Physiological, biochemical and agromorphological responses of five cowpea genotypes (Vigna unguiculata (L.) Walp.) to water deficit under glasshouse conditions
Département de Biologie. Faculté des Sciences. Université Abdou Moumouni. BP 10662, Niamey (Niger). E-mail : falalouh@yahoo.fr – Centre d’Etude régional pour l’Amélioration de l’Adaptation à la Sécheresse (CERAAS). Thiès. BP 3320. Thiès Escale, Thiès (Sénégal).
UFFR-SVT, Université de Ouagadougou. Laboratoire de Biologie et physiologie vegétales. 03 BP 7021. Ouagadougou 03 (Burkina Faso).
Centre d’Etude régional pour l’Amélioration de l’Adaptation à la Sécheresse (CERAAS). Thiès. BP 3320. Thiès Escale, Thiès (Sénégal).
Centre de Coopération internationale en Recherche agronomique pour le Développement (CIRAD). Avenue Agropolis, 34398. Montpellier Cedex 5 (France).
Received on 19 August 2005, accepted on 7 Septembre 2006.
Résumé
Réponses physiologique, biochimique et agromorphologique de cinq variétés de niébé (Vigna unguiculata) soumises à un déficit hydrique en serre. Cinq variétés de niébé (Vigna unguiculata), Bambey 21, Gorom local, KVX61-1, Mouride et TN88-63, cultivées en pots en serre ont été soumises à un déficit hydrique par suspension d’arrosage pendant 14 jours en phase végétative (T1) et 12 jours au stade floraison (T2). Les incidences de ce traitement sur le potentiel hydrique foliaire, les échanges gazeux, le volume racinaire, les teneurs en proline, en amidon et en protéines totales des feuilles, le rendement maximal photochimique (fp0) et les composantes de rendement ont été déterminées. Le potentiel hydrique n’a significativement baissé que chez Mouride et TN88-63 (de -0,55 à -0,92 MPa en moyenne) stressés en floraison, tandis que le volume racinaire, les échanges gazeux, ainsi que la teneur en amidon ont été significativement réduits chez les 5 variétés en conditions de stress aux 2 stades. (fp0) n’a pas été affecté par le stress en phase végétative. En phase floraison il a significativement baissé dès le 6e jour d’application chez Gorom, KVX61-1 et TN88-63 et au 10e chez Bambey 21 et Mouride. Une accumulation significative de la proline due au déficit hydrique a été observée chez les 5 variétés en phase T1 et T2, Mouride et TN88-63 ont les teneurs les plus élevées (respectivement 2,9 et 3,3 mg.g-1 MS) en phase floraison. La teneur en protéines totales n’a pas été significativement modifiée par le stress aux 2 stades. Nos résultats ont montré que les 5 variétés ont évité la déshydratation en baissant la conductance stomatique et la transpiration lors du stress en T1 et T2. L’accumulation de la proline, le maintien de la teneur en protéines totales et la baisse de la teneur en amidon chez les 5 génotypes en conditions de stress aux 2 stades pourraient contribuer au maintien de la turgescence cellulaire. En outre, ces solutés permettraient de protéger l’appareil photosynthétique (PSII) contre la dénaturation notamment durant le stress en floraison. Le nombre de graines par gousse et le nombre de graines par plante ont été réduits en conditions de déficit hydrique, la différence variétale observée a montré que Bambey 21 a été moins affecté que Gorom, TN88-63 et Mouride tandis que KVX61-1 s’est révélée la plus sensible. Bambey 21 s’est montrée tolérante au stress durant les 2 stades, Gorom, Mouride et TN88-63 ont été intermédiaires tandis que KVX61-1 a été la plus sensible.
