Growth, nitrate reductase activity and antioxidant system in cadmium stressed tomato (Lycopersicon esculentum Mill.) cultivars
Aligarh Muslim University. Plant Physiology Section. Department of Botany. IND-Aligarh 202 002 (India). E-mail: shayat@lycos.com
Aligarh Muslim University. Plant Physiology Section. Department of Botany. IND-Aligarh 202 002 (India).
Aligarh Muslim University. Plant Physiology Section. Department of Botany. IND-Aligarh 202 002 (India).
Received on March 6, 2010; accepted on December 7, 2010
Résumé
Croissance, activité de la nitrate réductase et du système antioxydant chez les cultivars de tomate (Lycopersicon esculentum Mill.) soumis à un stress au cadmium. Dix cultivars de tomate ont été soumis à différentes concentrations en cadmium (Cd2+) afin de déterminer leur degré de tolérance vis-à-vis de ces ions métalliques durant l'ontogénèse de la tomate. Des semences de cultivars de tomate (‘K-25’, ‘K-21’, ‘NTS-9’, ‘Kaveri’, ‘NBR-Uday’, ‘Swarnoday’, ‘Sarvodya’, ‘NBR-Uttam’, ‘Malti’ et ‘S-22’) ont été trempées dans 0, 50, 100 et 150 μM de Cd2+ pendant 0, 4, 8 et 12 h. Malgré des différences variétales évidentes, une augmentation de la concentration en Cd2+ et de la durée de trempage provoquent une décroissance linéaire de la croissance et de l'activité catalase et peroxydase chez toutes les variétés. La variété ‘K-25’ s'est montrée la plus résistante et présente aussi la plus grande activité des enzymes antioxydants, ce qui représente une des raisons possibles de sa résistance aux conditions de stress. À l’inverse, les semences de ‘S-22’ ne germent pas du tout en présence de Cd2+, même à faible concentration.
Abstract
Ten cultivars of tomato were subjected to different cadmium (Cd2+) concentrations, to find out their degree of tolerance towards these metal ions during the tomato ontogeny. Seeds of tomato cultivars (i.e. ‘K-25’, ‘K-21’, ‘NTS-9’, ‘Kaveri’, ‘NBR-Uday’, ‘Swarnodya’, ‘Sarvodya’, ‘NBR-Uttam’, ‘Malti’ and ‘S-22’) were soaked in 0, 50, 100 or 150 μM of Cd2+ for 0, 4, 8 or 12 h. Despite substantial varietal differences, increases in Cd2+ concentration and the soaking duration caused a linear decrease in growth and a reduced activity of catalase and peroxidase for all varieties. Variety ‘K-25’ was found to be the most resistant cultivar as it possessed maximum activity of antioxidative enzymes reflecting one of the possible reasons to overcome stress conditions. However, the seeds of ‘S-22’ could not germinate in the presence of even the lowest Cd2+ concentration.
1. Introduction
1Plants are good bioindicators as they play a significant role in food chain transfer and in defining environmental health (Gianazza et al., 2007). They are easy to grow and adaptable to environmental stress and also reflect toxicant damage in other organisms, such as animals (Minissi et al., 1997). Heavy metals in soil and plants have received increasing attention in recent years because of the harmful effects on dietary intake (Usha et al., 2002). Cadmium is one of the most toxic heavy metal pollutant in the environment, soil and water, and the sources of its contamination are fossil fuel, mining, waste water, household waste, municipal and industrial waste, use of metal containing pesticides and fertilizers in agricultural soil (Radotic et al., 2000; Akinola et al., 2006). Cadmium (Cd2+) is a non-essential and highly toxic metal ion that at higher concentrations inhibits growth and cell division (Liu et al., 2003). Moreover, species and cultivars display marked difference for Cd2+ tolerance (Wu et al., 2004; He et al., 2006; Hasan et al., 2009). Cd2+ causes severe morphological, physiological and biochemical effects on plants such as stunted growth (Ali et al., 2007; Hasan et al., 2008), leaf rolling and chlorosis (Ghani et al., 2007), interferes with uptake, transport and use of several elements (Cu, Zn, Ni, Pb and Cr) and that of water by plants (Das et al., 1997). At the cellular level Cd2+ interacts with biomolecules such as protein and nucleic acid, as it affects enzyme activities and causes alteration in the membrane permeability (Sanita di Toppi et al., 1999). Studies suggested that Cd2+ reduces ATPase activity of plasmalemma fraction (Astolfi et al., 2005), changes lipid composition by enhancing reactive oxygen species (ROS) production (Gomes-Junior et al., 2006). Moreover Cd2+ is an effective inhibitor of photosynthesis (Vessiliev et al., 1998; Hasan et al., 2009). A linear relationship between photosynthesis and inhibition of transpiration was observed, that suggests Cd2+ inhibited stomatal opening (Barcelo et al., 1986) and it also decreases the activity of several other enzymes (Hayat et al., 2007; Hasan et al., 2009).
