Contamination of poultry flocks by the human pathogen Campylobacter spp. and strategies to reduce its prevalence at the farm level
Gembloux Agricultural University – FUSAGx. Unité de Zootechnie. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: vandeplas.s@fsagx.ac.be
Gembloux Agricultural University – FUSAGx. Unité de Zootechnie. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Gembloux Agricultural University – FUSAGx. Unité de Bio-industries. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Gembloux Agricultural University – FUSAGx. Unité de Zootechnie. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Gembloux Agricultural University – FUSAGx. Unité de Bio-industries. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Gembloux Agricultural University – FUSAGx. Unité de Zootechnie. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Received on July 28, 2007, accepted on January 15, 2008
Résumé
Contamination des élevages de volailles par l'agent pathogène humain Campylobacter spp. et les stratégies pour réduire sa prévalence au niveau des élevages. Campylobacter spp. est une bactérie entérique pathogène pour l'homme qui contamine fréquemment les élevages de volailles. La consommation de produits d'origine aviaire peut ainsi entraîner une gastro-entérite bactérienne aiguë appelée campylobactériose, dont la prévalence augmente depuis une dizaine d'années en Europe. Cette synthèse bibliographique résume les données épidémiologiques sur Campylobacter, les facteurs de risque de contamination dans les élevages de volailles et les stratégies envisagées pour lutter contre ce pathogène.
Abstract
Enteric Campylobacter spp. bacteria are human pathogens that frequently contaminate poultry flocks. Consumption of products from poultry origin may then lead to acute bacterial enteritis called campylobacteriosis of which prevalence is increasing for about ten years in Europe. This review summarizes Campylobacter epidemiological data, risk factors for contamination in poultry flocks and conceivable strategies to control this pathogen.
1. Introduction
1Even though pig meat, with a worldwide share of about 50%, is by far the most preferred meat by European Union (EU) consumers, the poultry meat production has shown the more favourable progression, with a mean annual increase rate of 2.5% from 1992 to 2002 (European Commission, 2005); it recorded a worldwide share of around 26% in 2005 (i.e. 70 million tons). Moreover, world poultry production and consumption are predicted to still increase over the next seven years by more than 20%, i.e. an average annual growth of approximately 2.5%. This expansion is mainly driven by low poultry meat production costs (relative to beef and pork), strong consumer preference, increased use in food preparations and a high demand for low price proteins on the worldwide market. Furthermore, poultry meat has generally benefited from the Bovine Spongiform Encephalopathy and Foot-and-Mouth Disease outbreaks, in the past few years.
2Nevertheless, the avian sector has also faced several sanitary problems to which media coverage was given since a few years. In June 1999, the dioxin crisis in Belgium was caused by dioxin-contaminated food components. The widespread avian influenza epidemic since 2003 has completely disrupted production and trade in many areas of the world, notably South East Asia but also the US and Canada. Beside these time-limited outbreaks, poultry production is confronted with a major permanent problem that is much less known. Poultry remains an important vehicle of bacterial human pathogens, leading to foodborne diseases by contaminated poultry products consumption and incriminated by epidemiological reports all over the world. The most reported pathogen agent is Salmonella spp. but, over the last three decades, Campylobacter spp. has emerged as an increasing concern all over the world. It is a major cause of a human acute bacterial enteritis called campylobacteriosis (van Vliet et al., 2001). Unlike Salmonella, Campylobacter is mainly a problem in extensive poultry production, with up to 100% of organic farms being contaminated (Engvall, 2001). This review will focus on prophylactic measures and curative treatments developed to reduce the incidence of Campylobacter infections in broiler flocks, at the primary production level.
2. Campylobacteriosis
3Campylobacter spp. have been recognized as a cause of diarrhoeal illness in human since 1972. The Campylobacter species associated with food poisoning include Campylobacter jejuni, Campylobacter coli, Campylobacter lari and Campylobacter upsaliensis. C. jejuni is predominant while C. coli accounts for most of the remainder (Hariharan et al., 2004). According to a French study, C. jejuni was found in ca. 68% of the isolates from human campylobacteriosis cases (Dachet et al., 2004).
4Dose-response studies have shown that ingestion of about 10 (Ridley et al., 2004) to 500 cells (Rosenquist et al., 2003) could already be sufficient to infect the human host. Pathogens invade epithelial cells in the ileum and large intestine thanks to chemotaxis and high motility, which causes inflammatory diarrhoea with usually moderate uncharacteristic symptoms (van Vliet et al., 2001).
5Complications following Campylobacter infection are uncommon, but an association with certain neurological disorders is noteworthy (Butzler, 2004). It is estimated that one on 1000 Campylobacter infections lead to the Guillain-Barré syndrome, an acute demyelinating disease characterized by muscular paralysis and leading to 2-3% mortality (Allos, 1997). This syndrome is usually confined to very young or elderly patients or to immuno-compromised suffering people (Altekruse et al., 1999).
2.1. Public health impact
6In most industrialized countries, the reported incidence of campylobacteriosis has increased during the last decade. In 2004, a total of 183,961 cases of laboratory confirmed campylobacteriosis were recorded in the EU-25, compared to 120,462 cases in 1999. The overall incidence was 47.6 per 100,000 population, which is slightly higher than for Salmonella (42.2). This makes Campylobacter the most commonly reported gastrointestinal bacterial pathogen in humans in the EU (EFSA, 2006).
7On the other hand, in Belgium, Campylobacter infections represent the second cause of foodborne illness, just after Salmonella (CSH, 2005), with an estimated annual number of cases of about 65 per 100,000 population (Ducoffre, 2006). In 1984, the sentinel laboratories network recorded only just 1,703 cases of infection. During the nineties, campylobacteriosis incidence has continually increased to reach 7,473 cases in 2000, although the increase in the number of Campylobacter infections cases until 1996 could mainly be attributed to problems at the surveillance level (van Dessel, 2005). From 2000 to 2003, the illness incidence was reduced. However, it tends to increase again since 2004, without reaching the levels observed in 2000. It is usually estimated that 90% of Campylobacter contamination are due to meat consumption and 80% specifically come from poultry meat. Nevertheless, the rise of Campylobacter incidence observed for more than 20 years may also be partly due to an increase of the poultry meat consumption during this period, rather than only an increase in the proportion of contaminated poultry (ICGFI, 1999).
8The high incidence of Campylobacter spp. diarrhoea, its duration and possible sequelae, make campylobacteriosis important from a socio-economic perspective.
2.2. Economic and social importance
9Campylobacteriosis affects each year a significant proportion of humans worldwide. Foodborne gastrointestinal diseases are major burdens on society causing considerable suffering and loss of productivity. Besides the discomfort felt by sick people, these infections have major economic repercussions by direct illness costs (laboratory diagnosis, consultations, medical cares, hospitalization, etc.) and indirect costs (work inefficacy, days lost work, etc.) (ICGFI, 1999; Bogaardt et al., 2004). In The Netherlands, the economic costs of campylobacteriosis are estimated at 21 million € per year for a population of 16 million (Mangen et al., 2005a). Costs for campylobacteriosis are difficult to estimate because of differences in the simulation models used. Differences in one case cost according to two recent studies, i.e. 465 € in the United Kingdom (Roberts et al., 2003) and 77 € in The Netherlands (van den Brandhof et al., 2004) show the complexity of estimating these costs.
3. Campylobacter and the animal hosts
3.1. Characteristics of Campylobacter species
10Campylobacter species are Gram-negative, non-sporing, slender, helical or curved rods. In culture exposed to environmental stresses such as oxygen, the cells can change to spherical or coccal forms. Their polar flagellum conferred them a characteristic darting, and corkscrew-like motility. They are unable to oxidize or ferment carbohydrates but they reduce nitrate and nitrite. C. jejuni is the most frequent of the four thermophilic Campylobacter species that is isolated from human, and is one of 20 species and subspecies within the genus Campylobacter and family Campylobacteraceae. The other thermophilic species include C. coli, C. upsaliensis and C. lari. The thermophilic species are characterized by their ability to grow best between 37 and 42°C and their inability to grow at 25°C. For the most part, Campylobacter require a microaerobic atmosphere for growth and can be very difficult to work with in laboratory settings, due to their fragile nature. However, isolates are extremely diverse, compared to some other enteropathogens. There are more than 60 different heat-stable serotypes, more than 100 heat-labile serotypes, differences in adherence properties, invasive properties, toxin production, serum resistance, colonization potential, aerotolerance and temperature tolerance. This diversity may be partly explained by the genomic plasticity of Campylobacter. The high levels of multiple-strain colonization and high frequency of incidence in mammals and birds mean there is substantial opportunity for exchange of genetic material and explain the ability of bacteria to survive in extreme conditions.
3.2. Transmission vectors
11Campylobacter, as Salmonella, may be carried asymptomatically, as commensal organism, in the alimentary tract of all warm-blood animals. Because this pathogen can be transferred from animals to man, Campylobacter is considered as a zoonotic bacteria (WHO, 2000). Human infection may be caused by direct contact with contaminated animals or animal carcasses. In the case of domesticated animals as cattle, sheep, goats, pigs and especially poultry, pathogens can spread via the slaughter process to raw and finished products. Campylobacter may also be transferred to humans by consumption of undercooked or recontaminated meat, or the handling of raw products (Bryan et al., 1995). It is noteworthy that, despite the meat importance, this does not represent the only food vehicle for Campylobacter and large campylobacteriosis outbreaks are usually associated with contaminated drinking water or raw or contaminated milk (Friedman et al., 2004). According to Mead et al. (1999), food contamination could originate for 80% of Campylobacter infection cases. Regarding inter-humans transmission, it is considered to be relatively exceptional (Adak et al., 1995; Studahl et al., 2000; Winquist et al., 2001).