Abstract
Five genotypes of cowpea (Vigna unguiculata), Bambey 21, Gorom local, KVX61-1, Mouride and TN88-63, grown in pots under glasshouse conditions, were submitted to water deficit by withholding irrigation at vegetative stage (T1) for 14 days, and at flowering stage (T2) for 12 days. Effect of this stress on leaf water potential, gas exchanges, foliar proline, total protein and starch contents, maximal quantum yield of photochemistry (fp0), root volume and yield components was determined. Leaf water potential decreased significantly only for Mouride and TN88-63 (from -0.55 to -0.92 MPa on average) at T2 while root volume, gas exchanges and foliar starch content decreased for the five genotypes under water stress conditions at T1 and T2. fp0 was not affected during water deficit at T1. Significant decrease of fp0 was observed at T2 on the 6th day after stress induction (Dasi) for Gorom, KVX61-1 and TN88-63 and the 10th Dasi for Bambey 21 and Mouride. Proline was significantly accumulated during water stress at the 2 stages, Mouride and TN88-63 showed the highest contents in the case of T2 (2.88 and 3.3 mg.g-1 DM respectively). Water deficit did not affect significantly the total proteins contents for the 5 varieties at T1 and T2. Our results showed that the 5 varieties involved drought avoidance mechanism by decreasing stomatal conductance and transpiration at the 2 stages. Proline accumulation, maintenance of total protein content and starch decrease under stress conditions at T1 and T2 could probably contribute in turgor maintenance. In addition, these solutes contributed probably in the protection of photosynthetic apparatus (PSII) against denaturation notably during water stress at flowering stage. At the two stages water stress reduced significantly seed number per pod and seed number per plant but the genotypic variation observed revealed that Bambey 21 was less affected than Gorom, TN88-63 and Mouride whereas KVX61-1 was most affected. Bambey 21 proved to be tolerant to water stress at the two stages, Gorom, Mouride and TN88-63 were intermediate and KVX61-1 sensitive.
1. Introduction
1Environmental stresses have adverse effects on crop yield. Water deficit is one of the most important factors limiting yield of main grain legumes such as cowpea. This grain legume is the most economically important African indigenous legume crop (Langyintuo et al., 2003). Cowpea is widely cultivated to enhance the outputs of agricultural systems and the productivity in the sahelian regions where water deficit occurs almost each year. Cowpea has many mechanisms of response and survival to drought. These include some physiological, biochemical and agromorphological responses (Hall et al., 1997; Roy-Macauley et al., 1992). The understanding of the mechanism explaining the resistance of cowpea varieties to drought is of extreme importance for improving the production of this grain legume (Cruz de Cavarlho et al., 1998). The importance of these mechanisms in the breeding program for improving crops adaptation to drought was already reported (Ronald et al., 1986; Monneveux, 1997). They are mainly used in cereal breeding to improve adaptation and increase yield in drought environments (Turner et al., 2001). Physiological, biochemical and agromorphological mechanisms on many sahelian cowpea varieties submitted to water stress have been separately but largely investigated (Nwalozie, 1991; Zombré et al., 1994; Nwalozie et al., 1996; Pimmentel et al., 1999; Hamidou, 2000; Diallo et al., 2001; Sarr et al., 2001; Ogbannaya et al., 2003). Only a few of these mechanisms known as adaptative characteristics are successfully used in breeding programs to improve drought resistance and yield of cowpea varieties grown in the Sahel. As far as we know, these studies have not investigated the physiological, biochemical and agromorphological mechanisms altogether. Furthermore, the functional significance of the physiological and biochemical characteristics notably their relationships with yield and its components, are still not clearly established on the cultivars, mainly on those grown in the Sahel (Burkina, Niger and Senegal).
2The purpose of the present study is to analyze the physiological, biochemical and agronomical responses of 5 cowpea varieties to water deficit at vegetative and flowering stages, in order
3– to assess the effect of this stress on some parameters characterizing the adaptative response,
4– to compare the responses of the five varieties to water stress,
5– to identify among them the most tolerant variety(ies) to water deficit.
2. Material and methods
2.1. Plant material, cropping conditions and water stress treatments
6Five cowpea genotypes, Gorom local (Gorom) and KVX61-1 (KVX) from Burkina Faso, Bambey 21 (B21) and Mouride (Mou) from Senegal and TN88-63 (TN) from Niger, were grown in pots under glasshouse conditions at Centre d’Etude Régional pour l’Amélioration de l’Adaptation à la Sécheresse (CERAAS), Thiès, Senegal (latitude 14° 81’ North and Longitude 16° 28’ West). In the glasshouse, the plants experienced maximum day/night temperatures of 31.3/25°C and humidities of 48.1/77.4%. These 5 genotypes were chosen mainly because they are of the principal varieties cultivated and consumed in the Sahelian zone of these countries and their agronomical responses to water deficit are different. Pots were filled with 18 kg of sandy soil underlaid by 1 kg of gravel. One day before sowing, pots were watered at field capacity. Three seeds were sown in each pot. Neither fertilizer nor nutrient solution was brought to the plantlets in order to mimic the farmer’s practices. Ten days after sowing (DAS), pots were thinned to one plant per pot. The experimental design was a complete randomized block design with 4 replications studying 2 factors: genotype at five levels (varieties listed above) and water regime at three levels T0 (control = well watered conditions), T1 (water stress at vegetative stage), T2 (water stress at flowering). The experimental unit contained 3 pots, leading to a total of 180 pots. The water requirement of the plant was determined according to the equation: ETM = Kc × ETP, where ETM = maximum evapotranspiration, ETP = potential evapotranspiration and Kc = crop coefficient (Dancette, 1983). Plants were watered every two days at T0 (ETM). On the 24th DAS T1 was applied. At flowering stage (on 36th DAS for B21, 40th for Gorom, KVX, Mou and TN) T2 was applied. T1 and T2 treatments were applied for all genotypes by withholding watering for 14 days and 12 days, respectively for T1 and T2, before re-watering the plants. Measurements were done during application of stresses.