2The present study was conducted with the aim to find out the degree of tolerance among ten cultivars of tomato by soaking (i.e. shotgun approach) their seeds in varied cadmium chloride concentrations for different durations, and to find out the most sensitive variety and most resistant one by assessing the plant through its growth and antioxidative system.
2. Materials and methods
2.1. Biological materials
3The seeds of Lycopersicon esculentum L. cv. 'K-25', 'K-21', 'NTS-9', 'Kaveri', 'NBR-Uday', 'Swarnodya', 'Sarvodya', 'NBR-Uttam', 'Malti' and 'S-22' were purchased from National Seed Corporation Ltd., New Delhi, India. The healthy seeds were surface sterilized with 5% hypochlorite and were soaked in 0, 50, 100 or 150 μM of Cd2+ for 0, 4, 8 or 12 h. These seeds were sown in earthen pots (6 inches diameter) filled with sandy loam soil and farmyard manure mixed in a ratio of 6:1 to create a nursery. At 15 day stage these seedlings were uprooted and transplanted to the pots, under similar conditions as that of nursery. The plants were grown in a net house under natural environmental conditions. The average temperature, humidity, and day/night photoperiods were 26 ± 2°C, 65 ± 5% and 14/10 h, respectively. Plants were watered at regular intervals. The plants were removed at 30 day stage along with the soil and dipped in a bucket, filled with water, to remove the adhering soil particles, ensuring the safety of roots. The plants were blotted and the lengths of roots and shoots were measured, followed by their subsequent weighing to record their fresh mass.
2.2. Estimation of nitrate reductase (NR) activity
4The activity of NR was measured following the method adopted by Jaworski (1971). The fresh leaf samples were cut into small pieces and transferred to plastic vials, containing phosphate buffer (pH 7.5) followed by the addition of potassium nitrate and isopropanol solutions. The reaction mixture was incubated at 30°C, for 2 h followed with the addition of N-1 naphthylethylenediamine dihydrochloride and sulphanilamide. The absorbance of the color was read at 540 nm and was compared with that of the calibration curve. The activity of NR (nmol NO2.g-1.h-1) was computed on fresh mass basis.
2.3. Assay of antioxidative enzymes
5Leaf tissue (0.5 g) was homogenized in 5 ml of 50 M phosphate buffer (pH 7.0) containing 1% insoluble polyvinylpyrolidone. The homogenate was centrifuged at 15,000 rpm for 10 min and the supernatant was used as the source of enzyme. The extraction was carried out at 4°C. Peroxidase and catalase were assayed following the procedure described by Chance et al. (1955). Catalase was estimated by titrating the reaction mixture, consisting of phosphate buffer (pH 6.8), 0.1M H2O2 enzyme extract and 2% H2SO4 against potassium permanganate. The reaction mixture for peroxidase consisted of pyrogallol phosphate buffer (pH 6.8), 1% H2O2 and enzyme extract. Change in absorbance, due to catalytic conversion of pyrogallol to perpurogallin, was noted at an interval of 20 s for 2 h at 420 nm. A control set was prepared by using distilled water instead of enzyme extract. The activity of superoxide dismutase was assayed using the method of Beauchamp et al. (1971). The reaction mixture contained 50 mmol phosphate buffer (pH 7.8), 13 mmol methionine, 74 µmol NBT, 2 µmol riboflavin, 0.1 mmol EDTA and 0-50 µl enzyme extract, and was placed under 15W fluorescent lamp. The reaction was started by switching on the light and was allowed to run for 10 min. The reaction was stopped by switching off the light. Fifty per cent inhibition by light was considered as one enzyme unit.
