- Home
- Volume 17 (2013)
- numéro 3
- Culture of Spirogyra africana from farm ponds for long-term experiments and stock maintenance
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Culture of Spirogyra africana from farm ponds for long-term experiments and stock maintenance

Editor's Notes
Received on July 4, 2012; accepted on May 8, 2013
Résumé
Culture de Spirogyra africana provenant d’étangs d’irrigation en vue d’expériences à long terme et de la maintenance des stocks de culture. Spirogyra africana (Fritsch) Czurda est une algue verte filamenteuse ubiquiste qui pousse naturellement dans les étangs d’irrigation en Andalousie. La recherche sur cette macroalgue a révélé plusieurs applications biotechnologiques, notamment sa valeur pour la production de biocarburant. Cependant, les milieux de culture n’ont pas été encore normalisés. C’est pourquoi nous avons testé les trois milieux les plus couramment cités dans la littérature pour la croissance du genre Spirogyra, c’est-à-dire HSCHU#10, sD11 et l’eau de l’étang, lors d’une expérience de laboratoire de 10 semaines. Nous avons comparé les taux de croissance, les pourcentages de filaments vivants et le nombre de rhizoïdes par filament de S. africana cultivée dans chaque milieu. L’effet de la stérilisation pour obtenir des cultures monoalgales, suivie par la chlorination, a également été testé sur sa croissance. Après 10 semaines, S. africana a grandi dans HSCHU#10, tandis que l’eau de l’étang et sD11 ont montré des résultats plus médiocres. La chlorination des filaments avant l’inoculation a montré un effet positif sur la croissance, en particulier pendant plusieurs semaines après l’inoculation des algues. La genèse des rhizoïdes semblait dépendre d’autres facteurs externes, mais a répondu à la chlorination de façon positive. Nous recommandons l’utilisation de HSCHU#10 et la décontamination de surface des algues filamenteuses avec du chlore pour les cultures de Spirogyra, en particulier pour les expériences à long terme et le maintien des stocks de culture.
Abstract
Spirogyra africana (Fritsch) Czurda is a ubiquitous filamentous green alga that grows naturally in Andalusian farm ponds. Research on this macroalga has reported several biotechnological applications, emphasizing its value for biofuel production. However, culture media for growth of this species have not yet been tested for long-term experiments and stock maintenance. Here we test the three most common culture media cited in the literature for Spirogyra growth, i.e. HSCHU#10, sD11 and pond water, in a 10-week laboratory experiment. We compared growth rates, percentages of live filaments and number of rhizoids per filament of S. africana cultured in each medium. The effect of filament sterilization to obtain monoalgal cultures, i.e. chlorination, was also tested on its growth. After 10 weeks, S. africana grew in HSCHU#10, whilst FPW and sD11 showed poorer results. Filament chlorination prior to inoculation showed a positive effect on growth, especially several weeks after algal inoculation. Rhizoid genesis seemed to be dependent on additional external factors, but responded to chlorination positively. We recommend the use of HSCHU#10 and the surface decontamination of filamentous algae with chlorine for Spirogyra cultures, especially for long-term experiments and stock maintenance.
Table of content
1. Introduction
1Spirogyra africana (Fritsch) Czurda is a common filamentous macroalga found in freshwater habitats (Lee, 2008) that is worldwide distributed (Kadlubowska, 1984; Kim et al., 2004). Since it usually forms free-floating masses that anchor to the substratum (Kadlubowska, 1984; Lee, 2008), it may cause problems in certain human activities, such as pumping impairment for drip irrigation in farm ponds (Bonachela et al., 2013; Juan et al., 2012) or hampering traditional fishing in lakes (Onyema et al., 2009). In the case of Andalusian farm ponds, S. africana is one of the most common growing macroalgae, which may forms monoalgal mats during spring months (I. Gallego, unpubl. data).