2.2. Methods
7Physiological parameter measurements.
8Chlorophyll fluorescence parameter. During T1 and T2 treatments, chlorophyll fluorescence was measured with a fluorimeter PEA (Plant Efficiency Analyser, Hansatech Inst Ltd, King’s Lynn, England), between 11 h (am) and 2 h (pm) on the third leaf from the top of the plant. Among the different fluorescence parameters measured by the equipment, the maximal quantum yield of photochemistry (fp0) which turned out to be the most representative was retained.
9Gaseous echanges. The gaseous echanges were measured on the same leaf as fluorescence, with a portable infra red gas analyser of CO2 Licor LI-6400 (Licor Inc., Lincoln, Nebraska, USA). The measurements were done between 11 h (am) and 2 h (pm) on the 7 and 14th days after stress induction (Dasi) for T1 and 4 and 7th Dasi for T2. Measurements included net photosynthesis (Pn), stomatal conductance (Gs) and transpiration (Tr).
10Leaf water potential. The last day of T1 and T2 treatments, after measuring the above parameters, the third leaf was excised for measurement of leaf water potential (y1) with a Scholander pressure chamber (type PMS, model 650, Instrument Co, Corvalis Oregon USA).
11Biochemical parameters measurements. After leaf water potential measurement, the leaf was immediately frozen into liquid nitrogen and stored at –70°C, before extracting and measuring total soluble protein content, starch content and proline content according to the methods of Bradford (1976), Jarnis and Walker (1993) and Bates (1973), respectively.
12Agromorphological parameters measurements. After re-watering stressed plants, one plant of each experimental unit was sampled to measure root volume. Thus, soil of the pot was delicately rinsed and plant roots were removed. Root volume was determined by measuring the volume of water displaced by the root system into a test tube.
13At harvest, total pod number (TPN), number of seeds per pod (SN/Pd) and total seed number per plant (SN/Pt) were determined. The SN/Pt was used to assess the drought susceptibility index (DSI) of cultivars according to Fisher and Maurer (1978). DSI=(CI/CNI)/CI. Where CI=component value in well-watered conditions, CNI=component value in stressed conditions.
14Statistical analysis. Statistical analysis of the results was performed with SAS (SAS Statistical Institute, Cary, NC). The data were subjected to analysis of variance (ANOVA) procedure for a randomized complete block design. The Student-Newman Keuls test (for a 95% confidential level) was done to compare the means and determine whether there were any differences for the parameters measured considering the water regimes and varieties.
3. Results
3.1. Physiological parameters
15Gaseous echanges and chlorophyll fluorescence. The results of the gaseous echanges measurements during the first drought stress (Table 1) showed that under control conditions, the highest values (Pnmax) reach 25 µmol CO2 m-2.s-1 for the 5 varieties. Under T1 treatment, our results showed that Pn and Tr decreased from seven Dasi for the five varieties (Table 1). A genotypic variation was observed for stomatal conductance (Gs) under T1 conditions at this date. The stomatal conductance of Bambey 21, KVX61-1 and TN88-63 were significantly higher than those of Gorom and Mouride. Fourteen days after T1 imposition, values of Pn, Gs and Tr were closed to 0, showing that gaseous echanges were almost stopped (Table 1). For T2 treatment Pn, Gs and Tr of the five varieties were reduced significantly from 4th Dasi (Table 1). A genotypic variation was observed at this date. Gorom maintained the highest rate of Pn (5.64 µmol CO2 m2.s-1). For Gs and Tr measured 4 Dasi, there was an interaction between RH and Variety. No significant difference was noted among varieties under water stress conditions. But under well watered conditions, Bambey 21 reached the highest values of Gs and Tr which were significantly different from Gorom and KVX also different from Mouride and TN. Seven Dasi at flowering stage, an interaction between RH and Variety was observed for Pn, Gs and Tr. We noted significant differences among varieties only under well watered conditions. B21 had the highest gaseous echanges while TN exhibited lowest Pn, Gs and Tr.
