- Accueil
- volume 16 (2012)
- numéro 2
- Silk moths in Madagascar: A review of the biology, uses, and challenges related to Borocera cajani (Vinson, 1863) (Lepidoptera: Lasiocampidae)
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Silk moths in Madagascar: A review of the biology, uses, and challenges related to Borocera cajani (Vinson, 1863) (Lepidoptera: Lasiocampidae)

Notes de la rédaction
Received on December 3, 2010; accepted on December 8, 2011
Résumé
Les vers à soie endémiques de Madagascar : une synthèse bibliographique de la biologie, des utilisations et des perspectives associées à Borocera cajani (Vinson, 1863) (Lepidoptera : Lasiocampidae). Borocera cajani ou « Landibe » (nom vernaculaire) est l'un des papillons séricigènes sauvages dont la soie est la plus utilisée dans le domaine textile de Madagascar. Cette espèce endémique s'observe dans toute l'île, mais colonise particulièrement la forêt de Uapaca bojeri ou forêt de « Tapia » des hautes terres centrales. La forêt fournit les aliments à B. cajani tels que les feuilles de U. bojeri. L'espèce secrète la soie quand elle entre en nymphose et construit son cocon. Borocera cajani et son habitat naturel sont menacés par les destructions de l'Homme telles que les feux de brousse, la collecte de bois de chauffage, la production de charbon de bois et la surexploitation de leurs cocons. La production de soie sauvage est en constante diminution, alors que la filière soie implique beaucoup de gens dans l'île comme les collecteurs des cocons, les fileurs, les teinturiers, les tisseurs et les artistes qui transforment la soie en habits, en accessoires et objets. Revitaliser la filière soie est un moyen de favoriser la conservation de cette ressource naturelle et de son habitat forestier.
Abstract
Borocera cajani or “Landibe” (vernacular name) is the wild silk moth that is currently used to produce silk textiles in Madagascar. This species is endemic to Madagascar, and is distributed throughout the island, colonizing the Uapaca bojeri or “Tapia” forest of the central highlands. The forest provides food in the form of plants for B. cajani, including U. bojeri leaves. The species secretes silk at the onset of pupation and for making cocoons. Borocera cajani and its natural habitat are threatened by human destruction, such as bush fires, firewood collection, charcoal production, and the over-harvesting of their cocoons. Wild silk production largely disappeared when the silk industry utilized many people on the island as the collectors of cocoons, spinners, dyers, weavers, and artists who transform the silk into clothes, accessories, and objects. Therefore, it is important to study the biology of B. cajani to revitalize silk production in a way that helps conserve this species and the Tapia forest.
Table des matières
1. Introduction
1Madagascar is one of the most important centers of world biodiversity, with a high level of endemism, around 90% (Mittermeier et al., 2004). Many of the species are forest dwelling (Myers, 1988), with biodiversity encompassing all classes of animals, including insects. Among these insects, moths are used in the textile industry. Madagascan textile silk has a long history. Indeed, wild silk, termed “Landibe”, was exploited long before the introduction of “Chinese silk” from Bombyx mori during the 19th century (Rafidiarimalala, 1974; Costa, 2004). Wild silk in Madagascar has many origins, with many silk producing species being present on the island. One such species is Borocera cajani (ONU, 1991). Historically, the Madagascan population used B. cajani silk to create sumptuous shrouds. However, the continued use of this silk, along with the gradual disappearance of native forests, has negatively affected the distribution and population levels of this species. Therefore, it is necessary for researchers to study the biology of B. cajani to safeguard its future (Paulian, 1953).
2. Biology
2.1. Taxonomy
2The genus Borocera was originally described by Boisduval (1833), and the species cajani was described by Vinson (1863). This species has been confused with Borocera madagascariensis, which often resides along the coastal part of the island. The B. cajani silk moth belongs to the Lasiocampidae family, and to the Gonometinae subfamily. This subfamily is endemic to Madagascar, and includes the larger species of the Lasiocampidae (De Lajonquière, 1972; ONU, 1991). It is characterized by vein eight of the hindwings, which connects to vein seven at a distance from its base, and then forms a secondary cell of length that is nearly equal to the top of the median cell, with both cells being about the same width (Aurivillius, 1927).
2.2. Morphology
3The genus Borocera encompasses all moths with fasciculate antennae, which are openly uneven along the first third of the segment from the base. Moths of this genus also have a small head, no proboscis, and small, slightly prominent, eyes. Borocera cajani is the most widespread moth in Madagascar, and without doubt is one of the most abundant. It has many forms and landraces (De Lajonquière, 1972).