2The genus Spirogyra has recently drawn attention to researchers due to its various biotechnological and industrial applications, mostly based on its sorption properties. Spirogyra filaments remove heavy metals (Romera et al., 2007; Singh et al., 2007; Kaonga et al., 2008; Pribyl et al., 2008) and other toxic compounds from effluents (Aleissa et al., 2004; Çelekli et al., 2009); as well as they produce allelochemicals inhibiting microalgal growth (Zakaria, 2002; Trochine et al., 2011), implying important consequences for the management of aquatic ecosystems. Antiviral and antihelmintic properties have also been reported (Muller-Feuga et al., 2003; Pultz et al., 2004). More recently, Spirogyra biomass has been shown to be an efficient energy source for biofuel (Hossain et al., 2008; Eshaq et al., 2010; John et al., 2011).
3Both stock maintenance and growth of algal species are essential for their use in biotechnology. Therefore, the search of the most suitable culture media and ease of laboratory culture are relevant topics. Regarding genus Spirogyra, culture techniques were first reported early in the 20th century (see Kadlubowska, 1984 and references herein). Mainly three culture media have proved successful for Spirogyra growth under laboratory conditions in short-term culture: sD11 (Graham et al., 1995), Half-Strength CHU#10 (Nalewajko et al., 1989), and Artificial Pond Water (Inoue et al., 2002). However, their suitability for long-term experiments has not been tested so far, neither contrasting experiments on different culturing media have not been carried out under the same environmental conditions (e.g. irradiance, temperature).
4One of the most widely used media for Spirogyra cultures nowadays is water from the source ecosystem, e.g. Townsend et al. (2008), sometimes replaced by Artificial Pond Water (Inoue et al., 2002; Ikegaya et al., 2004; Yoshida et al., 2009). sD11 culture medium has also proved suitable for Spirogyra culturing (Nalewajko et al., 1989). It is derived from D11 medium, originally used for Cladophora Kützing (1843) and slightly modified for Spirogyra (Graham et al., 1995; O’Neal et al., 1995). Half-Strength CHU#10, henceforth HSCHU#10, has been widely used for a variety of green alga, including Spirogyra spp. (Saygideger, 1996).
5The presence of epibionts in Spirogyra in vitro cultures is one of the major problems in testing filaments growth, despite the continuous mucilage secretion from Spirogyra cell walls (Simons et al., 1990; Wöber et al., 2007). Among the several techniques used to remove epibionts from macroalgae, chlorination – an oxidant sterilization of filaments with a sodium hypochlorite solution – has been successful for epiphytic algae in seawater (Pang et al., 2007) and is commonly used for obtaining axenic cultures, e.g. García-Jiménez et al. (1999). To our knowledge, no evidence has been found for its use in Spirogyra cultures.
6Rhizoid production has also been included as a parameter for quantifying filament growth and survival, since rhizoids fix the filaments to the substratum and hence facilitate algal survival. Several factors have been reported as drivers of rhizoid genesis, such as substratum properties (Ikegaya et al., 2008) or the lowering of extracellular Ca2+ concentration (Inoue et al., 2002; Yamada et al., 2003). Release of single cells resulting from filament-severing (Inoue et al., 2002; Ikegaya et al., 2008) has also been reported as a triggering factor.
7In the present study, we measured growth rate, percentage of live filaments and rhizoid production of Spirogyra africana filaments over 10 weeks in three commonly-used culture media – sD11, HSCHU#10 and Filtered Pond Water (FPW) i.e. water from the source pond where the filaments were gathered – with the aim of culturing Spirogyra africana for long-term maintenance, thus facilitating biotechnological applications and stock maintenance. Additionally, we tested the effect of sterilization with NaClO (henceforth chlorination) as a procedure for assuring monoalgal cultures.