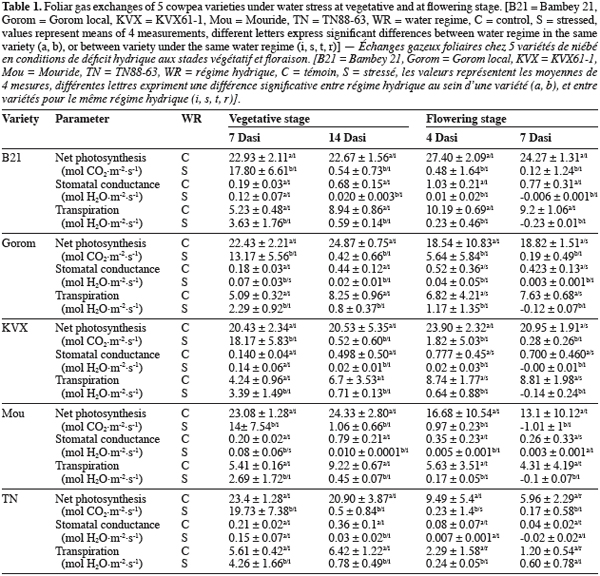
16Concerning chlorophyll fluorescence parameter fp0, well watered and stressed plants were not significantly (P = 0.563) different at vegetative stage (Figure 1a). At flowering stage (Figure 1b), there was significant (P = 0.0004) interaction between water regime and variety at 6th Dasi. Under well watered conditions, fp0 of B21 was the lowest and significantly different from the other varieties, while under stress Gorom, TN and KVX recorded the lowest fp0. At 10th Dasi significant water regime effect (P = 0.0009) was observed. fp0 of the five varieties decreased (5,76%) due to the water deficit.
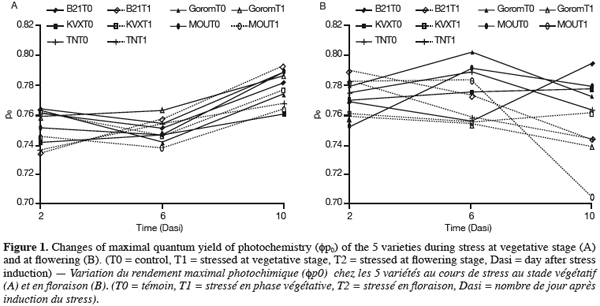
17Leaf water potential (y1). After the period of T1 application, yl of the five varieties was near -0.5 MPa for well-watered plants and -0.7 MPa for stressed plants (Figure 2). Statistical analysis revealed that these two yl were not significantly different. Further, no genotypic difference was observed. In our experimental conditions, either withholding watering during 14 days at vegetative stage was not long enough to result in a significant decrease in yl, either this parameter is not suitable for genotypes discrimination.
18For T2 (Figure 2), there was an interaction between water regime (RH) and variety (Var). Under control conditions (T0) there was no significant difference among varieties. Under stress conditions (T2), the results showed that Bambey 21 presented the highest yl (-0.43 MPa) and differed significantly from Gorom, KVX, Mouride and TN of which yl where respectively -0.85, -0.87, -0.90 and -1.06 MPa. Between water regimes in the same variety, significant difference in leaf water potential was noted only for Mouride and TN88-63.
3.2. Biochemical parameters
19Fourteen days after T1 treatment imposition, the total protein content in leaves was not significantly differed between well-watered and stressed plants for the 5 varieties (Table 2). However, water deficit induced significant increase of proline content. The proline content in leaves increased by 56% in stressed plants compared to well-watered plants. There was a significant interaction (P<0.0001) between RH and Var for starch content. Under well watered conditions, starch content of B21 and KVX was higher than for Gorom, Mouride and TN (Table 2), while under T1 conditions, B21 starch content (79.21 mg.g-1 DM) was significantly higher than Gorom (36.85 mg.g-1 DM) which was higher than KVX (15.92 mg.g-1 DM), TN (8.75 mg.g-1 DM) and Mouride (3,61 mg.g-1 DM). Starch content of KVX, Mouride and TN was reduced significantly under T1 conditions while it didn’t differ significantly from control for Gorom and Bambey 21.