12As mentioned above, the most important Campylobacter species known to cause human illness are the thermophilic species: C. jejuni, C. coli and C. lari. Birds, especially breeding poultry, appear to be the main reservoir for these pathogens, their internal temperature of 41-42°C being favourable for thermophilic Campylobacter proliferation (Hariharan et al., 2004). Therefore, foods of poultry origin have been identified as significant sources of human campylobacteriosis. In Belgium, more than 40% of campylobacteriosis cases would be associated to poultry meat consumption (Vellinga et al., 2002). In 1999, the finding of dioxin-contaminated feeding stuffs caused the Belgian authorities to withdraw all poultry meat and eggs from the market. The estimated reduction in campylobacteriosis cases during the following crisis months was 40% without any other explicative event that happened in this period. Furthermore, the Belgian poultry reintroduction 4 weeks later on the market lead back to the previous campylobacteriosis incidence situation.
13Another factor that could link together chicken consumption and human pathogen acquisition is the important similarity between human and poultry serotypes (Petersen et al., 2001). Nevertheless, it is advisable to relativize this affirmation. Several studies have shown that some Campylobacter strains colonizing chicken are not human pathogens while some human isolated strains are unable to colonize poultry (Corry et al., 2001).
3.3. Poultry colonisation
14Colonized chickens usually show no observable clinical symptoms of infection even when young animals are exposed to high doses under experimental conditions (Newell et al., 2003). Corry et al. (2001) reported furthermore possible observation of enteritis and hepatitis symptoms or excessive mortality of very young chicks.
15Experimentally, the dose of viable C. jejuni required to colonize chicks and chickens can be as low as 40 cfu even if numbers from 104 to 107 cfu can be frequently found in literature (Udayamputhoor et al., 2003; Bjerrum, 2005). Furthermore, a strain variability concerning the ability to colonize the chicken digestive tract is also reported (Stas et al., 1999). Infection pattern in poultry is also age-dependent. Actually, Campylobacter is not detected in chicks less than 2 to 3 weeks of age under commercial broiler production conditions, and that may be related to high levels of circulating Campylobacter-specific maternal antibodies in young chickens, which gradually decrease to undetectable levels at 2 to 3 weeks of age (Sahin et al., 2003).
16In chickens, C. jejuni colonizes the mucus overlying the epithelial cells primarily in the cæca and the small intestine but may also be recovered from elsewhere in the gut and from spleen and liver (Beery et al., 1988; Achen et al., 1998). The microorganism remains in the intestinal lumen at the crypts level, without adhesion. Once colonization is established, Campylobacter can rapidly reach extremely high numbers in the cæcal contents, from 105 to 109 cfu.g-1 of content (Schoeni et al., 1992; Achen et al., 1998; Woodall et al., 2005).
3.4. Poultry flock prevalence
17The reported proportion of Campylobacter-positive broiler chickens flocks (the flock prevalence) varies between countries, ranging from 5% to more than 90% (EFSA, 2005), as summarized in table 1. This apparent variation in the flock prevalence may reflect significant differences between countries, but is affected by sampling time, during the breeding period, and age and type of birds (conventional, free-range, organic). The method of detection (direct plating vs. enrichment), and type of sample (cæcal contents, fresh droppings, litter) also influence the detection of Campylobacter spp. (Jørgensen et al., 2002; Oyarzabal et al., 2005).
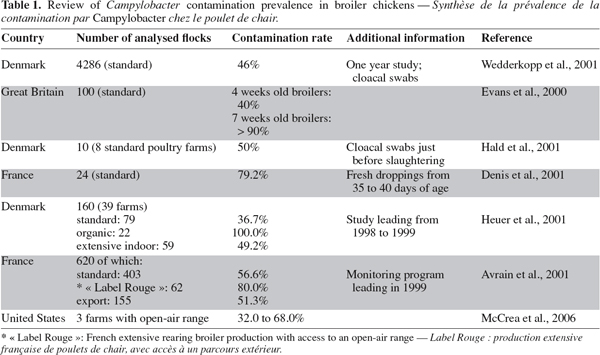
18Table 2 shows more frequently contaminated broilers flocks in extensive rearing systems, especially those allowing access of the birds to an open-air range (organic, “ Label Rouge ”, etc.). According to Heuer et al. (2001), the higher contamination rate of free-range broiler production could be explained by an unimpeded access to soil and water in the open-air range, a longer rearing period and differences in chicken host lineages.
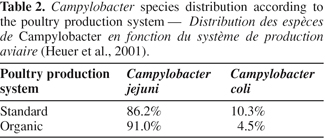
19Distribution of Campylobacter species is also dependent on the rearing system, as shown in table 2 and on the country. C. jejuni is the most frequently isolated species in poultry farms, whatever the production system. C. coli is less common although it is predominant in some EU Member States as Slovenia (Zorman et al., 2006). Moreover, this species tends to become more frequent from a few years (Desmonts et al., 2004). Finally, although relatively scarce, C. lari can also be isolated from poultry samples (Denis et al., 2001; Hald et al., 2001).
3.5. Risk factors for contamination at farm level
20Although several risk factors for infection of broilers with Campylobacter spp. have been identified, knowledge about the various routes by which flocks become infected and their relative importance is still incomplete. The risk factors that have repeatedly been identified are summarized below.
21Vertical Transmission. Campylobacter can be present in the poultry reproductive system. Nevertheless, several authors dismiss the assumption that vertical transmission is a major source of pathogen transmission (van de Giessen et al., 1992; Jacobs-Reitsma, 1995; Chuma et al., 1997; Sahin et al., 2003). The main reasons proposed are a poor Campylobacter survival on eggshells and inability to penetrate, to survive and to multiply into eggs in natural conditions. Meanwhile, some evidence could be found for vertical transmission of Campylobacter (Callicott et al., 2006).
22Horizontal transmission from the outer environment:
23Flock-to-flock transmission and litter role. Campylobacter transmission from a contaminated flock to the following flock seems to be not very important. Campylobacter is actually sensitive to detergents and disinfectants as well as dry conditions found in the poultry house during the empty period, although a little number of bacteria could survive during flocks intervals (Evans et al., 2000; Petersen et al., 2001).
24Dry and aerobic conditions of clean fresh litter are considered harmful to C. jejuni as reported by Newell et al. (2003) and Hutchinson et al. (2005). On the other hand, litter can be contaminated by broiler fæcal droppings and then favours pathogen transmission through the flock. Nevertheless, in Belgium, the problem of litter as contamination vector is not recognized because houses are generally cleaned and disinfected and the litter is replaced between two subsequent flocks.
25Dirty contaminated litter spread over the land can scatter the microorganism in the environment. Contaminated sewage are attractive for wild birds and insects that can be infected and then become Campylobacter vectors (Jones, 2001; Stanley et al., 2003).
26Environment and open-air range. Campylobacter is able to survive in the house surroundings soil (Bull et al., 2006) and the farmer can therefore act as a pathogen vector for Campylobacter entrance in the broiler house, for instance via farmer's boots (Newell et al., 2003). The open-air range to which broilers have access in free-range poultry production could also be a major environmental source for flock contamination. When Campylobacter is isolated from the open-air range soil or from stagnant water, before the birds go out, the precedent flock may be responsible for the contamination. Furthermore, even if the open-air range seems to be Campylobacter-free, it is possible that Campylobacter is present under a Viable but Non Culturable (VNC)-form. Induced through cell stress, particularly in drastic soil conditions (Rivoal et al., 2005), VNC represents a resting or dormant stage extremely difficult to detect, which could return to virulent form under appropriate conditions (Moore, 2001).
27This transmission route seems yet not negligible as shown by Rivoal et al. (2005). Among seven poultry farms sampled from 1996 to 1999, four had got information about the respect of strict biosecurity measures aimed at preventing the introduction of Campylobacter into flocks. In these farms, flock contamination appeared from six weeks of age, at the time of outdoor rearing period. In the three farms for which no biosecurity measures were applied, broiler contamination appeared from two weeks of age, then before the access to the open-air range. The influence of the open-air range on the contamination is yet not fully understood. According to a recent study of the " Agence Française de Sécurité Sanitaire des Aliments " (AFSSA), access to an open-air range is not the main Campylobacter contamination route of free-range broiler production. Among 73 farms, close to three quarters of flocks were contaminated before access to the open-air range. At the end of the rearing period, all the flocks were Campylobacter-positive, and concerned mainly C. jejuni (Huneau-Salaün et al., 2005).
28Feed and drinking water. Campylobacter can not survive in poultry feed because of a too low moisture rate (Altekruse et al., 1999; Newell et al., 2003) although feed, as drinking water, can be contaminated by fæcal droppings during the rearing period and can serve as transmission route (Bull et al., 2006). On the other hand, water can be a real contamination vector for broiler chickens, as shown by Shanker et al. (1990) who succeeded to infect broilers with artificially contaminated water.
29Air. Campylobacter can be isolated from air, both in the broiler house and from the house surroundings (Bull et al., 2006). Pathogens are entrapped in aerosols or dust (Berndtson et al., 1996), which could then be considered as pathogen transmission vector (Berrang et al., 2003). Nevertheless, there is an assumption that C. jejuni cannot survive for long period within the dehydrating conditions of dust. Saleha (2004) failed to isolate Campylobacter from 114 swabs samples of the walls, floors and dust from a total of 19 Malaysian chicken houses. According to Newell et al. (2003), the location of ventilation fans can affect the risk of flock positivity, and the use of air conditioning increased this risk.
30Wild and domesticated animal. Because of the pathogen unability to multiply outside warm-blooded animals, farm animals like poultry, cattle, pigs, sheep and goats (Oporto et al., 2007), pets like cats and dogs, and wild animals like birds and rodents, are often considered as important Campylobacter reservoir. Although the broiler contamination by wild and domesticated animals does not seem to be direct, except for the free-range broiler productions, animal bearing and fæcal shedding of the bacteria have been actually pointed out in several studies (Stanley et al., 2003; Hutchinson et al., 2005) as a potential origin of environmental contamination (Nicholson et al., 2005).
31Because of their microaerophilic metabolism and their inability to growth at temperatures below 31°C, the presence of Campylobacter in streams, rivers and ponds can then be taken as a sign of recent fæcal contamination by livestock or wild animal (Friedman et al., 2000) but can last up to four months (Rollins et al., 1986; Hazeleger et al., 1998). Campylobacter serotypes and genotypes are not systematically corresponding, and the wild animals role should be relatively limited after all (Petersen et al., 2001).