2. Materials and methods
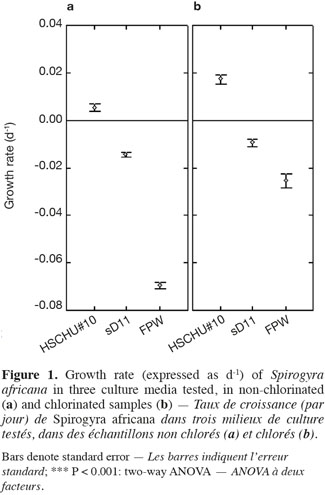
2.1. Sample collection and laboratory analyses
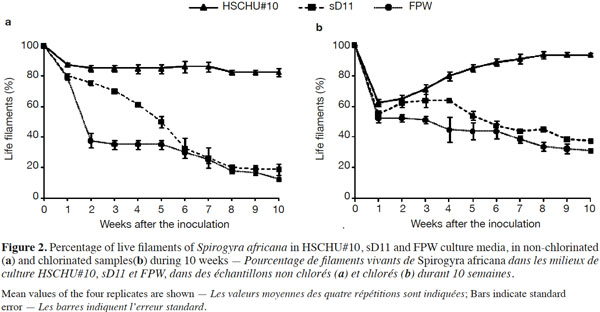
8Filaments were collected from a small agricultural pond measuring 20 x 18 x 1.75 m (L x W x D), located in Almería, SE Spain. As the filaments were anchored onto the substratum, a rake was used to collect them. Samples were rinsed in pond water to remove sediment and immediately taken to the laboratory. For the preparation and chemical analysis of FPW, 10 l water were taken with a centrifugal electric pump from the bottom of the pond, where Spirogyra filaments were growing.
2.2. Laboratory experiment
9The influence of culture media type and chlorination on Spirogyra africana growth and rhizoid production were investigated in a laboratory experiment with a 3 x 2 factorial design, with culture media and filament chlorination as independent factors. Four replicates per treatment were performed, with a total of 24 culture flasks.
10Three different culture media were analyzed: sD11, HSCHU#10 and FPW (pond water filtered through a 0.22 μm pore Millipore filter). sD11 and HSCHU#10 were prepared following Graham et al. (1995) and Nalewajko et al. (1989), respectively.
11Chemical analyses were performed for FPW nutrient content, as follows: sodium (Na+), potassium (K+), magnesium (Mg2+) and calcium (Ca2+) were analyzed by atomic absorption spectrometry (APHA, 2005). CO32- and HCO3- contents were obtained from alkalinity measurements, being determined by standard titration (Wetzel et al., 2000). Chloride (Cl-) was measured using the iodometric method (APHA, 2005), nitrate (NO3-) was determined by the sodium salicylate method (Monteiro et al., 2003), and phosphate (PO4-3) by ascorbic acid reduction (APHA, 2005). The pH of the culture media was measured with a pH-electrode (CRISON HA405) on experimental day 1. In the case of the two synthetic media, the pH was adjusted and controlled weekly with HEPES according to the requirements for each medium.
12For the factorial experiment, 48 samples of 0.150 ± 0.001 g (mean ± SD) fresh weight of S. africana were weighed with an analytical balance (ADA 120L, Adam Equipment). The filaments had been previously rinsed with distilled water and cut into 2-cm pieces. Excess water was removed with blotting paper. Twenty-four samples were inoculated inside 75 cm2 T-Flasks (vent cap), filled with 100 ml of culture medium and used for the experiment. The remaining 24 samples were dried at 60 ºC for 24 h and then weighed to obtain dry weight. To check if all the inoculated samples contained the same fraction of water, a correlation between dry and fresh weight was made to ensure that the fresh weight of S. africana was an accurate proxy of the dry weight, since fresh weight was necessary to calculate the growth rate.
13In order to analyze the influence of chlorination on S. africana growth, 12 replicates were submerged for 10 s in a solution of sodium hypochlorite containing 1.25‰ active chlorine and subsequently rinsed three times with distilled water prior to their inoculation. Different times and chlorine concentrations were previously tested to prevent cell damage (data not shown). However, both chlorine concentration and submersion time were too low to cause turgor pressure (Iwata et al., 2001). As controls, filaments of the remaining 12 replicates were rinsed with distilled water three times and inoculated into the T-Flasks.