20At flowering stage, protein content was not significantly different from stressed to well-watered plants. We observed genotypic difference showing that TN, B21 and Mouride reached significantly higher than Gorom and KVX (Table 2). Under water deficit, all varieties increased their foliar proline content. No genotypic difference was observed among control plants while under stress conditions TN accumulated more proline than Mouride, Bambey 21 and Gorom which accumulated more than KVX. At the same stage, water deficit significantly decreased starch content of the five varieties at flowering stage (Table 2). The diminution was -87%, -75%, -86%, -72% respectively for Bambey 21, Gorom, KVX and Mouride while it reached only -25% for TN88-63.
3.3. Agromorphological parameters
21Root volume. Stresses T1 and T2, caused significant reduction in root volume of the 5 genotypes (Table 3 and 4). This reduction reached -50% for Bambey, KVX, Mouride and TN88-63 and -34% for Gorom under T1 conditions. At flowering stage, there was an interaction between water regime and variety. Under well irrigated conditions, the genotypic difference observed revealed that root system of B21 was larger (volume = 40 ml) than other varieties (volumes≤25 ml). Under T2 conditions, root volume decreased significantly and no difference among the 5 varieties was noted.
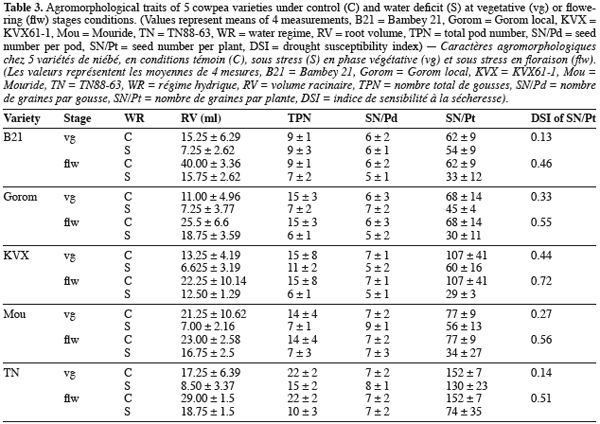
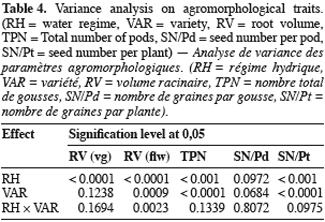
22Yield components. Water stress didn’t reduce significantly the seed number per pod (SN/Pd) but decreased significantly the total pod number per plant (TPN) and the seed number per plant (SN/Pt) (Table 3 and 4). The reduction of TPN and SN/Pt induced by T1 compared to control was -36% and -24% respectively for TPN and SN/Pt. It reached -56% for the 2 yield components under T2 conditions. Furthermore, the genotypic variation observed revealed that under control conditions TN produced significantly more pods (TPN) and seeds (SN/Pt) than the other varieties. The drought susceptibility index (DSI) concerning the SN/Pt (Table 3) in the 5 varieties was higher when stress occurs at flowering stage than during vegetative stage. At the 2 stages, B21 showed the lowest DSI while KVX presented the highest.
4. Discussion
23The gaseous echanges of the 5 varieties decreased under water stress conditions. This decrease was significant 7 Dasi for T1 and four Dasi for T2. The early effect of water deficit on gaseous echanges at flowering stage could be due, in one hand to the fact that flowering is a critical growth stage of plants (Zombre et al., 1994; Anyia et al., 2004), and in the other hand to the water requirement of plants which is more important at flowering stage due to higher biomass (roots and shoots) than at vegetative stage. After 4 days of stress occurring at flowering stage, Gorom maintained significantly the highest values of Pn (5.64 µmol CO2 m2.s-1), indicating that assimilation still occurred in spite of the constraint. As stress intensity increased (14 days at vegetative stage and 7 days at flowering), gaseous echanges of the 5 varieties decreased. Cowpea is known to have a high stomatal control leading to a rapid closure of stomata under water stress conditions (Hall et al., 1997; Scotti et al., 1999; Cruz de Carvalho, 2000; Sarr et al.; 2001, Ogbonnaya et al., 2003). The consequence of this stomatal closure is the reduction of net photosynthesis which was closed to 0 for the 5 varieties after 14 and 7 days of stress at vegetative and flowering stages, respectively. In addition to stomatal control, the decrease of net photosynthesis under water stress conditions could be due to some non-stomatal processes like the disturbance of the photochemical activities or damages of the photosynthetic apparatus (PSII) (Niinemets, 2002). Chlorophyll fluorescence parameters are direct indicators of the photosynthetic activity (Lichtenthaler et al., 2000) and give an indication of status of photosynthetic apparatus. The maximal quantum yield of photochemistry (fp0) of stressed and well watered plants was similar at vegetative stage for the 5 varieties. The lack of significant decrease of fp0 could indicate that the photochemical apparatus (PSII) was quite stable under water deficit, as it was previously demonstrated in the case of some moderate water deficits (Lu et al., 1999; Souza et al., 2004). At flowering stage, water stress decreased fp0 of the 5 varieties notably at 10th Dasi indicating that water deficit was much severe than at vegetative stage, and affected the photochemical activity. The decrease of fp0 could indicate a downregulation of PSII. Souza et al. (2004) suggested that under more severe water stress, the capacity of protective mechanisms in cowpea could be exceeded. Felexas et al., (1999) also reported that when water deficit becomes stronger, it induces a drastic downregulation of photosynthesis, a decrease of photon accumulation, a dissipation of important energy and a decrease of quantum yield of PSII electron transport. B21 and Mouride revealed less affected because their fp0 didn’t decease at 6th Dasi while it decreased significantly for the other varieties. Our results suggested that the decrease of the Pn under stress conditions at vegetative stage was mainly due to stomatal process. At flowering stage, in addition to the stomatal control process, the decrease of Pn was induced by perturbation of PSII activities due to water deficit. Present results also demonstrated that fp0 could be used to screen varieties under water deficit notably at flowering stage.