4Both sexes exhibit very high dimorphism in size and color. The genitalia remain however constant, regardless of the origin of species being examined (De Lajonquière, 1972). The wings are generally dark and fuzzy for the male and a uniform grey for the female (Paulian, 1950; Razafindraleva, 2001). Adult males and females have several main characteristics (Figure 1).

5Males are smallish, hairy, and thick-bodied moths. Their antennae are well developed and bipectinate. Their head, thorax, and legs are the same color as the forewing. The wingspan is about 38-52 mm (De Lajonquière, 1972). The forewings are oblong and well developed in width, while the apex is slightly sinuous or round. Their color is red ochracea to brown, with a sinuate submarginal line, a postmedial transverse line, and a stigma grayish point in the discal cell (Paulian, 1951). The form of the forewing varies for one egg-laying (Paulian, 1953). The color of the hindwings is quite similar to the forewings, but is often overshadowed in part or in whole. Like the forewings, the dorsal wings are sometimes barred transversely with an apparent median reddish shadow, and are sometimes obsolete (De Lajonquière, 1972; Razafindraleva, 2001). The genitalia are formed by a membrane that leads to a thicker penis and serrated edges. The penis is crossed by a prominent ejaculatory duct. Two valves are located on either side of the penis (De Lajonquière, 1972; Razafindraleva, 2001).
6Females are much larger; with a body size about three times that of the male. Their wingspan is about 70-75 mm, with a forewing length of 35 mm. The antennal shaft is black, with yellow pectination. These moths are hairy, with a thick bodied, grayish yellow tinted, bare thorax, and legs of the same color as the forewings. The hairy legs terminate in black tarsi. The wings are a dirty white to pale grey, silky, and shiny (De Lajonquière, 1972; Razafindraleva, 2001). The forewings are crossed by two transverse lines that spilt the wing into different spaces (Razafindraleva, 2001). The discal area has a dark lunule dot at the end of the cell. The color of the hindwings is the same as the forewings. The back of the four wings is yellowish sepia in color, which is consistent with much less apparent median gray shadows. The apex of the abdomen is characterized by two well developed thick ridges, with sensory hairs. The genital opening is formed by the superposition of two beads. This structure ensures coaptation during mating and ovoposition.
3. Life cycle and habitat
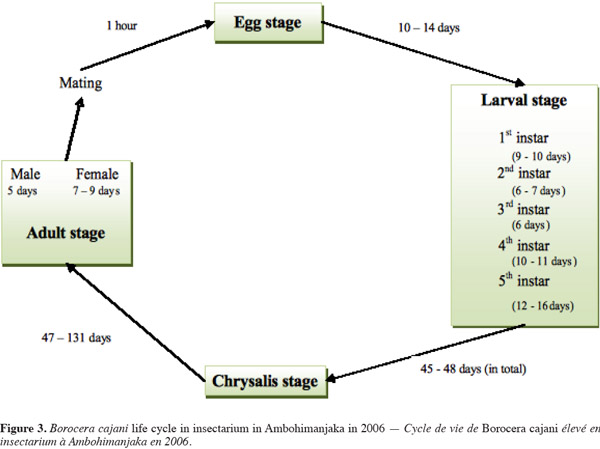
7Borocera cajani is bivoltine, meaning that it has two generations in a year (Paulian, 1953; Razafindraleva, 2001; Razafimanantsoa, 2008). The first generation of eggs hatches during the rainy season, between January (Razafimanantsoa, 2008) and March (Razafindraleva, 2001). The second generation of eggs hatches between the end of April and November (Razafindraleva, 2001; Razafimanantsoa, 2008). The life cycle of the silk moth comprises four stages: the egg, caterpillar or larva, chrysalis or pupa, and adult. After emergence, females attract males by releasing a sexual pheromone (Zborowski et al., 2007). For both sexes, emergence primarily occurs between two and six in the afternoon. Copulation is largely observed between one and seven in the afternoon, lasting up to one hour. One male may mate with up to three females. The adult does not feed. Eggs are laid from seven in the afternoon onwards of the same day that the adult female emerged and mated. Unfertile females may lay eggs, but these eggs do not hatch. The number of eggs that are laid varies from 250 to 845. All eggs are laid on the leaves, branches, and trunk surface of specific host plants (including U. bojeri) (Razafimanantsoa, 2008). Females lay eggs in captivity, even without the necessary host plants. The eggs of B. cajani are about 2 mm in size, quite hemispherical, with a hard shell and granular texture. The porcelain-like eggs are covered in a grey-greenish band. The most fertile eggs hatch after about 10 days, with a hatching success rate of about 89%. No parthenogenetic characteristics have been observed for B. cajani.