14Spirogyra growth is promoted by relative high temperatures, ranging 20-25 ºC (O’Neal et al., 1995; Berry et al., 2000), so a culture chamber (Medilow-M, JP Selecta) was set at 22 ± 1 ºC. Despite Andalusian ponds are shallow and irradiance levels are considerable high in this region (Casas et al., 2011; Bonachela et al., 2013), irradiance in the bottom of natural ponds often is < 100 μE·m-2·s1, because S. africana mats can be shaded by other macroalgae or even phytoplankton (M. Juan-Cazorla, unpubl. data). Indeed, previous experiments on several Spirogyra morphotypes (including those with similar width to S. africana) showed no significant differences in their growth rate when irradiances ranged from 60-900 μE·m-2·s1, although net photosynthesis increased with the lower irradiances (Berry et al., 2000). Thus, we selected an irradiance of 50 ± 10 μE·m-2·s1, using a Biospherical QSL-100 irradiance meter. The flasks were placed horizontally and parallel to the irradiation flux, in a culture chamber set at a 14:10 h L:D cycle. Two cool white fluorescent lamps (30 W) were used for lighting. Fifty percent of the volume of the media in the flasks was replaced weekly to minimize nutrient depletion. The medium was extracted with a sterile pipette to avoid loss of material. The experiment lasted for 10 weeks.
15The following equation was used to calculate the specific growth rate (μ) for each replicate, according to O’Neal et al. (1995):
16μ (d-1) = [ln (final fresh weight) - ln (initial fresh weight)] / (days of incubation)
17For this purpose, the fresh weight of each replicate was measured before its inoculation and after harvest. Mats of dead filaments (not coloured) were removed with forceps before weighing.
18Since the calculation of filament growth rate only considers the initial and final weights and requires manipulation, which could negatively affect their growth and/or survival, we included other variables, such as the percentage of live filaments and rhizoid production. The former was calculated by dividing the number of live filaments by the total amount of filaments. These were considered dead when chloroplasts were observed damaged (non-helical) or absent. The number of rhizoids per live filament was counted as well. For each replicate both parameters were taken weekly under the inverted microscope from 10 random fields prior to the replacement of culture media. The quantification of live filaments and rhizoids was carried out directly using an inverted microscope at x10 magnification (MOTIC AE31).
2.3. Statistical analyses
19Prior to the statistical analyses, variables were normalized by logarithmic transformation or by arcsine-square-root transformation in the case of the percentage of live filaments.
20A two-factor ANOVA was performed to analyze the effects of culture media and chlorination on S. africana growth rate. Repeated measures ANOVA were used to analyze the effect of the chlorination and culture media on the percentage of live filaments and number of rhizoids per filament. When ANOVAs showed significant differences between groups (culture media, chlorination conditions), Tukey’s HSD post-hoc tests were used. All analyses were performed with STATISTICA 7.1 software (Statsoft Inc., 2005).
3. Results
3.1. Effect of culture media on S. africana growth rate
21The three culture media showed substantial differences in nutrient composition (Table 1). HSCHU#10 and sD11 contained N:P molar ratios that varied from a P-impoverished FPW medium to a N-depleted ratio in sD11, whereas HSCHU#10 showed an N:P ratio nearest to the optimal Redfield ratio for phytoplankton, namely 16:1.
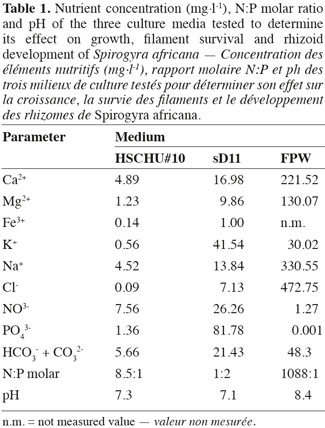
22At the end of the experiment, S. africana only showed a positive growth rate in HSCHU#10 (Figure 1). All replicates in sD11 and FPW showed negative growth rates. The two-factor ANOVA test showed significant differences in S. africana growth rate between culture media (F = 429.784; P < 0.001), between chlorination treatments (F = 152.025; P < 0.001) and in the interaction of both factors (F = 53.315; P < 0.001).
3.2. Effect of culture media on live filaments
23The type of culture media also affected the percentage of live filaments (ANOVA F = 150.093; P < 0.001), with HSCHU#10 proving to be the highest and FPW the lowest (Figure 2). Furthermore, the effect of culture media and time was significant (ANOVA F = 53.452; P < 0.001), since HSCHU#10 showed asymptotic values around 90% of the initial value after 10 weeks and both sD11 and FPW showed percentages close to 40% at the end of the experiment. Chlorination had no effect on the percentage of live filaments (ANOVA F = 2.665; P > 0.05) but the interaction of time and chlorination showed a positive effect (ANOVA F = 19.019; P < 0.001).