24The decrease of leaf water potential was only significant under water stress occurring at flowering stage. The senescence of leaves could explain these results. Indeed, previous works reported that age of leaves contributed to decrease leaf water potential notably under stress conditions (Anyia et al., 2004). Furthermore, reproductive stage of plants is critical due to important physiological activities occurring at that time, notably the transfer of assimilates to grains. The decrease was more important for Mouride and TN varieties. The maintenance of leaf water potential in drought conditions is considered to be associated with dehydration avoidance. A turgor maintenance of tissues, which could allow continuation of the physiological activities (Jongdee et al., 2002), could be done by osmolytes accumulation (Serraj et al., 2002). Our results showed that proline content increased for all cultivars in response to water stress at the 2 stages but it increased more for Mouride and TN than for Gorom, B21 and KVX. Proline accumulation is known to provide an efficient mechanism for cellular adaptation to osmotic stress (Martinez et al., 1995). In another hand, Marjorie et al. (2002) demonstrated that proline accumulated during water deficit has a minor contribution to total osmolytes in drought conditions but plays a key role after re-watering. At the same time, total protein content was not affected for any cultivars whatever the treatment. This could mean either that proteins were not degraded and their synthesis was inhibited during water stress, or that their degradation and de novo synthesis were similar. On the opposite, starch content decreased in the 5 varieties mainly at flowering stage. This decrease could be explained by a reduction of starch synthesis during water deficit due to the Pi increase and the inhibition of phosphorylase activity (Da Silva et al., 2004), and/or an increase of hydrolytic enzymes activities as amylase and acid invertase which hydrolyze starch into soluble sugars, sucrose and organic acids (Keller et al., 1993). These carbohydrates could compensate the lack of synthesis of carbohydrates due to photosynthesis inhibition caused by water stress and/or act as osmoticums and decrease hydric potential (Pelleshi et al., 1997; Sanchez et al., 1998).
25Under water deficit occurring at flowering stage, we observe simultaneously in the case of Mouride and TN a significant decrease of their leaf water potential, in a lesser extent a diminution of the starch content and an accumulation of proline higher than in Gorom, B21 and KVX. These observations indicate that the 2 genotypes, could have the capacity of osmoregulation, by increasing the solutes contents in leaf tissues, trough hydrolysis of starch for soluble sugars production and synthesis of proline, thus resulting in a decrease of osmotic potential and finally of leaf water potential. In the case of Gorom, B21 and KVX, the significant decrease of starch and the increase of proline content are not linked with a diminution of leaf water potential, which give food for thought that these 3 varieties would not be able to osmoregulate under water stress as Mouride and TN. In that case, proline accumulation and starch decrease observed under water stress should be considered as injury symptoms.
26For the 5 varieties, stress conditions resulted in low root volume indicating that the development of roots was inhibited or stopped during water stress. These could be explained by the pot culture conditions because Sarr et al. (2001) observed that cowpea increased root system under water stress in field conditions.
27The agronomical parameters measured on the 5 varieties were reduced under water stress conditions. The decrease was more important at flowering than at vegetative stage. This could be due to the fact that water stress occurring at flowering stage affects immediately the reproduction process, unlike stress at vegetative stage that can be compensated by the plant trough different mechanisms. Whatever the stress applied (T1, T2) the agronomical parameters of B21 were less reduced while those of KVX were most affected.