2.4. Statistical analysis
6The experiment was conducted according to simple randomized block design. A total of ten replicates for each treatment were taken. Treatment means were compared by analysis of variance using SPSS (SPSS, Chicago, IL, USA). Least Significance Difference (LSD) was calculated at the 5% level of probability.
3. Results
3.1. Root length
7The observations shown in table 1 clearly indicate that the Cd2+ treatment resulted in a significant decrease in the length of roots of the resulting plants. However, the response to the Cd2+ stress showed significant variation when the varieties were taken into consideration. The highest concentration of Cd2+ (150 μM) supplied for any of the duration (4, 8 or 12 h) of seed soaking caused the maximum reduction in the root length. The varieties which were severely affected by the Cd2+ were 'Sarvodya', 'NBR-Uttam' and 'Malti'. These varieties also experienced a significant damage at the lowest concentration (50 μM) supplied for minimum duration (4 h). Here the root length was 10.2%, 27.1% and 20.6% lower than the respective controls. The varieties 'K-25', 'K-21' and 'NTS-9' showed the maximum resistance to Cd2+, however the higher concentration (150 μM) of Cd2+ fed for 12 h reduced the root length by 42.5%, 46.1% and 54.1% compared to their controls. The pattern of resistance exhibited by three varieties were 'K-25' > 'K-21' > 'NTS-9'. The varieties 'Kaveri', 'NBR-Uday' and 'Swarnodya' showed maximum inhibition of root elongation at 100 μM Cd2+ concentration, soaked for 12 h. However, treating the seeds for shorter time duration (4 or 8 h) caused a lesser toxicity than 12 h soaking duration.
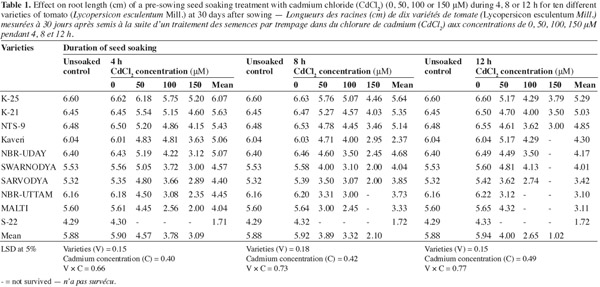
3.2. Root fresh mass
8The shotgun approach of Cd2+ (50, 100 or 150 μM) significantly reduced the root fresh mass and all the varieties differed significantly in their response to the metal concentrations (Table 2). The treatment combination of the highest concentration (150 μM) of Cd2+ and longest soaking duration (12 h) was most toxic, which was tolerated only by the varieties 'K-25', 'K-21' and 'NTS-9'. In response to this treatment combination these varieties exhibited 32.7%, 28.1% and 36.6% decrease, compared to their respective control. The combined effect of 50 μM of Cd2+ and 4 h soaking duration was the least damaging. The varieties 'Sarvodya', 'NBR-Uttam' and 'Malti' were so sensitive that even at the lowest level of Cd2+ (50 μM) and minimum soaking duration (4 h) showed 14.2%, 16.8% and 13.6% decrease, over their respective controls. The variety 'S-22' was the most sensitive that could not survive in the presence of any of the cadmium concentration.