3.3. Effect of culture media on rhizoid production
24Rhizoid genesis was observed from the first week after inoculation (Figure 3). The percentage of rhizoids per live filament was affected by the type of culture media used (ANOVA F = 4.044; P < 0.05). Tukey’s HSD post hoc test showed that sD11 was the medium with the lowest number of rhizoids per filament (P < 0.05), whereas both HSCHU#10 and FPW showed no significant differences (P > 0.05). Chlorination increased rhizoid production (ANOVA F = 23.956; P < 0.001). However, the interaction of medium and chlorination was not statistically significant (F = 1.731; P > 0.05).
4. Discussion
4.1. Suitability of the culture media
25Our results show that HSCHU#10 is the most suitable of the three assayed media for S. africana culture, since both growth rate and percentage of live filaments were higher in this medium compared to the others. This can be explained by the difference in the nutrient composition of the three culture media tested, particularly their contrasting N:P molar ratios, since both are essential macronutrients for algal primary production (Sterner et al., 2002; Klausmeier et al., 2004). In the field, a high growth of the Spirogyra fluviatilis Hilse complex was reported after fertilizing with a molar ratio N:P = 10:1 (Townsend et al., 2008). This approaches the HSCHU#10 of our experiments (N:P = 8.5:1) and both are relatively close to the optimal Redfield ratio of 16:1 proposed for phytoplankton (Klausmeier et al., 2004). However, the N:P supply ratios of FPW and sD11 significantly deviated from the ratio both Redfield ratio and the ratio proposed by Townsend et al. (2008), pointing respectively to a case of P- and N- impoverished media.
26Spirogyra africana mats grew in the pond with apparently no nutrient limitations during the 10 weeks of our experiment (I. Gallego, unpubl. data) while FPW showed the poorest result. It is likely that the sediment was an important P source for this benthic alga in the natural system (Townsend et al., 2008). The extremely low-P FPW probably resulted in a severe nutrient depletion in our experiment, where the pond sediment was not included.
27Contrary to our expectations, S. africana filaments did not grow in sD11 and the percentage of live filaments was relatively low. The specific sD11 medium has been reported as a successful medium to grow Spirogyra spp. in vitro cultures (Graham et al., 1995; O’Neal et al., 1995; Berry et al., 2000). However, we obtained poorer results with this medium compared to HSCHU#10, a medium commonly used on a wide range of algae (Saygideger, 1996). Our experimental conditions were similar to those used previously with Spirogyra spp. growing in sD11, i.e. temperature, irradiance and a weekly medium replacement. However, our experiment lasted for 10 weeks and half the medium was replaced weekly, whilst previous ones lasted only for 2-3 weeks (O’Neal et al., 1995; Berry et al., 2000) and media were entirely replenished every 7-10 days (Berry et al., 2000). The effect of N depletion on S. africana growth became evident several weeks after inoculation in sD11, while filaments in short-term cultures could thrive for a while on cellular N reserves. Our results show that despite the reportedly good results of the specific sD11 medium for S. africana cultures, filament growth for long-term cultures is not guaranteed.
28Indeed, mat cohesiveness was studied by Berry et al. (2000) as an indicator of filament decline that depends on irradiance. A higher filament entanglement was observed in our sD11 replicates than in FPW and HSCHU#10 (data not shown), despite all our replicates were exposed to the same irradiation. We suggest that cohesiveness may be also dependent on the medium nutrient content as well, although further study is required in this area.
29Since there is a lack of studies on irradiance and/or temperature using other culture media apart from sD11, we can only suggest that the observed mat cohesiveness and the low-N composition of sD11 may be detrimental for S. africana survival.
30The conditions for avoiding nutrient depletion in our experiment (weekly replacement of half the medium) were clearly insufficient in sD11 and FPW, but seemed to be adequate in HSCHU#10. Indeed, the effect of mat cohesiveness may interact with nutrient composition and nutrient depletion. Thus, long-term cultures can be performed in HSCHU#10 with less laboratory manipulation and effort, so we recommend this medium for long-term experiments or for the maintenance of stock cultures of S. africana.