5. Conclusion
28In our experimental conditions, water deficit affected almost all the physiological, biochemical and agronomical parameters we measured on the 5 varieties. The effect was more important at flowering than at vegetative stage. Genotypes avoided dehydration trough a decrease of stomatal conductance and transpiration. This adaptative strategy was associated with a net decrease of photosynthesis. The photochemical activities of PSII were maintained under water stress occurring at vegetative. But at flowering stage, the decrease of the maximal quantum yield of photochemistry proved that these processes were obviously disturbed for the 5 varieties. The starch content decreased and proline accumulated under water deficit conditions for the 5 genotypes but no significant change was observed for the total protein content. Mouride and TN88-63 accumulated more proline and less decreased starch than Bambey 21, Gorom and KVX61-1 under water stress at flowering stage. Due to their high accumulation of solutes, Mouride and TN88-63 have the lowest leaf water potential under stress conditions. Water deficit did not decrease significantly leaf water potential of Bambey 21, Gorom and KVX, showing that their osmoregulation capacity would be very limited. Our results indicated that Bambey 21 was tolerant to water deficit because its agronomical parameters were less affected; Gorom, Mouride and TN88-63 were intermediate while KVX61-1 was the most sensitive.
29Aknowledgement
30This work received a financial support from Agence Universitaire pour la Francophonie (AUF) and from Deutscher Akademischer Austausch Dienst (DAAD).
Bibliographie
Anyia AO., Herzog H. (2004). Genotypic variability in drought performance and recovery in cowpea under controlled environment. J. Agron. Crop Sci. 190, p. 151–159.
Bates LS. (1973). Rapid determination of free proline for water stress studies. Plant Soil 39, p. 205–209.
Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann. Biochem. 72, p. 248–254.
Cruz De Carvalho MH., Laffray D., Louguet P. (1998). Comparaison of physiological response of Phaseolus vulgaris and Vigna unguiculata cultivars when submitted to drought conditions. Env. Exp. Bot. 40, p. 197–207.
Cruz De Carvalho MH. (2000). Etude physiologique, biochimique et moléculaire de la réponse à la sécheresse chez Phaseolus vulgaris L. et Vigna unguiculata L. Walp. Implication de l’aspartique protéinase. Mise au point de l’étape préalable à la transgenèse : régénération in vitro des plantes. Thèse doct. physiol. cellulaire et moléculaire des plantes. Paris, France : univ. Paris VI, 180 p.
Dancette N. (1983). Contraintes pédoclimatiques et adaptation de l’agriculture à la sécheresse en zones intertropicales. Document CIRA-GERDAT-ISRA, p. 27–39.
Da Silva JM., Arrabaça MC. (2004). Contributions of soluble carbohydrates to the osmotic adjustement in the C4 grass Setaria sphacelata: A comparison between rapidly and slowly imposed water stress. J. Plant Physiol. 161, p. 551–555.
Diallo AT., Samb PI., Roy-Macauley H. (2001). Water status and stomatal behaviour of cowpea, Vigna unguiculata (L.) Walp., plants inoculated with two Glomus species at low soil moisture levels. Eur. J. Soil Biol. 37 (3), p. 187–196.
Felexas J., Escalona JM., Medrano H. (1999). Water stress induces different levels of photosynthesis and electron transport rate regulation in grapevines. Plant Cell Env. 22, p. 38–48.
Fischer RA., Maurer R. (1998). Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust. J. Agric. Res. 29, p. 897–912.
Hall AE., Thiaw S., Ismail AM., Ehlers JD. (1997). Water use efficiency and drought adaptation of cowpea. In Singh BB., Mohan RDR., Dashiell KE., Jackai LEN. Advances in cowpea research, IITA and JIRCAS, p. 87–98.
Hamidou F. (2000). Réponse physiologique du niébé (Vigna unguiculata L. Walp) au déficit hydrique au cours de deux stades de développement. Mémoire de Diplôme d’études Approfondies Physiologie végétale, Unité de formation et de recherche en sciences de la vie et de la terre. Ouagadougou, Burkina Faso, 66 p.
Jarnis CE, Walker JRL. (1993). Simultaneous, rapid spectrophotometric determination of total starch amylose and amylopectin. J. Sci. Food Agric. 63, p. 53–57.
Jongdee B., Fukai S., Cooper M. (2002). Leaf water potential and osmotic adjustment as physiological traits to improve drought tolerance in rice. Field Crops Res. 76, p. 153–163.
