V.M., Yridoe E.K. & Bennet-Lattey S.O., 2001. Enhancing sustainable production and genetic resource conservation of Bambara groundnut: a survey of indigenous agricultural knowledge systems. Outlook Agric., 30, 281-286.
Blakesey D., 1991. Uptake and metabolism of 6-benzyladenine in shoot proliferation of Musa and Rhododendron. Plant Cell Tissue Organ Culture, 25, 69-74.
Brar M.S., Al-Khayri J.M., Morelock T.E. & Anderson D.E., 1999. Genotypic response of cowpea Vigna unguiculata (L.) to in vitro regeneration from cotyledon explants. In Vitro Cell. Dev. Biol. Plant, 35, 8-12.
Brunning J.L. & Kintz B.L., 1977. Computational handbook of statistics. 2nd ed. Glenview, IL, USA: Scott Foresman.
Chand S., Pattnaik S. & Chand P.K., 2002. Adventitious shoot organogenesis and plant regeneration from cotyledons of Dalbergia sissoo (Roxb.), a timber yielding tree legume. Plant Cell Tissue Organ Culture, 68, 203-209.
Chandra M. & Pal A., 1995. Differential response of the two cotyledons of Vigna radiata (L.) in vitro. Plant Cell Rep., 15, 248-253.
Chandra A. & Pental D., 2003. Regeneration and transformation of grain legumes: an overview. Curr. Sci., 84(3), 381-387.
Compton M.E., 2000. Interaction between explant size and cultivar affects shoot organogenic competence of watermelon cotyledons. HortScience, 35, 749-750.
Coudert M.J., 1984. Market openings in West Africa for cowpea and Bambara groundnuts. Int. Trade Forum, 20, 14-19.
Gwekwerere Y., 1995. Pests and diseases of Bambara groundnut in Zimbabwe. In: Heller J., Begemann F. & Mushonga J., eds. Bambara groundnut (Vigna subterranea (L.) Verdc.) promoting the conservation and use of underutilized and neglected crops. 9. Proceedings of the workshop on conservation and improvement of Bambara groundnut (Vigna subterranea (L.) Verdc.), 14-16th November, 1995, Harare, Zimbawe, 84-86.
Hartweck L.M. et al., 1988. Auxin orientation effects on somatic embryogenesis from immature soybean cotyledons. In Vitro Cell. Dev. Biol. Plant, 24, 821-828.
Heller F., Begemann F. & Mushonga J., 1997. Bambara groundnut: Vigna subterranea (L.) Verdc. promoting the conservation and use of under-utilized and neglected crops. Vol. 9. Roma; Gatersleben, Germany: IPGRI.
Henry Y., Vain P. & De Buyser J., 1994. Genetic analysis of in vitro plant tissue culture responses and regeneration capacities. Euphytica, 79, 45-58.
Ignacimuthu S. & Francklin G., 1999. Regeneration of plantlets from cotyledon and embryonal axis explants of Vigna mungo (L.) Hepper. Plant Cell Tissue Organ Culture, 55, 75-78.
Khawar K.M., Sancak C., Uranbey S. & Ozcan S., 2004. Effect of thidiazuron on shoot regeneration from different explants of lentil (Lens culinaris Medik.) via organogenesis. Turk. J. Bot., 28, 421-426.
Koné M. et al., 2007. In vitro morphogenesis from cotyledon and epicotyl explants and flow cytometry distinction between landraces of Bambara groundnut [Vigna subterranea (L.) Verdc], an under-utilised grain legume. Plant Cell Tissue Organ Culture, 88, 61-75.
Koné M. et al., 2009a. Factors affecting regeneration of Bambara groundnut [Vigna subterranea (L.) Verdc.] from mature embryo axes. In Vitro Cell. Dev. Biol. Plant, 45, 769-775.
Koné M. et al., 2009b. In vitro plantlets regeneration in Bambara groundnut [Vigna subterranea (L.) Verdc. (Fabaceae)] through direct shoot bud differentiation on hypocotyl and epicotyl cuttings. Afr. J. Biotechnol., 8, 1466-1473.
Lacroix B., Assoumou Y. & Sangwan R.S., 2003. Efficient in vitro direct shoot organogenesis of fertile plants from embryo explants of Bambara groundnut [Vigna subterranea (L.) Verdc.]. Plant Cell Rep., 21, 1153-1158.