32Insects. Some authors have made the assumption that insects like flies could play a part in the Campylobacter epidemiology (Skov et al., 2004). They could act as mechanical vectors, transmitting pathogens from reservoir environment or animals to broiler flocks (Ekdahl et al., 2005; Nichols, 2005). Nevertheless, insects seem to be contaminated by the broilers and may act as pathogen vector only afterwards.
4. European legislation
33Following several different sanitary crises, the Community legislation on food hygiene has been progressively restructured and strengthened in order to establish a coherent and consistent network of hygiene rules based on an integrated approach covering the whole food chain " from stable to table ". The new legal instrument on food hygiene ensures that the Member States comply with the Good Hygiene/Farming Practices (GHP) in livestock production, as applied in Belgium. The reflection of the Commission on the new approach to food safety, covering the entire production chain of all foodstuffs, both of animal and of plant origin, resulted in the adoption of the White Paper on food safety in January 2000.
34The main principles depicted in the White Paper are: the assurance of a high standard of food safety; the responsibility for food safety primarily upon food businesses, including feed manufacturers and farmers; the assurance of a " farm to table " policy; the possibilities for traceability and transparency and the possibilities to take into account the precautionary principle and other legitimate factors, where appropriate.
35These rules would be essential to prevent contamination and spread of zoonotic agents in farms and are the basis of the European legislation concerned with the monitoring and control of zoonoses and zoonotic agents at the primary production, transformation and distribution levels. With the aim of decreasing the incidence of zoonoses in humans, of improving the control of zoonoses in the primary production and of strengthening the collection of relevant data to support risk assessment activities and risk management decisions, the European Union has decided more recently to integrate and to standardize the different national monitoring and survey plans by the establishment of the Directive 2003/99/CE and the Regulations (EC) n°2160/2003 and n°1003/2005.
36The specific purpose of these Regulations is " to ensure that proper and effective measures are taken to detect and to control Salmonella and other zoonotic agents, particularly at the level of primary production, in order to reduce their prevalence and the risk they pose to public health ". Salmonella is the primary zoonotic agent targeted at primary production as it represents an important burden to public health. From 2010, poultry meat containing Salmonella in 25 g shall not be placed on the market without any industrial treatment able to eliminate Salmonella.
37Such measures are not yet implemented for Campylobacter at this time but are actually examined by The Community Economic and Social Committee, a small number of Member States and at a preliminary stage the European Parliament (Kremer, 2005). It is within this context that the European Food Safety Authority (EFSA) has formulated several recommendations in its Scientific Report in 2005 (EFSA, 2005). They concern particularly the intensification of epidemiological studies about Campylobacter and the reduction of the proportion of Campylobacter-infected poultry farms, by the application of strict biosecurity measures.
38Since 1996 in Belgium, the " Institut d'Expertise vétérinaire " that became included in the " Agence Fédérale pour la Sécurité de la Chaîne alimentaire " (AFSCA), with the help of Universities and Community Reference Laboratories, has setting up an annual monitoring program of zoonotic agents in human and animal products. Since 1998, the survey program, intended for all foodborne pathogens including Campylobacter, is coupled with a hygiene plan based on biosecurity measures at primary production level, which aims to reduce contamination from live animals. Such interventions measures can lead to additional production costs that are at the moment difficult to estimate. The Dutch CARMA Project has tried to evaluate these costs by means of an economic model. According to Mangen et al. (2005b), the annual income of broiler farmers could not bear increased production costs without any additional bonus, and this situation is all the more actual for extensive small-sized poultry farms.
5. Integrated approach to reduce flock contamination
39Given the public health and economic problem represented by Campylobacter, and the strengthening of the European legislation relating to animal products contamination by zoonotic agents, it is important to take measures in order to reduce Campylobacter prevalence throughout the poultry production chain leading to a reduced incidence of the human illness. In a recent risk evaluation, the CSH (2005) showed that the risk to contract illness decreases significatively if the proportion of contaminated meat-based preparations may be limited or eliminated in the food distribution chain. Moreover, it is not just presence or absence of pathogenic bacteria that is important, but also the amounts in which they are present. Dutch (Nauta et al., 2007) and Danish (Rosenquist et al., 2003) studies have particularly developed quantitative microbiological risk assessment models based on mathematical dose-response model to estimate the relationship between ingested dose and the probability of developing campylobacteriosis.
40Many broiler flocks can become infected with Campylobacter spp. at many stages of the poultry production chain. Therefore, the only intervention strategy to reduce the exposure of humans to Campylobacter spp. seems to be an integrated approach (Snijders et al., 2002), with multiple control measures along the poultry production chain, for instance at farm level, during transport, at the slaughterhouse and/or at the product transformation step (Line, 2002; Hariharan et al., 2004; Whitaker, 2006).
41Risk factors and sanitary measures for contamination during catching and transportation have been presented by Ramabu et al. (2004) and Rasschaert et al. (2007). The risk factors associated with the slaughter operations on the contamination of carcasses have been studied by Rosenquist et al. (2006) and EFSA (2005) have reviewed the risk management options available at this level. Furthermore, techniques of preventing contamination or decontaminating raw meat and poultry meat products in the food processing industry have been discussed by several authors (Huffman, 2002; Woteki et al., 2003; Dinçer et al., 2004). Woteki et al. (2003) have also presented in details necessary strategies at the consumer level.
6. Usual prevention methods
6.1. Hygiene measures
42Practical biosecurity measures at the farm level have been determined as the primary strategy to prevent colonisation of housed broiler flocks with Campylobacter entering the processing plant and hence the food chain (van de Giessen et al., 1992; ICGFI, 1999; Gibbens et al., 2001; Rivoal et al., 2005). Nevertheless, many authors have shown that biosecurity measures are only partly effective in controlling Campylobacter contamination (Pattison, 2001; Sahin et al., 2003; Van Gerwe et al., 2005).
43Measures that are important to protect the flock include the washing of hands, the wearing of protective clothing and dedicated footwear, the respect of house cleaning and disinfection protocoles, provision of Campylobacter-free water, feed and the removal of spent litter between two flocks. Details about biosecurity measures designed to control Campylobacter have been reported by Allen et al. (2005).
44The limited action of hygiene procedures is based on the fact that in conditions where broilers are confronted with environmental factors that are scarcely controllable (open-air range, wild birds, domesticated animals faeces, etc.), i.e. organic and free-range flocks, biosecurity is difficult to apply. In these production systems, Rivoal et al. (2005) have shown that, even if strict hygiene measures allow broiler flocks to be Campylobacter-negative during the first weeks of age (the indoor period), birds are almost always colonised at slaughter, after the access of birds to the open-air range.
45Nevertheless, even if high levels of environmental exposure to Campylobacter may overwhelm best practice biosecurity measures and that these practices can not guarantee infection prevention, they can help to delay the onset of Campylobacter colonisation and are consequently essential.
6.2. Antibiotics use
46The use of antibiotics in modern intensive animal production as growth-promoters and for therapy and prevention of diseases could not be a rational solution to reduce Campylobacter incidence. Several studies have actually pointed out the partial association between the veterinary use of antibiotics and the emergence of resistant strains of Campylobacter related to human enteritis (Pezotti et al., 2003; Desmonts et al., 2004; Luangtongkum et al., 2006). Nevertheless, Bywater (2004) assessed the sum total contribution of antibiotics use in animal production to human bacterial resistance as < 4%. Moreover, variation is seen in antibioresistance in different countries, reflecting various veterinary practices in antimicrobial usage. Whatever the opinion we have in this debate, these antibiotics have been banned in the EU since January 2006, according to the " Precautionary Principle ".
6.3. Acidification
47It is generally acknowledged that Campylobacter is sensitive to acid conditions (AFSCA, 2006). Several strategies developed to reduce Campylobacter populations are based on the acidification of the pathogen environment.
48Drinking water and feed acidification. The in vitro studies realized by Chaveerach et al. (2002) have pointed out the bactericidal activity of organic acids used individually or in combination. The four studied acids (formic, acetic, propionic and hydrochloric), alone or in combination at different formulation ratios, were mixed with a commercial broiler feed into bottles containing 250 ml of tap water. The acid combinations have shown an interesting bactericidal activity at pH 4.0 with Campylobacter numbers declining below 1 log cfu.ml-1 within 1 h, and the reduction was higher than the decreasing effect observed with the different acids used individually.
49Water being an efficient Campylobacter vector, Chaveerach et al. (2004) studied in vivo the drinking water acidification by the same four organic acids as a prophylactic measure. During all the experiment, no Campylobacter was found in acidified drinking water. Although acidification seems to be an effective measure to control water as a prominent contamination vector, most chickens were infected at the end of the experiment, demonstrating the impact of other contamination ways. Byrd et al. (2001) have also studied drinking water acidification during pre-slaughter feed withdrawal. The addition of 0.5% lactic acid in drinking water significantly reduced crop contamination with Campylobacter as compared with the controls (62.3% vs 85.1%).
50Another study by Heres et al. (2004) has tested fermented feed containing high concentrations of organic acids (5.7% lactic and 0.7% acetic) on susceptibility of chickens to Campylobacter and Salmonella. Broilers fed with fermented feed until 21 days of age needed a ten times higher dose of Campylobacter to achieve the same proportion of infected chickens as the control population. Nevertheless, the protective effects seem relatively limited and dependent on the infection dose according to the pathogen inoculated.
51Litter acidification. Acidification of poultry litter has also been suggested as a method to limit pathogen proliferation in breeding flocks. Line (2002) assessed two commercially available litter treatments (aluminium sulfate and sodium bisulfate) on Campylobacter prevalence and cæcal colonization of broilers. For example, treatment of pine shavings litter with the lowest level of aluminium sulfate, i.e. 3.63 kg per 4.6 m² litter significantly reduced cæcal Campylobacter colonization frequency by 65% and effected a 3.4 log reduction in cæcal pathogen populations. Nevertheless, it is noteworthy that, even at the lowest treatment level, such high concentrations are difficult to include in an environmental-respectful rearing system.