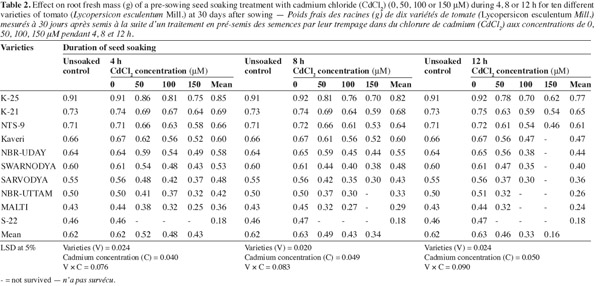
3.3. Shoot length
9The data in table 3 indicate that the pre-sowing seed soaking treatment (shotgun approach) caused a significant decrease in the shoot length of the resulting plants. The decrease was proportionate to the concentration of the metal as well as to the duration of soaking. The treatment for the longest duration (12 h) caused the maximum damage. The variety 'S-22' could not survive in the presence of any of the Cd2+ concentration, whereas the varieties 'NBR-Uttam' and 'Malti' could not survive to the pre-sowing soaking with Cd2+ 100 and/or 150 μM for 12 or 8 h, respectively. The variety 'K-25' was the most resistant that exhibited the minimum damage in response to the stress. This variety showed 8.2%, 12.6%, 21.4%; 11.4%, 15.5%, 25.4% and 17.2%, 26.0% and 41.3% reduction in response to 50, 100 or 150 μM of Cd2+ supplemented for 4, 8 or 12 h, respectively. The variety 'K-21' showed a response comparable to that of the most resistant one ('K-25'). The varieties 'Kaveri', 'NBR-Uday' and 'Swarnodya' were moderately affected by the cadmium. However, they could not resist the pre-sowing soaking in 150 μM of Cd2+ for 12 h. The varieties 'Sarvodya', 'NBR-Uttam' and 'Malti' experienced a severe damage and among these three varieties the order of susceptibility/sensitivity was 'Sarvodya' > 'NBR-Uttam' > 'Malti'.
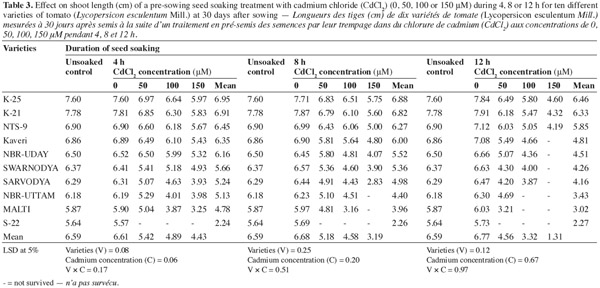
3.4. Shoot fresh mass
10The plants raised from the seeds soaked in different concentrations of Cd2+ for 12 h duration exhibited maximum inhibition in shoot fresh mass production (Table 4). The varieties 'K-25', 'K-21' and 'NTS-9' showed least inhibition to 12 h soaking in 150 μM of Cd2+ where the values were 51.8%, 59.6% and 55.2% below their respective controls. However the other varieties could not survive in higher concentration of the metal. The varieties 'Kaveri', 'NBR-Uday', 'Swarnodya' and 'Sarvodya' were moderately affected by Cd2+ and showed 31.6%, 31.1%, 45.6% and 44.1%; 49.5%, 50.0%, 52.7% and 63.9%, decrease compared to their respective controls when Cd2+ (150 μM) was given for 4 or 8 h respectively.
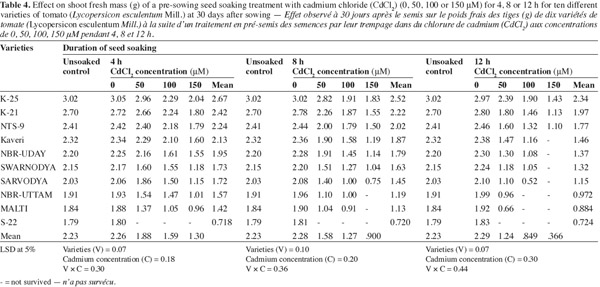
3.5. Nitrate reductase (NR) activity
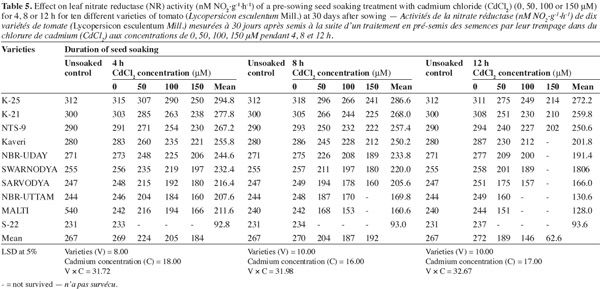
11Table 5 shows that the activity of NR significantly decreased with the increasing concentration of Cd2+ as well as duration of soaking. The lowest concentration of Cd2+ (50 μM) was least toxic for all the varieties. The other two concentrations (100 and 150 μM) showed a higher magnitude of toxicity, which shifted from variety to variety. Among the varieties 'K-25', 'K-21' and 'NTS-9' were comparatively resistant in terms of the NR activity. The varieties 'Kaveri', 'NBR-Uday' and 'Swarnodya' were neither too resistant nor too sensitive. In these three varieties seed soaking in 100 μM of Cd2+ for 4, 8 or 12 h inhibited the activity of NR by 16.9%, 20.2%, 26.1; and 17.5%, 24.3%, 27.7%; and 14.4%, 23.3%, 26.7% compared to their respective control. The varieties 'Sarvodya', 'NBR-Uttam' and 'Malti' were highly sensitive to the cadmium ion.