31Effectiveness of chlorination on S. africana growth. Chlorination had a positive effect on filaments growth, while the effect of chlorination on the percentage of live filaments was only significant when interacted with time. Chlorine is reported as a traditional sterilizing agent for removing epibionts from macroalgae (García-Jiménez et al., 1999; Pang et al., 2007) and free chlorine also inactivates bacteria and cyanoprokariota (Daly et al., 2007; Amiri et al., 2010). The elimination of these nutrient competitors by chlorination probably favored Spirogyra growth. In spite of this apparently positive effect, sodium hypochlorite can react with polysaccharides present in the mucilaginous sheath of the Zygnemataceae (Cheli et al., 1989) and even damage the cell walls. Thus, the higher mortality observed in chlorinated samples at the beginning of the experiment could be attributed to this treatment. However, at the end of the experiment, this likely damage might be counteracted by the elimination of competitors.
4.2. Rhizoid genesis in S. africana
32Our results on rhizoid production are not as clear as those on growth and percentage of live filaments. The N:P ratio does not seem to be involved in rhizoid production, since both HSCHU#10 and FPW showed similar results. A low extracellular Ca2+ concentration and release of single cells have also been reported as triggering factors of rhizoid formation (Nalewajko et al., 1989; Ikegaya et al., 2004). Nonetheless, the lowest concentration of Ca2+ occurred in HSCHU#10, which showed high rhizoid formation, while single cells released from filaments were observed in both sD11 and FPW (I. Gallego, unpubl. data), which paradoxically showed different results. We suggest that other unidentified factors, not described in the literature so far, may be involved in the process of rhizoid genesis. However, chlorination increased rhizoid genesis, supporting our previous results on the positive effect of chlorine sterilization on Spirogyra filaments.
5. Conclusion
33The search of the most suitable culture media for stock maintenance and optimal growth of S. africana is essential for its subsequent use in biotechnology. For this purpose, we tested the suitability of the three culture media most commonly used for S. africana growth.
34Half-Strength CHU#10 was the most suitable of the media assayed for S. africana growth and it seems to be appropriate for long-term experiments. The widely used sD11 specifically modified for Spirogyra showed poorer results.
35Filament chlorination prior to experiment inoculation showed a positive effect on S. africana growth and rhizoid production.
36We recommend the use of both Half-Strength CHU#10 and surface decontamination of filamentous algae with NaClO for Spirogyra cultures. Thus, longer-term cultures can be performed with less laboratory manipulation and effort.
37Abbreviations
38HSCHU#10: Half-Strength CHU#10
39FPW: Filtered Pond Water
40Acknowledgements
41This research has been funded by the Andalusian Regional Government (Junta de Andalucía, Consejería de Medio Ambiente y Consejería de Innovación, Ciencia y Empleo) as part of the project P06-RNM01709. We thank the pond owner for allowing us access and sampling. We sincerely thank Enric Descals for editorial assistance.
Bibliographie
Aleissa K.A., Shabana E.S.I. & Al-Masoud F.I.S., 2004. Accumulation of uranium by filamentous green algae under natural environmental conditions. J. Radioanal. Nucl. Chem., 260, 683-687.
Amiri F., Mesquita M.M.F. & Andrews S.A., 2010. Disinfection effectiveness of organic chloramines, investigating the effect of pH. Water Res., 44, 845-853.
APHA, 2005. Standard methods for the examination of the water and wastewater. 22th ed. Washington, DC: APHA.
Berry H.A. & Lembi C., 2000. Effects of temperature and irradiance on the seasonal variation of Spirogyra (Chlorophyta) population in a Midwestern lake (USA). J. Phycol., 36, 841-851.
Bonachela S. et al., 2013. Pond management and water quality for drip irrigation in Mediterranean intensive horticultural systems: multifunctional pond management using submerged aquatic plants. Irrig. Sci., 31, 769-780.