7. Complementary developing strategies
7.1. Non antagonism-based studies
52Active and passive immunity. Vaccination of poultry against Campylobacter has been considered to be a more effective measure than strict hygiene practices by some studies (de Zoete et al., 2007), because of the observation of a Campylobacter-specific immune response in chickens (Rice et al., 1997).
53So, the study of Wyszynska et al. (2004) has shown that chicken immunization with a virulent Salmonella vaccine strain carrying C. jejuni cjaA gene, encoding highly immunogenic proteins, may be an attractive and efficient approach for bird vaccination.
54About the passive immunization, Sahin et al. (2003) have observed that C. jejuni-specific maternal antibodies have a role in protection against colonization in young Campylobacter-negative chicks. Furthermore, Tsubokura et al. (1997) showed a prophylactic and therapeutic effects against C. jejuni, for at least 5 days post-infection, by oral administration of bovine and chicken immunoglobulin preparations to 22-days-old chickens. Nevertheless, the use of maternal antibodies could be hindered by their short protection period, unable to cover the whole rearing period. Wilkie (2006) purified and concentrated egg yolk antibodies from C. jejuni vaccinated hens. Three hours after experimentally infecting day-of-hatch broiler chicks with 5⋅107 cfu C. jejuni, yolk antibodies were administered via oral gavage or in the feed at a final concentration of 0.5% (w/w) until day 11 post-challenge. Despite measurable antibody activity in vitro, no significant reduction in the intestinal colonization by C. jejuni could be demonstrated.
55Bacteriophage therapy. The use of Campylobacter-specific bacteriophages has been attempted by several authors to face pathogens in poultry farms (Goode et al., 2003; Carrillo et al., 2005; Wagenaar et al., 2005). Atterbury et al. (2005) demonstrated a correlation between the presence of natural environmental phage and a reduction in the Campylobacter population colonizing broiler chicken caeca. Although it is a relatively new developing technique, it has already given some interesting results. However, Goode et al. (2003) emphasize the limitation of phage use at farm level i.e. the potential for fast selection of resistant Campylobacter following the simultaneous pathogen and bacteriophage release. These authors would limit consequently the bacteriophages use at the slaughter stage. On the other side, Wagenaar et al. (2005) consider the release of phage-infected Campylobacter in the environment to be acceptable, since phages have been shown to reside in Campylobacter populations present on naturally infected poultry.
56Diet modification. Heres et al. (2003) have studied the effect of feed fermentation on the Campylobacter contamination of broiler chickens. They used a moistened commercial standard broiler feed (feed: water ration = 1 : 1.4) supplemented with a Lactobacillus plantarum strain to ferment the mixture. The resulting product, named FLF (fermented liquid feed), lead to a significant reduction of Campylobacter susceptibility in chickens. This reported effect was particularly due to the high organic acids concentrations and the resulting pH decrease in the feed. FLF had also an effect on the chicken intestinal microflora (Heres, 2004).
57Cereal-based broiler diets contain anti-nutritive Non-Starch Polysaccharides (NSP) that increase intestinal viscosity, impairing digestion and reducing broiler performances (Bedford, 2001). Addition of exogenous enzymes, in particular xylanases and glucanases, reduces anti-nutritive effects of NSP and improves zootechnical poultry performance. Moreover, growth-promoting enzymes have also shown interesting antagonistic effect against Campylobacter. By reducing viscosity of the intestinal contents, xylanases can induce modifications of the chickens flora (Vahjen et al., 1998) and reduce C. jejuni contamination when these enzymes are added to the broiler diet, as shown by Fernandez et al. (2000). These authors have found significant reductions of the C. jejuni cæcal colonization (from 0.3 to 0.5 log cfu.g-1 cæcal content on average) by 0.1% xylanases supplementation of the diet. This reduction can be due to a lower intestinal viscosity as well as to the reduction of the digesta transit time, leading to a too short time for the pathogen establishment. Viscosity reduction could stimulate mucin production in the small and large intestines and in the caeca, as well as changes in the mucin composition. Some mucin glycoproteins are responsible for the protective properties of the mucus gels in the gastrointestinal tract.
58It is however important to point out that the use of feed additives is subjected to strict European legislations. Regulation (EC) n°1831/2003 of the European Parliament and of the Council of September 22, 2003 on additives for use in animal nutrition, including enzymes, lays down rules governing the Community authorization of the additives and, in particular, defines the conditions that a substance or a product should meet to be granted authorization, and the labelling conditions for these additives. Authorization of the additive needs to pass the risk assessment by EFSA. To be legally placed on the market and used, feed additives must be proved to have a favourable effect on the characteristics of the feed to which it is added or on animal production, to have no harmful effect on animal health, human health or the environment and that the presentation of the additive or alteration of the features of the products to which it is added does not harm or mislead the consumer. All these procedures are expensive and time-consuming so that enzymes approach may only be attractive if the purpose of pathogen prevention is combined with performance improvement.
7.2. Microbiological competition
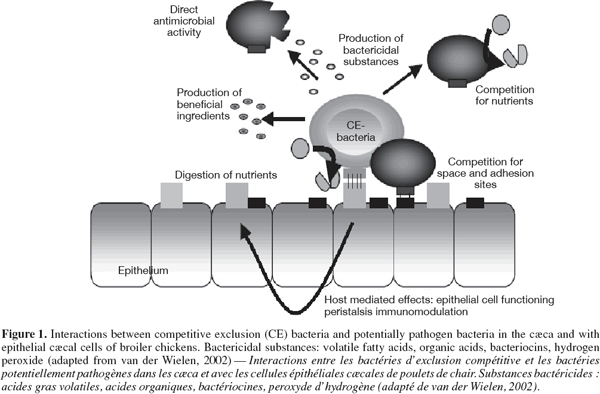
59Competitive exclusion flora. Competitive exclusion (CE) is a concept taking advantage of bacterial antagonism to reduce animal intestinal colonization by pathogenic microorganisms. The study of defined or undefined flora acting by competitive exclusion mechanisms was first initiated in the 1970s by Nurmi et al. (1973). They observed that introduction of gut contents originating from adult cocks to 1-2 d old chicks can protect young birds against Salmonella infantis infection. Figure 1, adapted from van der Wielen (2002), summarizes possible interactions between competitive exclusion flora and potential pathogens in broiler caeca. A twofold competition may operate in the gastrointestinal tract, i.e. competition for nutriments and for adhesion sites. Moreover, CE bacterial formulations may have a direct antimicrobial effect by the production of lactic acid, volatile fatty acid, hydrogen peroxide or bacteriocins.
60Afterwards, such CE floras have displayed variable results according to the experiments, generally because of their undefined composition. Oral treatment of newly-hatched chicks, challenged at day 24 with 5.7⋅104 cfu, 5.4⋅104 cfu or 7.3.103 cfu C. jejuni, with the commercial CE Broilact® reduced both the proportion of positive chicks from 100% to 0-62% and the numbers of the challenge organism in the caeca by 108 to 109-fold according to the infection dose (Hakkinen et al., 1999). Aho et al. (1992) also observed a reduction in Campylobacter cæcal population with Broilact-treated chicks. Stern et al. (2001) showed a Campylobacter average reduction of 0.38 log cfu.g-1 and 2.01 log cfu.g-1 cæcal material in 6-days chicks treated with CE and a mucosal CE cultures respectively. The average incidence colonization reduction observed in CE- and MCE-treated birds was 2.2% and 15.6%, respectively. On the other hand, Laisney et al. (2003) failed to show beneficial effect of cæcal CE flora on broiler infection with 102-103 cfu C. jejuni at 15 days of age. Because of the limited advantage for the poultry producers, the practical application of CE has only a great success in Finland.
61Furthermore, it is difficult to ensure the absence of potentially pathogen organisms in the bacterial compositions. It is noteworthy that Chen et al. (2001) aimed to prevent Campylobacter colonization of the chickens intestinal tract by early inoculation in these chickens of non-pathogenic C. jejuni strains used as defined CE preparation. Nevertheless, some authors predict a promising future for CE (Schneitz, 2005), among others owing to the ban of growth-promoting antibiotics in animal production and sanitary requirements that become more and more strict.
62Acidifying bacteria. Because of the CE disadvantages, the current trend is now the development of defined flora although the work is made complicated by lack of knowledge of the mechanism of CE and of the type of bacteria involved in the process (Chaveerach et al., 2004; Bjerrum, 2005). Acidifying bacteria, particularly lactic acid bacteria (LAB), contribute since several thousand years to preserve food. Nevertheless, their antimicrobial properties are not limited to the food industry field. Several in vitro and in vivo studies, summarized in tables 3 and 4, have investigated the bacterial antagonistic activities against Campylobacter.
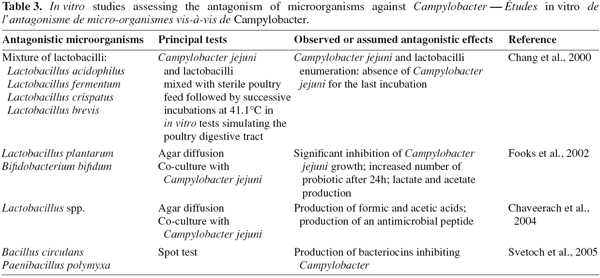
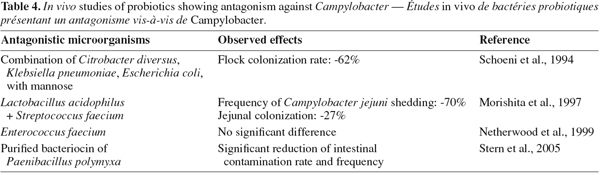
63Lactobacilli are frequently used in these in vitro studies. Chaveerach et al. (2004) have assessed the inhibitory activity of a Lactobacillus fermentum (P93) strain isolated from the chicken gut on ten C. jejuni/coli strains by diffusion agar assay and co-culture in anaerobic conditions. The experiment revealed an antagonistic effect of the L. fermentum strain against all the ten Campylobacter tested strains, which decreased of 4.10 ± 2.15 log cfu.ml-1 during 24 h of co-culture incubation. The authors have suggested that the inhibitory effect of Lactobacillus (P93) on Campylobacter growth could be explained mainly by organic acids production, resulting in pH reduction. Furthermore, the inhibitory effect was enhanced when the pH level in the culture media was low. Levels and types of organic acids produced depend on bacterial species or strains, culture composition and growth conditions (Ammor et al., 2006). According to van der Wielen et al. (2000) and Chaveerach et al. (2004), the acid dissociation stage is an essential factor for antagonism effect. van der Wielen et al. (2000) stated that the undissociated form of these short-chain acids can diffuse freely across the bacterial membrane and dissociates inside the cell, thereby reducing the internal pH and causing internal pathogen cell damage. Some authors mention also the damage caused by the anion itself as well, and in particular the inhibition of fundamental metabolic functions (van der Wielen et al., 2000; Chaveerach et al., 2002).