3.6. Antioxidative enzyme activities
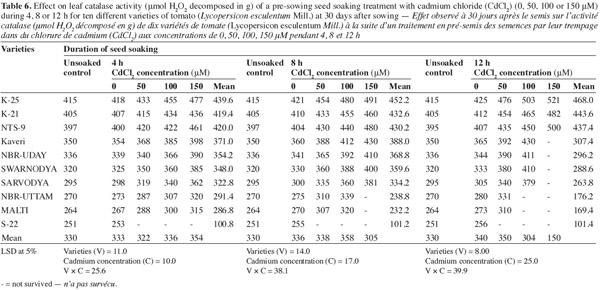
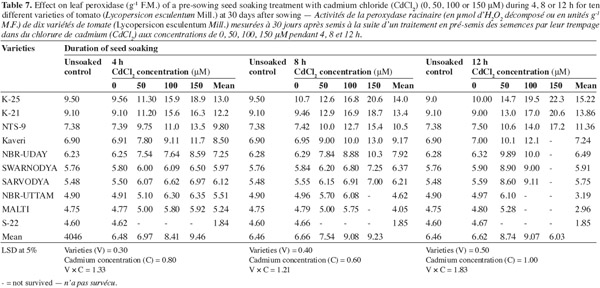
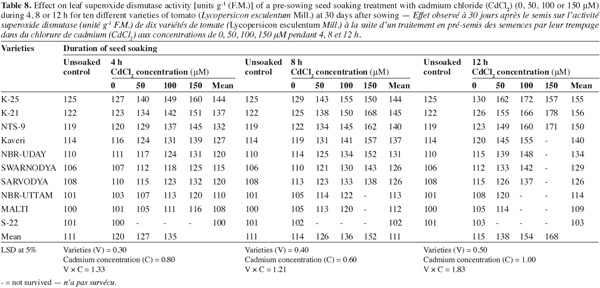
12The activities of catalase (CAT), peroxidase (POX) and superoxide dismutase (SOD) were positively affected by the Cd2+ as well as its duration of soaking in all the varieties (Tables 6, 7, 8) and this increase was directly proportional to the both treatment components (concentration and duration of soaking). The activity of the enzymes was maximum in variety 'K-25', at all the Cd2+ levels. The plants of 'K-25' raised from the seeds exposed to 50, 100 or 150 μm of Cd2+ for 12 h possessed the activity of the enzymes which was 12.0%, 18.3% and 22.5% higher for catalase and 47.5%, 95.0% and 123.9% higher for peroxidase over the control, respectively. The varieties 'Sarvodya', 'NBR-Uttam' and 'Malti' possessed least value among all the varieties. The treatment combination of Cd2+ (100 μM) with 8 h soaking duration elevated the level of the enzymes, which were 20%, 23.2% and 18.5% higher for catalase, 24.5%, 22.5% and 20.0% higher for peroxidase and 24.6%, 32.3% and 20.7% higher for SOD in the varieties 'K-25', 'NBR-Uttam' and 'Malti', respectively. This increase was maximum for these three varieties among all the Cd2+ doses and the soaking durations. The varieties 'Kaveri', 'NBR-Uday' and 'Swarnodya' showed an intermediate response, which possessed the maximum activities of the enzymes at 100 μM Cd2+ supplied for 12 h treatment. The trend followed by different treatment durations was 12 h > 8 h > 4 h.