Casas J.J. et al., 2011. Artificial ponds in a Mediterranean region (Andalusia, southern Spain): agricultural and environmental issues. Water Environ. J., 25, 308-317.
Çelekli A., Yavuzatmaca M. & Bozkurt H., 2009. Kinetic and equilibrium studies on the adsorption of reactive red 120 from aqueous solution on Spirogyra majuscula. Chem. Eng. J., 152, 139-145.
Cheli F. & De Vecchi L., 1989. An ultrastructural and cytochemical study on Conjugatophycean cell wall. Caryologia, 42, 127-137.
Daly R.I., Ho L. & Brookes J.D., 2007. Effect of chlorination on Microcystis aeruginosa cell integrity and subsequent microcystin release and degradation. Environ. Sci. Technol., 41, 4447-4453.
Eshaq F.S., Ali M.N. & Mohd M.K., 2010. Spirogyra biomass a renewable source for biofuel (bioethanol) production. Int. J. Environ. Sci. Technol., 2, 7045-7054.
García-Jiménez P., Marian F.D., Rodrigo M. & Robaina R.R., 1999. Sporulation and sterilization method for axenic culture of Gelidium canariensis. J. Biotechnol., 70, 227-229.
Graham J.M., Lembi C.A., Adrian H.L. & Spencer D.F., 1995. Physiological responses to temperature and irradiance in Spirogyra (Zygnematales, Charophyceae). J. Phycol., 31, 531-540.
Hossain A.B.M.S. et al., 2008. Biodiesel fuel production from algae as renewable energy. Am. J. Biochem. Biotechnol., 4, 250-254.
Ikegaya H., Yamada S.Y., Sonobe S. & Shimmen T., 2004. Saponin-induced release of single cells from filaments and rhizoid differentiation in Spirogyra. J. Plant Res., 117, 443-447.
Ikegaya H. et al., 2008. Presence of xyloglucan-like polysaccharide in Spirogyra and possible involvement in cell-cell attachment. Phycol. Res., 56, 216-222.
Inoue N., Yamada S., Nagata Y. & Shimmen T., 2002. Rhizoid differentiation in Spirogyra: position sensing by terminal cells. Plant Cell Physiol., 43, 479-483.
Iwata K., Tazawa M. & Itoh T., 2001. Turgor pressure regulation and the orientation of cortical microtubules in Spirogyra cells. Plant Cell Physiol., 42, 594-598.
John R.P., Anisha G.S., Nampoothiri K.M. & Pandey A., 2011. Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour. Technol., 102, 186-193.
Juan M. et al., 2012. Construction characteristics and management practices of in-farm irrigation ponds in intensive agricultural systems. Agronomic and environmental implications. Irrig. Drain., 61(5), 657-665.
Kadlubowska J.Z., 1984. Süsswasserflora von Mitteleuropa. Band 16, Chlorophyta VIII. Conjugatophyceae I: Zygnemales. Stuttgart, Deutschland: Gustav Fischer Verlag.
Kaonga C.C. et al., 2008. Levels of cadmium, manganese and lead in water and algae; Spirogyra aequinoctialis. Int. J. Environ. Sci. Technol., 5, 471-478.
Kim J.-H., Kim Y.H. & Lee I.K., 2004. Morphotaxonomy of the genus Spirogyra (Zygnemataceae, Chlorophyta) in Korea. Algae, 19, 91-105.
Klausmeier C.A., Litchman E., Daufresne T. & Levin S., 2004. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature, 429, 171-174.
Lee R.E., 2008. Phycology. 4th ed. Cambridge, UK: Cambridge University Press.
Monteiro M.I.C., Ferreira F.N., De Oliveira N.M.M. & Ávila A.K., 2003. Simplified version of the sodium salicylate method for analysis of nitrate in drinking waters. Anal. Chim. Acta, 477, 125-129.
Muller-Feuga A., Moal J. & Kaas R., 2003. The microalgae for aquaculture. In: Stottrup J.G. & McEvoy L.A., eds. Life feeds in marine aquaculture. Oxford, UK: Blackwell.
Nalewajko C. & O’Mahony M.A., 1989. Photosynthesis of algal cultures and phytoplankton following an acid pH shock. J. Phycol., 25, 319-325.