64The in vitro study realised by Fooks et al. (2002) aimed to investigate antagonistic effects of lactobacilli (L. plantarum, L. pentosus, L. acidophilus, L. reuteri). L. plantarum 0407 showed the most promising inhibitory activity on Campylobacter growth, both using plate assays and co-culture. This antimicrobial activity appeared to depend on the carbohydrate source supplied in vitro, suggesting that a suitable carbohydrate substrate supplementation may enhance competitive exclusion by lactobacilli. The experiment of Chang et al. (2000) tried to get closer to in vivo conditions, by investigating the impact of a selected lactobacilli mixed culture (L. acidophilus, L. fermentum, L. crispatus, L. brevis) on C. jejuni in simulated chicken digestive tract. The C. jejuni and lactobacilli were mixed with sterile poultry feed and incubated at 41.1°C for various lenghts of time and pH values, simulating five segments of the digestive tract. All the tested Lactobacillus spp. showed an antagonistic effect on Campylobacter in individual sections and the whole simulated digestive tract models.
65Then, several studies have pointed out the bactericidal activity of hydrogen peroxide (H2O2) produced by LAB in the presence of oxygen (Felten et al., 1999; Strus et al., 2006). Hydrogen peroxide may inhibit growth of bacteria that do not possess protective mechanisms like catalase or peroxidase. Its antimicrobial effect may result mainly from oxidation phenomenons causing denaturing of a number of enzymes and from the peroxidation of membrane lipids and proteins leading to an increased membrane permeability (Edens, 2003; Ammor et al., 2006). Zhao et al. (2006) showed that incubation of 7.0 log cfu.ml-1 C. jejuni with 0.1 and 0.2% H2O2 in suspension reduced C. jejuni populations by ca. 2.0 and 4.5 log cfu.ml-1, respectively. Furthermore, some authors studied the efficacy of broiler carcasses decontamination with H2O2 during the slaughter processing. Although Wagenaar et al. (2004) observed that immersion of carcasses in 1, 2, 3 and 4% H2O2 solutions containing glycerol resulted in average reductions of 0.3 up to 1.4 log cfu for the mesophilic aerobic counts, they did not measure Campylobacter loads on carcasses. Moreover, Dickens et al. (1997) demonstrated that addition of up to 1.5% H2O2 to sprays waters during defeathering had no effect on total aerobic plate counts of picked uneviscerated carcasses when compared to the water control.
66Besides organic acids and H2O2, bacteriocins are the third kind of compounds that may help to inhibit Campylobacter growth, as shown by Stern et al. (2006) for a bacteriocin produced by a Lactobacillus salivarius strain. Bacteriocins are peptidic compounds with antimicrobial properties produced by some bacteria. Their target is mainly the cytoplasmic membrane, forming pores that allow the unregulated outflow of essential ions, leading to bacteria death (Papagianni, 2003). The bacteriocins have often a relatively restricted spectrum of activity against bacteria strains closely related to the producing strain. Particularly, the genus Paenibacillus has been pointed out by Russian and American researchers. Svetoch et al. (2005) have revealed the production, by three Paenibacillus polymyxa strains, of bacteriocins effective against Campylobacter. One of these bacteriocins, secreted by P. polymyxa NRRL-B-30509, was purified and microencapsulated to evaluate a bacteriocin-based treatment to reduce C. jejuni colonization in poultry (Stern et al., 2005). The purified preparation was incorporated in chicken feed at the rate of 0.25 g.kg-1. One day old chicks were orally infected with 108 cfu C. jejuni and were provided from day seven to chicken feed containing or not (control) bacteriocin. Ten days after C. jejuni challenge, comparison of cæcal contamination rate between control and treated chickens showed that bacteriocin treatment reduced levels of intestinal colonization by C. jejuni from 4.6 to 6.3 log cfu.g-1 of fæces (P ≤ 0.05).
67Probiotics. The probiotic notion derives directly from the competitive exclusion concept. Unlike the CE treatments, probiotics are compositions containing one or several well-defined strains. Several descriptions have been proposed for probiotics (Jin et al., 1997) but they may globally be defined as living microorganisms that, once ingested, beneficially affect the host animal by improving its microbial balance (Fuller, 1989). The main expected characteristics and functions for an efficient probiotic strain in poultry production, presented in table 5, include maintaining normal intestinal microflora by competitive exclusion and antagonism, altering metabolism by increasing digestive enzyme activity, improving feed intake and digestion and neutralizing enterotoxins and stimulating the immune system (Ghadban, 2002). The use of probiotic microorganisms in animal production is well controlled and is considered, as enzymes and feed additives, by Regulation (EC) n°1831/2003 of the European Parliament and of the Council of September 22, 2003.
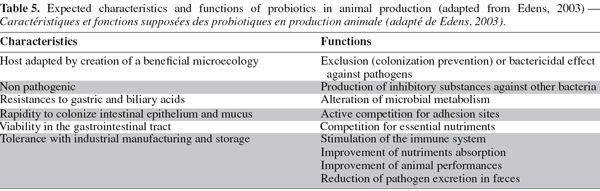
68About the in vivo studies, Morishita et al. (1997) have assessed the antagonistic effect of probiotic containing a L. acidophilus strain combined with a Streptococcus faecium. This avian-specific probiotic was given to chicks from day one to day three; moreover, birds were challenged with C. jejuni 6 h after the first oral administration of probiotic. At 40 days of age, the probiotic-treated group had a 70% (P = 0.0001) decreased number of birds shedding C. jejuni when compared with the control group given distilled water instead of probiotic. They also found a 27% (P = 0.0001) reduction in the number of chickens that were colonized in the jejunum at slaughter in comparison with the controlled birds.
7.3. Prebiotics and synbiotics
69Prebiotics are defined as poorly digestible food ingredients, that beneficially affect the hosts by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon (Gibson et al., 1995). Among the mostly reported prebiotics are polyols (xylitol, etc.), or di-, oligo- and polysaccharides (lactilol, fructo-oligosaccharides, inulin, etc.) (Šušković et al., 2001).
70Some specific carbohydrates used as prebiotics, like mannanoligosaccharides (Spring et al., 2000) and isomaltooligosaccharides (Chung et al., 2004), have been shown to reduce Salmonella colonization in the caeca of poultry. Such carbohydrate substrates are fermented in the latter intestinal segments and give rise to a mixture of carbon dioxide, hydrogen and short-chain fatty acids (Grizard et al., 1999; MacFarlane et al., 2006) that lead to intestinal pH reduction and may partially explain the pathogen antagonism.
71Combinations of prebiotics and probiotics, for example Lactobacillus and lactitol, are known as synbiotics, and may have antimicrobial activity (Klewicki et al., 2004). Then, the survival and the development of the probiotic organism could be improved, because its specific substrate is readily available (Collins et al., 1999). Fooks et al. (2002) have yet recorded a C. jejuni inhibition in vitro, with a population reduction below detectable level after 24 h culture, with a L. plantarum or Bifidobacterium bifidum, when combined with oligofructose or an oligosaccharide: xylo-oligosaccharide mixture (50 : 50, w/w) at 10 g⋅l-1. The observed antagonistic effect was related to a pH decrease of the cell culture.
8. Conclusion
72Zoonose, particularly food pathogen transmission from animals to man, is a major concern of food safety. Consequently, the European Union has recently established the Directive 2003/99/CE and the Regulations (EC) n°2160/2003 and n°1003/2005, in the way to decrease the incidence of zoonoses in humans, to improve their control in the food chain and to strengthen the collection of relevant data to support risk management decisions. Salmonella is the primary zoonotic agent targeted at primary animal but similar measures and recommendations are actually examined for Campylobacter by the European authorities. Campylobacter is one of the main recognized causes of human acute enterocolitis called " campylobacteriosis ". Foods of poultry origin appear to be the main source of this pathogen. In order to reduce the exposure of humans to Campylobacter spp., an integrated approach including control measures implemented throughout the poultry production chain (chicken meat and eggs) appears to be the only effective intervention strategy. At the primary production level, biosecurity measures are only partly effective and subtherapeutic antibiotics, which were used as growth promoting but also helped to prevent pathogen contamination, are baned in the EU since January 2006. Many alternative procedures have been investigated. They are based on active/passive immunity, on bacteriophage, NSP-hydrolysing enzymes or bacteriocins incorporated in chicken feed, or on diet modification. Nevertheless, direct and indirect acidification- and antagonism-based measures seem to be the more promising strategies. Beside competitive exclusion flora, defined bacterial strains like probiotics and acidifying bacteria have shown interesting in vitro and in vivo antagonistic effects against Campylobacter spp., especially by organic acids production and pH reduction. Several studies have shown that synbiotics, i.e. combinations of probiotics and prebiotics that can be used specifically as substrate by probiotics, may also have antimicrobial activity. Feed additives, i.e. components other than feedstuffs like probiotics, synbiotics, bacteriophage or exogenous enzymes, are yet subjected to strict European legislations. With the cost inherent to these authorisation procedures, application of monitoring plans and developed measures to control Campylobacter contamination in poultry farms will be expensive for the producer. Only the strategies that combine low cost and efficacy to prevent or reduce Campylobacter contamination in broiler flocks, in order to fit the EU Directives and Regulations, would be applicable in practice.