8The highest mortality occurs during the first instar (Paulian, 1953). At this stage, the larvae disperse by dropping from twigs on a long silken thread (Piney, 1975), but which may result in their landing on a plant of the wrong food type. If the first instar does not eat within 4 days of hatching, it cannot survive (Paulian, 1953). As the caterpillars develop through five stages, they change in size and shape. The second instar larvae develop four spiny bouquets toward the thoracic region. The spiny bouquets progressively develop in size with each instar and, when alarmed, the caterpillars expose four “pom-pom” like spiny bouquets, which are red at the base and black on top. Some larvae show only the blackish bristle. The fifth instar caterpillar (Figure 2) is very hairy at the lateral face, and is immense, reaching over 12 cm long (Grangeon, 1906; Razafimanantsoa, 2008; Rakotoniaina, 2009). In the wild, the fifth instar is remarkably well camouflaged to mimic the bark of their plant food. Their color is grey-blue.

9The cocoon of B. cajani is oval in shape and grayish in color, and projects urticant (i.e., itchy) hairs. The size of female cocoons is about 50 mm in length, and about 25 mm in diameter, and is smaller for males. The cocoon has three layers, weighing 300-400 mg for females and 90-200 mg for males. The prepupa stage lasts 3 to 5 days (Grangeon, 1906; Razafimanantsoa, 2008).
10As with many moths, B. cajani is preyed on by many predators and is subject to many diseases, with all stages being at risk of attack (Zborowski et al., 2007). Bird predators includ Acridithores tristis, Cuculus rochii, Centropus toulou, Corvus alba, and Hypsipetes madagascariensis. However, other predators also exist, including bats, reptiles (Furcifer lateralis), the praying mantis, ants, and spiders. Parasites are the most invasive, and mostly comprise wasps (Ichneumonidae: Pimpla, Xanthopimpla hova, Brachymeria borocera, Ophion sp., Braconidae: Apanteles borocerae and Apanteles decaryi). Another invasive species is Synthesiomyia nudiseta (Diptera: Tachinidae), which, while it attacks the larvae, may still emerge in a subsequent stage (Grangeon, 1906; Grangeon, 1907; Paulian, 1953; Razafindraleva, 2001; Razafimanantsoa, 2008). There is also a wide range of fungal, bacterial, and viral diseases, such as “pebrine, muscardine and flasherie” (Grangeon, 1907).
11The life cycle of B. cajani requires 102 to 193 days to complete (Razafindraleva, 2001; Razafimanantsoa, 2008) (Figure 3). This major variation in the days required to reach the pupal stage is due to diapause during the dry season.
12Borocera cajani colonizes the U. bojeri (or “Tapia”) forest, which is found in the central highland zones (Imamo, Itremo) and Isalo zones of southwest of Madagascar (Gade, 1985). The “Tapia” forest constitutes one of the naturally formed forests of the island, primarily containing just one tree species, U. bojeri (Rakotoarivelo, 1993; figure 4). This plant is one of the major plant foods of B. cajani. The silk moths are very polyphagous, and are found inhabiting many habitats of Madagascar, including Diego Suerez, Montagne d'Ambre, Nosy Be, Anjozorobe, Analavory, Ankazobe region, Antananarivo, Manjakatompo, Ankarana's massif, Ambohimahasoa, Andringitra's massif, Bekily, Majunga, Ankarafantsika's Region, Antsingy, Antsalova region, and Morondava (De Lajonquière, 1972). They also feed on Psidium guajava L., Psidium cattleianum Sabine, Aphloia theiformis (Vahl) Benn., Dodonaea madagascariensis Radlk., Cajanus indicus Spreng., Harungana madagascariensis Lam. ex Poir., Acacia dealbata Link, Schefflera sp. J.R.Forst. & G.Forst., Eucalyptus sp. L'Hér., Tamarindus indica L., Avicennia officinali L., Salíx babylonica L., and Terminalia catappa L. (Paulian, 1953; De Lajonquière, 1972; Rakotoarivelo, 1993; Razafindraleva, 2001; Razafimanantsoa, 2008; Rakotoniaina, 2009).

13The herbaceae stratum in the “Tapia” forest has an important role in the life cycle of B. cajani. This stratum serves as place for chrysalis nidification (i.e., chrysalis construction) (Razafimanantsoa, 2008).