4. Discussion
13Plants have a well equipped natural antioxidative defense system to maintain the redox equilibrium. In non-stress conditions, ROS and other oxidants are balanced by the antioxidative defense system, which is composed of enzymes (CAT, POX and SOD) and metabolites (tocopherol, ascorbate and proline). However under stress this redox equilibrium is disturbed and the increased ROS accumulation causes a specific oxidative stress response (Cuypers et al., 2001). In the present study, the increase in the activity of SOD and CAT is a consequence of dis-equilibrium, provoked by the increased availability of the Cd2+. Previous studies have also shown that Cd2+ is able to induce oxidative stress, which provokes an increase in metabolite content as well as the activation of several antioxidative enzymes (Smeets et al., 2007; Hasan et al., 2008). This increase in SOD and CAT activities with the increasing concentration of cadmium (Tables 6, 7, 8) indicated that these cultivars had the capacity to adapt to different Cd2+ concentrations by developing an antioxidative defence system. However the varieties differed widely in their ability to tolerate Cd2+ stress. The variety 'S-22' could not tolerate the presence of even the lowest concentration of Cd2+. 'Sarvodya', 'NBR-Uttam' and 'Malti' are also severely affected by cadmium, however 'Kaveri', 'NBR-Uday' and 'Swarnodya' experienced moderate damage. Moreover, varieties 'K-25', 'K-21' and 'NTS-9' showed maximum resistance to cadmium concentration as they possessed highest level of the antioxidative enzymes.
14The activity of NR enzyme decreased with the increasing concentration of cadmium where Cd2+ is known to restrict the uptake of nitrate by the roots by damaging the normal function of plasma-membrane bound proton pump (Obata et al., 1996) and the fluidity of membrane (Meharg, 1993). Therefore, the restricted supply of the NR inducer and the substrate hamper the activity of NR. Moreover, toxicity generated by Cd2+ impaired root and shoot growth in all the tomato cultivars. However, it should be noted that although both root and shoot growth were affected by Cd2+ treatment but the decrease in the root length and its fresh mass was stronger than in shoot. The primary reason for high root sensitivity to Cd2+ might be related to the fact that root is the first organ exposed to Cd2+ and hence accumulates metal at much higher concentrations than the shoot (Tiryakioglie et al., 2006). Secondly Cd2+ is known to cause physiological drought by altering plant water balance, nutrient uptake and permeability of plasma membrane (Barcelo et al., 1990; Hernandez et al., 1996) which in turn affect cell enlargement and resulted in stunted growth. The extent of changes in growth attributes and enzymatic activity revealed the existence of great varietal differences throughout the tomato ontogeny for Cd2+ tolerance.
15Acknowledgements
16This work was funded by University Grants Commission [Project No 32-403/ 2006 (SR)], New Delhi, India.
Bibliographie
Akinola M.O. & Ekiyoho T.A., 2006. Accumulation of lead, cadmium and chromium in some plants cultivated along the bank of river Rabillia at Odonla area of Ikirodu, Lagos state, Nigeria. J. Environ. Biol., 27, 597-599.
Ali B., Hayat S. & Ahmad A., 2007. 28-Homobrassinolide ameliorates the saline stress in chickpea (Cicer arietinum L.). Environ. Exp. Bot., 59, 217-233.
Astolfi S., Zuch S. & Passera C., 2005. Effect of cadmium on H+-ATPase activity of plasma membrane vesicles isolated from roots of different S supplied maize (Zea mays L.) plants. Plant Sci., 169, 361-368.
Barcelo J., Poschenrieder C., Andreu I. & Gunse B., 1986. Cadmium induced decrease of water stress resistance in bush bean plants (Phaseolus vulgaris L. cv. 'Contender'). I. Effect on water potential, relative water content and cell wall elasticity. J. Plant Physiol., 125, 17-25.
Barcelo J. & Poschenrieder C., 1990. Plant water relations are affected by heavy metal stress: a review. Plant Nutr., 13, 1-37.
Beauchamp C.O. & Fridovich I., 1971. Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Anal. Biochem., 44, 276-287.
Chance B. & Maehly A.C., 1955. Assay of catalase and peroxidase. Methods Enzymol., 2, 764-775.