73Acknowledgements
74The authors would like to thank the Ministry of the Walloon Region – Directorate-General of Agriculture (D.G.A.), within the grant D31-1093, for financial support.
75List of abbreviations
76AFSCA: Agence Fédérale pour la Sécurité de la Chaîne alimentaire
77AFSSA: Agence Française de Sécurité Sanitaire des Aliments
78C.: Campylobacter
79CE: Competitive Exclusion
80cfu: Colony Forming Unit
81EFSA: European Food Safety Authority
82EU: European Union
83GHP: Good Hygiene/Farming Practices
84ICGFI: International Consultative Group on Food Irradiation
85L.: Lactobacillus
86MIC: Minimum Inhibitory Concentration
87VNC: Viable but Non Culturable
88WHO: World Health Organization
Bibliographie
Achen M., Morishita T.Y. & Ley E.C., 1998. Shedding and colonization of Campylobacter jejuni in broilers from day-of-hatch to slaughter age. Avian Dis., 42(4), 732-737.
Adak G.K., Cowden J.M., Nicholas S. & Evans H.S., 1995. The Public Health Laboratory Service national case-control study of primary indigenous sporadic cases of Campylobacter infection. Epidemiol. Infect., 115(1), 15-22.
AFSCA, 2006. Appréciation des risques alimentaires liés aux campylobacters – Application au couple poulet / Campylobacter jejuni. Http://www.afssa.fr/Ftp/afssa/22208-I.pdf, (20/03/06).
Aho M., Nuotio L., Nurmi E. & Kiiskinen T., 1992. Competitive exclusion of campylobacters from poultry with K-bacteria and Broilact®. Int. J. Food Microbiol., 15, 265-275.
Allen V. & Newell D.G., 2005. Food standards agency report: evidence for the effectiveness of biosecurity to exclude Campylobacter from poultry flocks. Http://www.food.gov.uk/multimedia/pdfs/biocampy.pdf, (04/07/06).
Allos B.M., 1997. Association between Campylobacter infection and Guillain-Barré Syndrome. J. Infect. Dis., 176 (Suppl 2), S125-128.
Altekruse S.F., Stern N.J., Fields P.I. & Swerdlow D.L., 1999. Campylobacter jejuni - An emerging foodborne pathogen. Emerging Infect. Dis., 5(1), 28-35.
Ammor S., Tauveron G., Dufour E. & Chevallier I., 2006. Antibacterial activity of lactic acid bacteria against spoilage and pathogenic bacteria isolated from the same meat small-scale facility 1- Screening and characterization of the antibacterial compounds. Food Control, 17, 454-461.
Atterbury R.J. et al., 2005. Correlation of Campylobacter bacteriophage with reduced presence of host in broiler chicken cæca. Appl. Environ. Microbiol., 71(8), 4885-4887.
Avrain L. et al., 2001. Etude de l'antibiorésistance des campylobacters de la filière avicole. In : Compte-rendu des 4è Journées de la Recherche Avicole, Nantes, France, 27-29 mars 2001, 281-284.
Bedford M.R. & Partridge G.G., 2001. Enzymes in farm animal nutrition. Oxon, UK: CABI Publishing.
Beery J.T., Hugdahl M.B. & Doyle M.P., 1988. Colonisation of gastrointestinal tract of chicks by Campylobacter jejuni. Appl. Environ. Microbiol., 54(10), 2365-2370.
Berndtson E., Danielsson-Tham M.-L. & Engvall A., 1996. Campylobacter incidence on a chicken farm and the spread of Campylobacter during the slaughter process. Int. J. Food Microbiol., 32, 35-47.
Berrang M.E. et al., 2003. Presence of Campylobacter in the respiratory tract of broiler carcasses before and after commercial scalding. Poult. Sci., 82, 1995-1999.
Bjerrum L., 2005. The intestinal microflora of broiler chickens: investigations on the microbial community and experimental infection studies. Thesis: Danish Institute for Food and Veterinary Research, Aarhus (Denmark).
Bogaardt M.J. et al., 2004. Controlling Campylobacter in the chicken meat chain. RIVM Report, Http://www.rivm.nl/bibliotheek/rapporten/250911005.pdf, (16/01/07).
Bryan F.L. & Doyle M.P., 1995. Health risks and consequences of Salmonella and Campylobacter jejuni in raw poultry. J. Food Prot., 58(3), 326-344.
Bull S.A. et al., 2006. Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Appl. Environ. Microbiol., 72(1), 645-652.
Butzler J.P., 2004. Campylobacter, from obscurity to celebrity. Clin. Microbiol. Infect., 10, 868-876.
Byrd J.A. et al., 2001. Effect of lactic acid administration in the drinking water during pre-slaughter feed withdrawal on Salmonella and Campylobacter contamination of broilers. Poult. Sci., 80, 278-283.
Bywater R. et al., 2004. A European survey of antimicrobial susceptibility among zoonotic and commensal bacteria isolated from food-producing animals. J. Antimicrob. Chemother., 54, 744-754.
Callicott K.A. et al., 2006. Lack of evidence for vertical transmission of Campylobacter spp. in chickens. Appl. Environ. Microbiol., 72(9), 5794-5798.
Carrillo C.L. et al., 2005. Bacteriophage therapy to reduce Campylobacter jejuni colonisation of broiler chickens. Appl. Environ. Microbiol., 71(11), 6554-6563.
Chang M. & Chen T.C., 2000. Reduction of Campylobacter jejuni in a simulated chicken digestive tract by Lactobacilli cultures. J. Food Prot., 63(11), 1594-1597.
Chaveerach P. et al., 2002. In vitro study on the effect of organic acids on Campylobacter jejuni/coli population in mixtures of water and feed. Poult. Sci., 81, 621-628.
Chaveerach P., Lipman L.J.A. & van Knapen F., 2004. Antagonistic activities of several bacteria on in vitro growth of 10 strains of Campylobacter jejuni/coli. Int. J. Food Microbiol., 90, 43-50.
Chen H.C. & Stern N., 2001. Competitive exclusion of heterologous Campylobacter spp. in chicks. Appl. Environ. Microbiol., 67(2), 848-851.
Chuma T., Makino K., Okamoto K. & Yugi H., 1997. Analysis of distribution of Campylobacter jejuni and Campylobacter coli in broilers by using restriction fragment length polymorphism of flagellin gene. J. Med. Vet. Sci., 59(11), 1011-1015.
Chung C.-H. & Day D.F., 2004. Efficacy of Leuconostoc mesenteroides (ATCC 13146) isomaltooligosaccharides as a poultry prebiotic. Poult. Sci., 83(8), 1302-1306.
Collins D.M. & Gibson G.R., 1999. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr., 69(5), 1052S-1057S.
Corry J.E.L. & Atabay H.I., 2001. Poultry as a source of Campylobacter and related organisms. J. Appl. Microbiol., 90, 96S-114S.
CSH - Conseil Supérieur d'Hygiène, 2005. Contribution à l'évaluation du risque présenté en Belgique par les Campylobacter spp. dans les préparations de viande à base de viande hachée de volaille. Avis du Conseil Supérieur d'Hygiène n°7947. Bruxelles : Conseil Supérieur d'Hygiène.
Dachet F. et al., 2004. Identification par PCR en temps réel et FRET des Campylobacters les plus fréquents. In : 6th National Congress of the French Society of Microbiology, Bordeaux, France, 10-12 May 2004, 444.
Denis M. et al., 2001. Campylobacter contamination in French chicken production from farm to consumers. Use of a PCR assay for detection and identification of Campylobacter jejuni and Campylobacter coli. J. Appl. Microbiol., 91, 255-267.
Desmonts M.-H., Dufour-Gesbert F., Avrain L. & Kempf I., 2004. Antimicrobial resistance in Campylobacter strains isolated from French broilers before and after antimicrobial growth promoter bans. J. Antimicrob. Chemother., 54, 1025-1030.
de Zoete M.R., van Putten J.P.M. & Wagenaar J.A., 2007. Vaccination of chickens against Campylobacter. Vaccine, doi: 10.1016/j.vaccine.2006.12.002.
Dickens J.A. & Whittemore A.D., 1997. Effects of acetic acid and hydrogen peroxide application during defeathering on the microbiological quality of broiler carcasses prior to evisceration. Poult. Sci., 76, 657-660.
Dinçer A.H. & Baysal T., 2004. Decontamination techniques of pathogen bacteria in meat and poultry. Crit. Rev. Microbiol., 30, 197-204.
Ducoffre G., 2006. Surveillance des maladies infectieuses par un réseau de laboratoires de microbiologie. Tendances épidémiologiques 1983-2004, Rapport D/2006/2505/29. Bruxelles: Institut Scientifique de Santé Publique, Section d'Epidémiologie. http://www.iph.fgov.be/epidemio/EPIFR/plabfr/plabanfr/index05.htm, (10/04/07).
Edens F.W., 2003. An alternative for antibiotic use in poultry: probiotics. Rev. Bras. Cienc. Avicola, 5(2).
EFSA – European Food Safety Authority, 2005. Scientific report of the scientific panel on biological hazards on the request from the Commission related to Campylobacter in animals and foodstuffs. EFSA J., annexe 2004, 173, 1-105.
EFSA – European Food Safety Authority, 2006. Trends and sources of zoonoses, zoonotic agents and antimicrobial resistance in the European Union in 2004. Http://www.efsa.europa.eu/etc/medialib/efsa/science/monitoring_zoonoses/reports/1277.Par.0022.File.dat/zoonoses2004-levels1-2-part11.pdf, (12/09/06).
Ekdahl K., Normann B. & Andersson Y., 2005. Could flies explain the elusive epidemiology of campylobacteriosis ? BMC Infect. Dis., 5(11).