4. Importance
14The Gonometinae species are currently used for wild silk production in Madagascar, with the silk being primarily spun from B. cajani (De Lajonquière, 1972). The size of the harvest varies greatly from year to year, reaching 10 t to 43 t for the whole island (Gade, 1985; CITE/BOSS CORPORATION, 2009). Wild silk production is a source of income for the communities living adjacent to the Tapia forest (Kull, 2003). Silk production provides an alternative source of income to the poorest and most disadvantaged people, especially during “lean periods” (Kull et al., 2005). The villagers gather cocoons and sell them. The chrysalis is removed and eaten, while the empty cocoons are cooked, spun, and woven into silk fabrics (Kull, 2003). Thirty-four percent of households in Ambohimanjaka and 65% of households in Ilaka earn cash incomes from the harvest of B. cajani cocoons (Kull, 1998; Kull, 2002). The larger rainy season silk harvest provides an important cash income during the meager months before the rice harvest. Collecting Borocera cocoons, processing them, unraveling the filaments to spin into thread, and weaving them into silk have been part-time activities of highland rice growing peasants for several hundred years (Gade, 1985).
15Silk production involves many Madagascan people, from the wild silk harvesters to the spinners, dyers, weavers, and craftsmen who transform the silk into clothing, accessories, and decorative items.
16Borocera pupae are also a preferred delicacy. Besides animal protein, the insects provide occasional variation in texture and taste to the daily diet, which primarily constitutes rice and manioc (Decary, 1937; Gade, 1985; Guigou, 1989; Kull et al., 2005).
17Borocera cajani silk is processed to produce ritual burial shrouds and clothing items throughout the highlands as part of the area's culture. The “Landibe” shroud is essential for any respected dead person. To be wrapped by many “Lambamena” (red silk shrouds) is a sign of supreme honor according to the Madagascan adage: “Izay sahy maty mifono lambamena” (literally translated as “the intrepid facing the death wears one “Landibe” shroud”) (Rakotoniaina, 2009). The “Landibe” shroud is preferred over that made with Bombyx mori for Madagascan ritual burials and for exhumations or “Famadihana” (a Madagascan habit to renew the shroud of mortal remains every 5 to 10 years) (CITE/BOSS CORPORATION, 2009) (Figure 5). Indeed, wild silk is precious for this secular usage (Rakotoniaina, 2009), even if few people can afford this type of cloth. The cloth costs 25 to 71 Euros (CITE/BOSS CORPORATION, 2009). The silk produced from B. cajani cocoons is very remarkable for its glow, tenacity, elasticity, and solidity (Coquerel, 1854; Guigou, 1989). For this reason, the Madagascan people seek this silk over other silks. However, some people also use Bombyx mori for shrouds.

5. Problems
18Estimates show that between 150,000 and 200,000 ha of Madagascan forest is lost per year (Minten et al., 2003). Poverty is one root cause of the loss of biodiversity. As with many species in Madagascar, B. cajani is critically endangered due to the destruction of their native forest (Razafimanantosoa et al., 2006). In 1933, the Imamo “Tapia” forest was viewed as being important, and was recognized for the collection of cocoons. However, deforestation over the subsequent 20 years (until the 1950s) resulted in this area becoming a sparse forest (Paulian, 1953). Consequently, since the 1960s, this zone ceased to produce “Landibe” (Razafintsalama et al., 1999; Kull, 2003; Kull et al., 2005). The Isalo and Itremo zones remain the producing centers of wild silk in Madagascar (Paulian, 1953). However, Madagascan wild silk production continues to decline. In 1902, the Island produced 103 t of empty cocoons versus an estimated 43 t was produced in 2009 (CITE/BOSS CORPORATION, 2009). In addition, in Itremo, cocoons are only abundant in cycles of one year out of five. As a result, the sparse cocoon populations are not worth the harvest effort (Gade, 1985). There are many contributors to the decline of the silk moth, and hence wild silk production, including:
19– the destruction of preferred moth habitats caused by the way that harvesters gather cocoons in the forest by breaking many branches (Grangeon, 1906);
20– the proliferation of introduced species, such as Pinus sp., which outcompetes native plants, such as U. bojeri, by its quick growth and alteration of microhabitats due to its heliophile characteristics, leading to changes in soil quality (Kull et al., 2005);
21– bush fires, which kill B. cajani and destroy young U. bojeri trees, seedlings, and sprouts (Grangeon, 1910; Perrier de la Bâthie, 1921; Paulian, 1953; Vignal, 1963; Gade, 1985; Guigou, 1989; Kull et al., 2005);
22– the overharvesting of pupae for food consumption (Paulian, 1953; Kull et al., 2005; Razafimanantosoa et al., 2006; Razafimanantsoa, 2008).