Cuypers A., Vangronsveld J. & Clijsters H., 2001. The redox status of plant cells (asa and gsh) is sensitive to zinc imposed oxidative stress in roots and primary leaves of Phaseolus vulgaris. Plant Physiol. Biochem., 39, 657-669.
Das P., Samantaray S. & Rout G.R., 1997. Studies on cadmium toxicity in plants. A review. Environ. Pollut., 98, 29-36.
Ghani A. & Wahid A., 2007. Varietal difference for cadmium induced seedling mortality and foliar toxicity symptoms in mungbean (Vigna radiate). Int. J. Agric. Biol., 9, 555-558.
Gianazza E. et al., 2007. Growth and protein profile changes in Lepidium sativum L. plantlets exposed to cadmium. Environ. Exp. Bot., 59, 179-187.
Gomes-Junior R.A. et al., 2006. Antioxidant metabolism of coffee cell suspension cultures in response to cadmium. Chemosphere, 65, 1330-1337.
Hasan S.A., Hayat S., Ali B. & Ahmad A., 2008. 28-Homobrassinolide protect chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidant. Environ. Pollut., 151, 60-66.
Hasan S.A., Hayat S. & Ahmad A., 2009. Screening of tomato (Lycopersicon esculentum) cultivars against cadmium through shotgun approach. J. Plant Interact., 4, 187-201.
Hayat S., Ali B., Hasan S.A. & Ahmad A., 2007. Brassinosteroids enhanced the level of antioxidant under cadmium stress in Brassica juncea. Environ. Exp. Bot., 60, 33-41.
He J. et al., 2006. Genotypic variation in grain cadmium concentration of lowland rice. J. Plant Nutr. Soil Sci., 169, 711-716.
Hernandez L.E., Carpena-Ruiz R. & Garate A., 1996. Alteration mineral nutrition of pea seedlings exposed to cadmium. J. Plant Nutr., 19, 1581-1598.
Jaworski E.G., 1971. Nitrate reductase assay in intact plant tissue. Biochem. Biophys. Res. Commun., 43, 1274-1279.
Liu D.H., Jiang W.S. & Gao X.Z., 2003. Effects of cadmium on root growth, cell division and nuclei in root tip cells of garlic. Biol. Plant., 47, 78-83.
Meharg A.A., 1993. Integrated tolerance mechanisms constitutive and adaptive plant response to elevated metal concentrations in the environment. Plant Cell Environ., 17, 989-993.
Minissi S. & Lombi E., 1997. Heavy metal content and mutagenic activity, evaluated by Vicia faba micronucleus test, of Tiber river sediments. Mutat. Res., 393, 17-21.
Obata H., Inone N. & Umebayshi M., 1996. Effect of cadmium on plasma membrane ATPase from plant root differing in tolerance to cadmium. Soil Sci. Plant Nutr., 42, 361-366.
Radotic K., Ducic T. & Mutavdzic D., 2000. Changes in peroxidase activity and iso-enzymes in spruce needles after exposure to different concentrations of cadmium. Environ. Exp. Bot., 44, 105-113.
Sanita di Toppi L. & Gabbrielli R., 1999. Response to cadmium in higher plants. Environ. Exp. Bot., 41, 105-130.
Smeets K. et al., 2007. Cadmium induced transcriptional and enzymatic alterations related to oxidative stress. Environ. Exp. Bot., 63, 1-8.
Tiryakioglie M. et al., 2006. Antioxidant defence system and cadmium uptake in barley genotype differing in cadmium tolerance. J. Trace Elem. Med. Biol., 20, 181-190.
Usha M., Venugopoj V.K. & Saraswathi P., 2002. Cadmium content of plants as affected by soil application of cadmium and farmyard manure. J. Trop. Agric., 40, 70-80.
Vessiliev A., Berora M. & Zlateve Z., 1998. Influence of Cd2+ on growth, chlorophyll content and water relations in young barley plant. Biol. Plant., 41, 601-606.
Wu F., Wu H., Zhang G. & Bachir D.M.L., 2004. Differences in growth and yield in response to cadmium toxicity in cotton genotypes. J. Plant Nutr. Soil Sci., 167, 85-90.