Engvall A., 2001. May organically farmed animals pose a risk for Campylobacter infections in humans ? Acta Vet. Scand., 95 (suppl.), 85-87.
European Commission, 2005. Prospects for agricultural markets and income, 2005–2012. Http://ec.europa.eu/agriculture/publi/caprep/prospects2005/fullrep.pdf, (06/02/07).
Evans S.J. & Sayers A.R., 2000. A longitudinal study of Campylobacter infection of broiler flocks in Great Britain. Prev. Vet. Med., 46(3), 209-223.
Felten A. et al., 1999. Lactobacillus species identification, H2O2 production, and antibiotic resistance and correlation with human clinical status. J. Clin. Microbiol., 37(3), 729-733.
Fernandez F., Sharma R., Hinton M. & Bedford M.R., 2000. Diet influences the colonisation of Campylobacter jejuni and distribution of mucin carbohydrates in the chick intestinal tract. Cell. Mol. Life Sci., 57, 1793-1801.
Fooks L.J. & Gibson G.R., 2002. In vitro investigations of the effect of probiotics and prebiotics on selected human intestinal pathogens. FEMS Microbiol. Ecol., 39, 67-75.
Friedman C.R., Neimann J., Wegener H.C. & Tauxe R.V., 2000. Epidemiology of Campylobacter jejuni infection in the United States and over industrialised nations. In: Nachamkin I. & Blaser M.J. Campylobacter, 2nd edition. Washington, DC, USA: ASM Press, 121-138.
Friedman C.R. et al., 2004. Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clin. Infect. Dis., Suppl. 3, S285-296.
Fuller R., 1989. Probiotics in man and animals. J. Appl. Bacteriol., 66, 365-378.
Ghadban G.S., 2002. Probiotics in broiler production – a review. Arch. Geflügelk., 66(2), 49-58.
Gibbens J.C. et al., 2001. A trial of biosecurity as a means to control Campylobacter infection of broiler chickens. Prev. Vet. Med., 48(2), 85-99.
Gibson G.R. & Roberfroid M.B., 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr., 125, 1401-1412.
Goode D., Allen V.M. & Barrow P.A., 2003. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophage. Appl. Environ. Microbiol., 69(8), 5032-5036.
Grizard D. & Barthomeuf C., 1999. Non-digestible oligosaccharides used as prebiotic agents: mode of production and beneficial effects on animal and human health. Reprod. Nutr. Dev., 39, 563-588.
Hakkinen M. & Schneitz C., 1999. Efficacy of a commercial competitive exclusion product against Campylobacter jejuni. Brit. Poult. Sci., 40, 619-621.
Hald P., Rattenborg E. & Madsen M., 2001. Role of batch depletion of broiler houses on the occurence of Campylobacter spp. in chicken flocks. Lett. Appl. Microbiol., 32, 253-256.
Hariharan H., Murphy G.A. & Kempf I., 2004. Campylobacter jejuni: public health hazards and potential control methods in poultry: a review. Vet. Med. – Czech, 49(11), 441-446.
Hazeleger W.C., Wouters J.A., Rombouts F.M. & Abee T., 1998. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl. Environ. Microbiol., 64(10), 3917-3922.
Heres L., 2004. Campylobacter and Salmonella control in chickens and the role of fermented food. Tijdschr. Diergeneeskd., 129(10), 332-335.
Heres L. et al., 2003. Effect of fermented feed on the susceptibility for Campylobacter jejuni colonisation in broiler chickens with and without concurrent inoculation of Salmonella enteritidis. Int. J. Food Microbiol., 87, 75-86.
Heres L. et al., 2004. Effect of acidified feed on susceptibility of broiler chickens to intestinal infections by Campylobacter and Salmonella. Vet. Microbiol., 99, 259-267.
Heuer O.E., Pedersen K., Andersen J.S. & Madsen M., 2001. Prevalence and antimicrobial susceptibility of thermophilic Campylobacter in organic and conventional broiler flocks. Lett. Appl. Microbiol., 33, 269-274.
Huffman R.D., 2002. Current and future technologies for the decontamination of carcasses and fresh meat. Meat Sci., 62, 285-294.
Huneau-Salaün A., Denis M., Balaine L. & Salvat G., 2005. Facteurs de risque de contamination par Campylobacter spp. des élevages de poulets de chair élevés en plein air à la fin de la période de claustration. In : Compte-rendu des 6è Journées de la Recherche Avicole, Saint-Malo, 30-31 mars 2005. Saint-Malo, France : Journées de la Recherche Avicole 6, 459-463.
Hutchinson M.L. et al., 2005. Analyses of livestock production, waste storage, and pathogen levels and prevalences in farm manures. Appl. Environ. Microbiol., 71(3), 1231-1236.
ICGFI – International Consultative Group on Food Irradiation, 1999. Safety of poultry meat: from farm to table. Http://www-naweb.iaea.org/nafa/fep/public/poultrymeat.pdf, (18/12/06).
Jacobs-Reitsma W., 1995. Campylobacter bacteria in breeder flocks. Avian Dis., 39, 355-359.
Jin L.Z., Ho Y.W., Abdullah N. & Jalaludin S., 1997. Probiotics in poultry: modes of action. World's Poult. Sci. J., 53, 351-368.
Jones K., 2001. Campylobacters in water, sewage and the environment. J. Appl. Microbiol., 90, 68S-79S.
Jørgensen F. et al., 2002. Prevalence and numbers of Salmonella and Campylobacter spp. on raw, whole chickens in relation to sampling methods. Int. J. Food Microbiol., 76, 151-164.
Klewicki R. & Klewicka E., 2004. Antagonistic activity of lactic acid bacteria as probiotics against selected bacteria of the Enterobaceriacae family in the presence of polyols and their galactosyl derivatives. Biotechnol. Lett., 26, 317-320.
Kremer F., 2005. Perspectives d'évolution des critères microbiologiques en France. Colloque Sécurité et Qualité des Aliments, Paris, le jeudi 1er décembre 2005. Nouveaux critères microbiologiques européens: quels enjeux et implications pratiques pour les acteurs de la filière agroalimentaire ? Paris : Silliker, 13.
Laisney M-J., Gillard M-O. & Salvat G., 2003. Efficacité d'une flore de barrière contre Campylobacter en fonction de l'origine génétique des poulets. In : Compte-rendu des 5è Journées de la Recherche Avicole, Tours, France, 26-27 mars 2003, 457-460.
Line J.E., 2002. Campylobacter and Salmonella populations associated with chicken raised on acidified litter. Poult. Sci., 81(10), 1473-1477.
Luangtongkum T. et al., 2006. Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of Campylobacter spp. in poultry. Appl. Environ. Microbiol., 72(5), 3600-3607.
MacFarlane S., MacFarlane G.T. & Cummings J.H., 2006. Review article: prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Ther., 24, 701-714.
Mangen M.J.J. et al., 2005a. The costs of human Campylobacter infections and sequelae in The Netherlands: a DALY and cost-of-illness approach. Acta Agric. Scand. C Food Econ., 2, 35-51.
Mangen M.J.J., Havelaar A.H. & Poppe K.J., 2005b. Controlling Campylobacter in the chicken meat chain – Estimation of intervention costs. Report 6.05.01. Http://www.rivm.nl/carma/resultaten/LEI%20rapport%206.05.pdf, (06/07/07).
Mc Crea B.A. et al., 2006. Prevalence of Campylobacter and Salmonella species on farm, after transport, and at processing in speciality market poultry. Poult. Sci., 85, 136-143.
Mead P.S. et al., 1999. Food-related illness and death in the United States. Emerg. Infect. Dis., 5, 607-625.
Moore J.E., 2001. Bacterial dormancy in Campylobacter: abstract theory or cause for concern ? Int. J. Food Sci. Technol., 36, 593-600.
Morishita T.Y. et al., 1997. Evaluation of an avian-specific probiotic to reduce the colonization and shedding of Campylobacter jejuni in broilers. Avian Dis., 41(4), 850-855.
Nauta M.J., Jacobs-Reitsma W.F. & Havelaar A.H., 2007. Special issue on Campylobacter Risk Management and Assessment (CARMA). A risk assessment model for Campylobacter in boiler meat. Risk Anal., 27(4), 845-861.
Netherwood T., Gilbert H.J., Parker D.S. & O'Donnell A., 1999. Probiotics shown to change bacterial community structure in the avian gastrointestinal tract. Appl. Environ. Microbiol., 65(11), 5134-5138.
Newell D.G. & Fearnley C., 2003. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol., 69(8), 4343-4351.
Nichols G.L., 2005. Fly transmission of Campylobacter. Emerg. Infect. Dis., 11(3), 361-364.
Nicholson F.A., Groves S.J. & Chambers B.J., 2005. Pathogen survival during livestock manure storage and following land application. Bioresource Technol., 96(2), 135-143.
Nurmi E. & Rantala M., 1973. New aspects of Salmonella infection in broiler production. Nature, 241, 210-211.
Oporto B. et al., 2007. Prevalence and strain diversity of thermophilic campylobacters in cattle, sheep and swine farms. J. Appl. Microbiol. Http://www.blackwell-synergy.com/doi/full/10.1111/j.1365-2672.2007.03328.x?prevSearch=allfield%3A%28Oporto%29, (01/08/07).
Oyarzabal O.A., Macklin K.S. & Barbaree J.M., 2005. Evaluation of agar plates for direct enumeration of Campylobacter spp. from poultry carcass rinses. Appl. Environ. Microbiol., 71, 3351-3354.
Papagianni M., 2003. Ribosomally synthesized peptides with antimicrobial properties: biosynthesis, structure, function, and applications. Biotechnol. Adv., 21, 465-499.
Pattison M., 2001. Practical intervention strategies for Campylobacter. J. Appl. Microbiol., 90, 121-125.
Petersen L., Nielsen E.M. & On S.L., 2001. Serotype and genotype diversity and hatchery transmission of Campylobacter jejuni in commercial poultry flocks. Vet. Microbiol., 82(2), 141-154.