O’Neal S.W. & Lembi C.A., 1995. Temperatures and irradiance effects on growth of Pithophora oedogonia (Chlorophyceae) and Spirogyra sp. (Charophyceae). J. Phycol., 31, 720-726.
Onyema I.C. & Emmanuel B.E., 2009. Spirogyra africana (Fritsch) Czurda bloom and associated fishing impairment in a tropical freshwater lagoon. Est. J. Ecol., 58, 18-26.
Pang S.J., Zhang Z.H., Zhao H.J. & Sun J.Z., 2007. Cultivation of the brown alga Hizikia fusiformis (Harvey) Okamura: stress resistance of artificially raised young seedlings revealed by chlorophyll fluorescence measurement. J. Appl. Phycol., 19, 557-565.
Pribyl P., Cepák V. & Zachleder V., 2008. Cytoskeletal alterations in interphase cells of the green alga Spirogyra decimina in response to heavy metals exposure: II. The effect of aluminium, nickel and copper. Toxicol. in Vitro, 22, 1160-1168.
Pultz O. & Gross W., 2004. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol., 65, 635-648.
Romera E. et al., 2007. Comparative study of biosorption of heavy metals using different types of algae. Bioresour. Technol., 98, 3344-3353.
Saygideger S., 1996. Bioaccumulation and toxicity of Zinc in Spirogyra fluviatilis Hilse (Chlorophyta). Water Air Soil Pollut., 101, 323-331.
Singh A., Kumar D. & Gaur J.P., 2007. Copper(II) and lead(II) sorption from aqueous solution by non-living Spirogyra neglecta. Bioresour. Technol., 98, 3622-3629.
Sterner R.W. & Elser J.J., 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton, NJ, USA: Princeton University Press.
Townsend S.A., Schult J.H., Douglas M.M. & Skinner S., 2008. Does the Redfield ratio infer nutrient limitation in the macroalga Spirogyra fluviatilis? Freshwater Biol., 53, 509-520.
Trochine C. et al., 2011. Filamentous green algae inhibit phytoplankton with enhanced effects when lakes get warmer. Freshwater Biol., 56, 541-553.
Wetzel R.G. & Likens G.E., 2000. Limnological analysis. 3rd ed. New York, USA: Springer Verlag.
Wöber J. & Schagerl M., 2007. Strategies of Spirogyra against epiphytes. Algological Stud., 123, 57-72.
Yamada S.Y., Sonobe S. & Shimmen T., 2003. Synthesis of a callosic substance during rhizoid differentiation in Spirogyra. Plant Cell Physiol., 44, 1225-1228.
Yoshida K. & Shimmen T., 2009. Involvement of actin filaments in rhizoid morphogenesis of Spirogyra. Physiol. Plant., 135, 98-107.
Zakaria A.M., 2002. Allelopathic activity of Spirogyra sp.: stimulating bloom formation and toxin production by Oscillatoria agardhii in some irrigation canals, Egypt. J. Plankton Res., 24, 137-141.
To cite this article
About: Irene Gallego
University of Almería. Department of Biology and Geology. Campus Excelencia Internacional (ceiA3). Ctra. Sacramento, s/n. E-04120 Almería (Spain). E-mail: igallego@ual.es
About: J. Jesús Casas
University of Almería. Department of Biology and Geology. Campus Excelencia Internacional (ceiA3). Ctra. Sacramento, s/n. E-04120 Almería (Spain).
About: Francisca Fuentes-Rodríguez
University of Almería. Department of Biology and Geology. Campus Excelencia Internacional (ceiA3). Ctra. Sacramento, s/n. E-04120 Almería (Spain).
About: Melchor Juan
University of Almería. Department of Biology and Geology. Campus Excelencia Internacional (ceiA3). Ctra. Sacramento, s/n. E-04120 Almería (Spain).
About: Pedro Sánchez-Castillo
University of Granada. Faculty of Science. Department of Botany. Fuentenueva, s/n. E-18071 Granada (Spain).
About: Carmen Pérez-Martínez
University of Granada. Institute of Water Research. E-18071 Granada (Spain).