Keller F., Ludlow MM. (1993). Carbohydrates metabolism in drought-stressed leaves of pigeonpea (Cajanus cajan L.). J. Exp. Bot. 44, p. 1351–1359.
Langyintuo AS., Lowenberg-Deboer J., Faye M, Lambert G., Ibro G., Moussa B., Kergna A., Kushwaha S., Musa S., Ntoukam G. (2003). Cowpea supply and demand in west and central Africa. Field Crops Res. 82 (2), p. 215–231.
Lichtenthaler HK., Babani F. (2000). Detection of photosynthetic activity and water stress by imaging the red chlorophyll fluorescence. Plant Physiol. Biochem. 38, p. 889–895.
Lu C., Zhang J. (1999). Effects of water stress on photosystem II photochemistry and its thermostability in wheat plants. J. Exp. Bot. 50, p. 1199–1206.
Marjorie JR., Smirnoff N. (2002). Proline metabolism and transport in maize seedlings at low water potential. Ann. Bot. 89, p. 813–823.
Martinez CA., Guerrero C., Moreno U. (1995). Diurnal fluctuations of carbon exchange rate, proline content and osmotic potential in two water-stressed potato hybrids. Rev. Bras. Fisiol. Veg. 7 (1), p. 27–33.
Monneveux P. (1997). La génétique face au problème de la tolérance des plantes cultivées à la sécheresse : espoirs et difficultés. Sécheresse 2, p. 29–37.
Niinemets U. (2002). Stomatal conductance alone does not explain the decline in foliar photosynthetic rates with increasing tree age and size in Picea abies and Pinus sylvestris. Tree Physiol. 22, p. 515–535.
Nwalozie MC. (1991). Adaptation of cowpea to drought: physiological aspects. Final report CR000907. Thiès, Sénégal: CERAAS, 14 p.
Nwalozie MC., Annerose DJM. (1996). Stomatal behaviour and water status of cowpea and peanut at low soil moisture levels. Acta Agron. Hung. 44, p. 229–236.
Ogbonnaya CI., Sarr B., Brou C., Diouf O., Diop NN., Roy-Macauley H. (2003). Selection of cowpea genotypes in hydroponics, pots and field for drought tolerance. Crop Sci. 43, p. 1114–1120.
Pelleschi S., Rocher JP., Prioul JL. (1997). Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves. Plant Cell Env. 20, p. 493–503.
Pimentel C., Roy-Macauley H., Abboud AC. de S., Diouf O., Sarr B.(1999). Effect of irrigation regimes on the water status of cowpea cultivated in the field. Physiol. Mol. Biol. Plants 5, p. 153–159.
Ronald JN., Shyamala B., Jeffrey DP., Smith HR. (1986). Physiological changes in cultured sorghum cells in response to induced water stress: soluble carbohydrates and organic acids. Plant Physiol. 81, p. 626–629.
Roy-Macauley H., Zuily-Fodil Y., Kidric M., Pham Thi AT., Vieira da Silva J. (1992). Effect of drought stress on proteolytic activities in Phaseolus and Vigna leaves from sensitive and resistant plants. Physiol. Plant. 85, p. 90–96.
Sanchez FJ., Manzanares M., Eusebio F. de A., Tenorio JL., Ayerbe L. (1998). Turgor maintenantce, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crops Res. 59, p. 225–235.
Sarr B., Diouf O., Diouf M., Roy-Macauley H. (2001). Utilisation des paramètres agromorphologiques comme critères de résistance à la sécheresse chez trois variétés de niébé cultivées au Sénégal et au Niger. Sécheresse 12 (4), p. 253–266.
Scotti CP., Ramalho JC., Lauriano JA., Silva LJ., Céu-Matos DM. (1999). Effects of drought on phosynthetic performance and water relations of four Vigna genotypes. Photosynthetica 36 (1-2), p. 79–87.
Serraj R., Sinclair TR. (2002). Osmolyte accumulation: can it really help increase crop yield under drought conditions. Plant Cell Env. 25, p. 333–341.
Souza RP., Machado EC., Silva JAB., Lagôa AMMA., Silveira JAG. (2004). Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Env. Exp. Bot. 51, p. 45–56.
Turner NC., Wright GC., Siddique KHM. (2001). Adaptation of grain legumes (Pulses) to water-limited environments. Adv. Agron. 71, p. 193–231.
Zombre G., Zongo JD., Sankara ETP. (1994). Réponse physiologique du niébé au déficit hydrique s’exerçant uniformément au cours du cycle de développement. African Crop Sci. J. 2, p. 225–231.