Pezzotti G. et al., 2003. Occurrence and resistance to antibiotics of Campylobacter jejuni and Campylobacter coli in animals and meat in northeastern Italy. Int. J. Food Microbiol., 82, 281-287.
Ramabu S.S., Boxall N.S., Madie P. & Fenwick S.G., 2004. Some potential sources for transmission of Campylobacter jejuni to broiler chickens. Lett. Appl. Microbiol., 39(3), 252-256.
Rasschaert G., Houf K. & De Zutter L., 2007. External contamination of Campylobacter-free flocks after transport in cleaned and disinfected containers. J. Food Prot., 70(1), 40-47.
Rice B.E. et al., 1997. Campylobacter jejuni in broiler chickens: colonization and humoral immunity following oral vaccination and experimental infection. Vaccine, 15(17-18), 1922-1932.
Ridley A.M. & Newell D.G., 2004. Campylobacter jejuni: control and prevention of a major public health problem. In: Proceedings of an international EU-RAIN conference, Padua, 2-3 December 2004. Padua, Italy: Teagasc–The National Food Centre, 103-120.
Rivoal K. et al., 2005. Genomic diversity of Campylobacter coli and Campylobacter jejuni isolates recovered from free-range broilers farms and comparison with isolates of various origins. Appl. Environ. Microbiol., 71(10), 6216-6227.
Roberts J.A. et al., 2003. The study of infectious disease in England: socio-economic impact. Epidemiol. Infect., 130(1), 1-11.
Rollins D.M. & Colwell R.R., 1986. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl. Environ. Microbiol., 52(3), 531-538.
Rosenquist H. et al., 2003. Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int. J. Food Microbiol., 83, 87-103.
Rosenquist H., Sommer H.M., Niels N.L. & Christensen B.B., 2006. The effect of slaughter operations on the contamination of chicken carcasses with thermotolerant Campylobacter. Int. J. Food Microbiol., 108, 226-232.
Sahin O., Kobalka P. & Zhang Q., 2003. Detection and survival of Campylobacter in chicken eggs. J. Appl. Microbiol., 95(5), 1070-1079.
Saleha A.A., 2004. Epidemiological study on the colonization of chickens with Campylobacter in broiler farms in Malaysia: possible risk and management factors. Int. J. Poult. Sci., 3(2), 129-134.
Schneitz C., 2005. Competitive exclusion in poultry - 30 years of research. Food Control, 16, 657-667.
Schoeni J.L. & Doyle M.P., 1992. Reduction of Campylobacter jejuni colonisation of chicks by cecum colonizing bacteria producing anti-Campylobacter jejuni metabolites. Appl. Environ. Microbiol., 58(2), 664-670.
Schoeni J.L. & Wong A.C., 1994. Inhibition of Campylobacter jejuni colonization in chicks by defined competitive exclusion bacteria. Appl. Environ. Microbiol., 60(4), 1191-1197.
Shanker S., Lee A. & Sorrell T.C., 1990. Horizontal transmission of Campylobacter jejuni amongst broiler chicks: experimental studies. Epidemiol. Infect., 104(1), 101-110.
Skov M.N. et al., 2004. The role of litter beetles as potential reservoir for Salmonella enterica and thermophilic Campylobacter spp. between broiler flocks. Avian Dis., 48, 9-18.
Snijders J.M.A. & van Knapen F., 2002. Prevention of human diseases by an integrated quality control system. Livest. Prod. Sci., 76, 203-206.
Spring P., Wenk C., Dawson K.A. & Newman K.E., 2000. The effects of dietary mannanoligosaccharides on cæcal parameters and the concentrations of enteric bacteria in the cæca of Salmonella-challenged broiler chicks. Poult. Sci., 79, 205-211.
Stanley K. & Jones K., 2003. Cattle and sheep as reservoirs of Campylobacter. J. Appl. Microbiol., 94, 104S-113S.
Stas T., Jordan F.T.W. & Woldehiwet Z., 1999. Experimental infection of chickens with Campylobacter jejuni: strains differ in their capacity to colonise the intestine. Avian Pathol., 28, 61-64.
Stern N.J. et al., 2001. Comparison of mucosal competitive exclusion and competitive exclusion treatment to reduce Salmonella and Campylobacter spp. colonization in broiler chickens. Poult. Sci., 80, 156-160.
Stern N.J. et al., 2005. Paenibacillus polymyxa purified bacteriocin to control Campylobacter jejuni in chickens. J. Food Prot., 68(7), 1450-1453.
Stern N.J. et al., 2006. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob. Agents Chemother., 50(9), 3111-3116.
Strus M. et al., 2006. The in vitro effect of hydrogen peroxide on vaginal microbial communities. FEMS Immunol. Med. Microbiol., 48, 56-63.
Studahl A. & Andersson Y., 2000. Risk factors for indigenous Campylobacter infection: a Swedish case-control study. Epidemiol. Infect., 125, 269-275.
Šušković J., Kos B., Goreta J. & Matošić S., 2001. Role of lactic acid bacteria and bifidobacteria in symbiotic effect. Food Technol. Biotechnol., 39(3), 227-235.
Svetoch E.A. et al., 2005. Isolation of Bacillus circulans and Paenibacillus polymyxa strains inhibitory to Campylobacter jejuni and characterization of associated bacteriocins. J. Food Prot., 68(1), 11-17.
Tsubokura K. et al., 1997. Oral administration of antibodies as prophylaxis and therapy in Campylobacter jejuni infected chickens. Clin. Exp. Immunol., 108, 451-455.
Udayamputhoor R.S. et al., 2003. Effects of diet formulations containing proteins from different sources on intestinal colonisation by Campylobacter jejuni in broiler chicken. Can. J. Vet. Res., 67, 204-212.
Vahjen W., Gläser K., Schäfer K. & Simon O., 1998. Influence of xylanase-supplemented feed on the development of selected bacterial groups in the intestinal tract of broiler chicks. J. Sci. Food Agric., 130, 489-500.
van de Giessen A. et al., 1992. Study on the epidemiologily and control of Campylobacter jejuni in poultry broiler flocks. Appl. Environ. Microbiol., 58(6), 1913-1917.
van den Brandhof W.E., De Wit G.A., de Wit M.A. & van Duynhoven Y.T., 2004. Costs of gastroenteritis in The Netherlands. Epidemiol. Infect., 132(2), 211-221.
van der Wielen P.W., 2002. Role of volatile fatty acids in competitive exclusion of Salmonella enteritidis. Thesis: Faculty of Veterinary Medicine, Utrecht (The Netherlands).
van der Wielen P.W. et al., 2000. Role of volatile fatty acids in the development of the cæcal microflora in broiler chickens during growth. Appl. Environ. Microbiol., 66(6), 2536-2540.
van Dessel P., 2005. Qualité sanitaire des produits de porcs et de volailles - Evolution en matière de santé publique. In : Compte-rendu des 5è Journées de productions porcines et avicoles, Gembloux, 19 octobre 2005. Gembloux, Belgique: Centre wallon de Recherches agronomiques.
Van Gerwe T.J. et al., 2005. Quantifying transmission of Campylobacter spp. among broilers. Appl. Environ. Microbiol., 71(10), 5765-5770.
van Vliet A.H.M. & Ketley J.M., 2001. Pathogenesis of enteric Campylobacter infection. J. Appl. Microbiol., 90, 45S-56S.
Vellinga A. & Van Loock F., 2002. The dioxin crisis as experiment to determine poultry-related Campylobacter enteritis. Emerg. Infect. Dis., 8(1), 19-22.
Wagenaar C.L. & Snijders J.M.A., 2004. Decontamination of broilers with hydrogen peroxide stabilised with glycerol during processing. Int. J. Food Microbiol., 91, 205-208.
Wagenaar J.A. et al., 2005. Phage-therapy reduces Campylobacter jejuni colonisation in broilers. Vet. Microbiol., 109, 275-283.
Wedderkopp A., Gradel K.O., Jorgensen J.C. & Madsen M., 2001. Pre-harvest surveillance of Campylobacter and Salmonella in Danish broiler flocks: a two-year study. Int. J. Food Microbiol., 68, 53-59.
Whitaker S., 2006. Controlling pathogens. 1 February 2006. Food Safety Network. Http://archives.foodsafetynetwork.ca/animalnet/2006/2-2006/animalnet_feb_2-2.htm, (22/05/06).
WHO – World Health Organization, 2000. Campylobacter. Fact Sheet n°255. Http://www.who.int/mediacentre/factsheets/fs255/en/print.html, (07/08/06).
Wilkie D.C., 2006. Non-antibiotic approaches to control pathogens in the gastrointestinal tract of the broiler chicken. Thesis: University of Saskatchewan, Saskatoon (Canada).
Winquist A.G. et al., 2001. Outbreak of campylobacteriosis at a senior center. J. Am. Geriatr. Soc., 49(3), 304-307.
Woodall C.A. et al., 2005. Campylobacter jejuni gene expression in the chick cecum: evidence for adaptation to a low oxygen environment. Infect. Immun., 73(8), 5278-5285.
Woteki C.E. & Kineman B.D., 2003. Challenges and approaches to reduce foodborne illness. Annu. Rev. Nutr., 23, 315-344.
Wyszynska A., Raczko A., Lis M. & Jagusztin-Krynicka E.K., 2004. Oral immunisation of chickens with avirulent Salmonella vaccine strain carrying C. jejuni 72Dz/92 CjA gene elicits specific humoral immune response associated with protection against challenge with wild type Campylobacter. Vaccine, 22, 1379-1389.
Zhao T. & Doyle M.P., 2006. Reduction of Campylobacter jejuni on chicken wings by chemical treatments. J. Food Prot., 69(4), 762-767.
Zorman T., Heyndrickx M., Uzunović-Kamberović S. & Smole Možina S., 2006. Genotyping of Campylobacter coli and Campylobacter jejuni from retail chicken meat and humans with campylobacteriosis in Slovenia and Bosnia and Herzegovina. Int. J. Food Microbiol., 110, 24-33.