23The demise of the industry has also been affected by the lack of modernization of native silk manufacture and the high cost of “Landibe” burial shrouds. About 53 t per year of silk is produced from the domesticated Chinese silkworm Bombyx mori (CITE/BOSS CORPORATION, 2009). However, the price of thread and clothes made with this silk is almost the same as for B. cajani products. Bombyx mori is produced with cheap labor, as this species can be easily reared in large quantities. This results in an esthetically superior fabric, which is exported in a market where B. cajani does not compete (Rafidiarimalala, 1974; Gade, 1985). The “Landibe” cocoons cannot be spun. It must be skinned off its chrysalis by cocoon incision. The spinning-mill is made using distaff, and productivity is limited (Rakotoniaina, 2009).
24Management methods including GELOSE (“Gestion locale Sécurisée,” meaning secure local management) and GCF (“Gestion Contractualisée des Forêts,” meaning contractual management of forests) are now entrusted to local based communities to manage large parts of the “Tapia” forest. Local based communities, with legal constitutions, are composed of residents in a hamlet, village, or group of villages. These volunteers are united by common interests and are willing to obey the rules of common life, and run the natural resource management associations (GELOSE, 1999; Robsomanitrandrasana, 2008). Their role is to ensure the self-sustainable management of natural resources that are present on their territory, including the forest and fauna, such as the silkworm (Razafindrakoto, 2005; Robsomanitrandrasana, 2008). Currently, due to community management, conservation, and recovery of the forest, these organizations have proved a successful transfer of good management, with the cessation of charcoal production (Consortium RESOLVE/PCP/IRD, 2005; Robsomanitrandrasana, 2008). However, some villagers do not wish to join the association (local community based) to avoid following the rules.
6. Conclusion and recommendations
25Although sericulture continues to play a minor role in Madagascar at an international level (Krishnaswami et al., 1974), it remains a limited source of income for local people who depend on it. More than 10,000 families work in the silk industry (CITE/BOSS CORPORATION, 2009). Therefore, its revitalization, along with careful management, could be an important factor for the future conservation of silk moths, such as B. cajani. In recent years, human pressures on B. cajani and its habitat have decreased through applying management methods led by local communities. However, it remains a challenge to convince people who do not wish to join the associations, to maintain the same level of management and thus conservation, to ensure everyone has the same conviction. Therefore, a comprehensive study on the biology and ecology of the insect and its habitat is necessary to establish long-term management protocols.
26The permanent cessation of bush fire practice and logging, the reforestation of U. bojeri, and elimination of introduced plants (such as Pinus) within the “Tapia” forest are very important actions towards maintaining the natural habitats of B. cajani (ONU, 1991; Razafimanantsoa, 2008). Restrictions on the methods of collecting cocoons, such as avoiding breaking branches, and the timing of cocoon gathering, to ensure the establishment of the next generation, must be adhered to. The repopulation of B. cajani in the wild requires an understanding of the various biological and human impacts on the population for successful production. The provision of information through environmental education to the children of the region where B. cajani is abundant would help safeguard the future of this industry. Through this mechanism, the Madagascan people may develop an understanding of the importance of B. cajani as a cultural heritage.
Pour citer cet article
A propos de : Tsiresy M. Razafimanantsoa
Université de Antananarivo. Faculté des Sciences. Département de Biologie animale, Écologie et Conservation. BP 906. Antananarivo (Madagascar).
A propos de : Gabrielle Rajoelison
Université de Antananarivo. École supérieure des Sciences agronomiques. Département des Eaux et Forêts. BP 175. Antananarivo 101 (Madagascar).
A propos de : Bruno Ramamonjisoa
Université de Antananarivo. École supérieure des Sciences agronomiques. Département des Eaux et Forêts. BP 175. Antananarivo 101 (Madagascar).
A propos de : Noromalala Raminosoa
Université de Antananarivo. Faculté des Sciences. Département de Biologie animale, Écologie et Conservation. BP 906. Antananarivo (Madagascar).
A propos de : Marc Poncelet
Univ. Liège. Département de Sociologie du Développement. Boulevard du Rectorat, 7. B-4000 Liège 1 (Belgium).
A propos de : Jan Bogaert
Université libre de Bruxelles. Service d'Écologie du Paysage et Systèmes de Production végétale. Avenue Franklin Roosevelt, 50. B-1050 Brussels (Belgium).
A propos de : Éric Haubruge
Univ. Liege - Gembloux Agro-Bio Tech. Unité d'Entomologie fonctionnelle et évolutive. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
A propos de : François J. Verheggen
Univ. Liege - Gembloux Agro-Bio Tech. Unité d'Entomologie fonctionnelle et évolutive. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: fverheggen@ulg.ac.be