Martin K.P., Joseph D., Massadery J. & Philip V.J., 2003. Direct shoot regeneration from lamina explants of two commercial cut flower cultivars of Anthurium andraeanum (Hort.). In Vitro Cell. Dev. Biol. Plant, 39, 500-504.
Massawe F.J., Schenkel W., Basu S. & Temba E.M., 2003. Artificial hybridisation in Bambara groundnut. In: Proceedings of the International Bambara groundnut symposium, 8-12 August 2003, Botswana College of Agriculture, Gaborone, Botswana, 193-210.
Massawe F.J., Mwale S.S., Azam-Ali S.S. & Roberts J.A., 2007. Towards genetic improvement of Bambara groundnut [Vigna subterranea (L.) Verdc.]. In: Ochatt S. & Mohan Jain S., eds. Breeding of neglected and under-utilized crops, spices and herbs. Plymouth, UK: Science Press, 61-80.
Mohan M.L. & Krishnamurthy K.V., 2000. Plant regeneration in pigeon pea (Cajanus cajan L. Millsp.) Plant Sci., 150, 41-49.
Murashige T. & Skoog F., 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant., 15, 473-497.
Murthy B.N.S., Murch S.J. & Saxena P., 1995. Thidiazuron-induced somatic embryogenesis in intact seedlings of peanut (Arachis hypogaea L.): endogenous growth regulator levels and significance of cotyledons. Physiol Plant., 94, 268-276.
Ncube B. et al., 2007. Raising the productivity of smallholder farms under semi-arid conditions by use of small doses of manure and nitrogen: a case of participatory research. Nutr. Cycling Agroecosyst., 77, 53-67.
Ochatt S.J., Pontécaille C. & Rancillac M., 2000. The growth regulators used for bud regeneration and shoot rooting affect the competence for flowering and seed set in regenerated plants of protein peas. In Vitro Cell. Dev. Biol. Plant, 36, 188-193.
Ochatt S.J., Abirached-Darmency M., Marget P. & Aubert G., 2007. The Lathyrus Paradox “Poor men’s diet” or a remarkable genetic resource for protein legume breeding? In: Ochatt S.J. & Jain S.M., eds. Breeding of neglected and under-utilised crops, spices and herbs. Plymouth, UK: Science Press, 41-60.
Odumodu C.U., 1992. Antinutrients content of some locally available legumes and cereals in Nigeria. Trop. Geogr. Med., 44, 260-263.
Quintero-Jiménez A. et al., 2010. Enhanced shoot organogenesis and regeneration in the common bean (Phaseolus vulgaris L.). Plant Cell Tissue Organ Culture, 102, 381-386.
Saini R. & Jaiwal P.K., 2002. Age, position in mother seedling, orientation, and polarity of the epicotyl segments of black gram (Vigna mungo L. Hepper) determines its morphogenic response. Plant Sci., 163, 101-109.
Shri P.V. & Davis T.M., 1992. Zeatin-induced shoot regeneration from immature chickpea (Cicer arietinum L.) cotyledons. Plant Cell Tissue Organ Culture, 28, 45-48.
Sujatha K., Mohan Panda B. & Hazra S., 2008. De novo organogenesis and plant regeneration in Pongamia pinnata, oil producing tree legume. Trees, 22, 711-716.
Susan E., Suchita T. & Leela G., 1998. Thidiazuron-induced shoot regeneration in pigeon pea (Cajanus cajan L.). Plant Cell Tissue Organ Culture, 53, 217-220.
Tivarekar S. & Eapen S., 2001. High frequency plant regeneration from immature cotyledon of mung bean. Plant Cell Tissue Organ Culture, 66, 227-230.
Vega T.A., Nestares G.M., Zorzoli R. & Picardi L., 2006. Responsive regions for direct organogenesis in sunflower cotyledons. Acta Physiol. Plant., 28, 427-432.
Vengadesan G., Ganapathi A., Amutha S. & Selvaraj N., 2003. High frequency plant regeneration from cotyledon callus of Acacia sinuata (Lour) Merr. In Vitro Cell. Dev. Biol. Plant, 39, 28-33.






