VOC emissions and protein expression mediated by the interactions between herbivorous insects and Arabidopsis plant. A review
Received on March 20, 2013; accepted on February 13, 2014
Résumé
L’émission des COVs et l’expression des protéines induites par des interactions entre les insectes herbivores et la plante Arabidopsis (synthèse bibliographique). Il est bien connu que les insectes herbivores, comme ceux qui se nourrissent de sève phloémienne et les insectes broyeurs, induisent une réponse de résistance chez les plantes. Une hypothèse formulée de longue date signale que les herbivores augmentent l’émission de composés organiques volatils (COVs) des feuilles d’Arabidopsis. Cependant, la plupart des travaux se sont restreints à l’étude de la régulation des COVs et, dans certains cas, aux effets de certains insectes sur l’émission des COVs. Souvent, ces travaux ne mettent pas en relation la production quantitative et qualitative des COVs des plantes avec les dégâts causés par les insectes qui leur sont inféodés. De plus, on en sait beaucoup moins sur ce qu’il se produit au niveau de l’expression du codage des protéines par la plante en conditions de stress. Dans cette synthèse bibliographique, nous résumons les effets d’insectes spécifiques se nourrissant de sève phloémienne ou d’insectes de type broyeurs, sur les émissions de COVs d’Arabidopsis thaliana Col-0 et sur la production de protéines en réponse au stress. Des recherches approfondies sur les effets des insectes herbivores amélioreront notre connaissance sur les profils et les fonctions des volatils émis par les plantes ainsi que la synthèse des protéines en relation avec la pathogenèse.
Abstract
Herbivorous insects, such as phloem-sap feeders and chewers, induce resistance response in plants. There is a long-standing hypothesis that herbivores increase the emission of volatile organic compounds (VOCs) in the Arabidopsis plant model. However, most works were restricted to the study of the regulation of plant VOC emissions and only in some cases to the effects of insects on such emissions. Often these investigations do not establish a link between quantitative and qualitative emission of plant VOCs with actual damages caused by insects. Moreover, information remain limited about the processes that occur at the protein level encoded of the host plant under stress conditions. Here, we briefly summarize the effects of specific chewing and phloem-sap feeding insects on the emission of VOCs by Arabidopsis thaliana Col-0, and review some predictions about pathogenesis-related proteins, based on current evolutionary hypotheses. Further investigation of the effects of herbivorous insects on VOC emissions and protein expression is expected to improve our knowledge about their patterns and functions in plant responses to stresses.
1. Introduction
1A significant aspect of ecology involves understanding how plants induce resistance against abiotic and biotic stress factors, such as temperature, drought, salt, insects, and pathogens. Induced resistance involves the use of defense mechanisms, resulting in the production and/or translocation of secondary products within plants that might act directly and/or indirectly on pathogens and insects. Abiotic factors may influence both primary and secondary metabolism. In the former, they change the photochemical or biochemical reactions of the photochemical cycle, while, in the latter, they affect the production of volatile emissions (e.g., terpenoids, green-leaf volatiles; Loreto et al., 2010). There is evidence that plants respond to biotic stresses by emitting a specific blend of volatiles through the expression of specific sets of genes (Walling, 2000; Thompson et al., 2006; Van Poecke, 2007; Ahuja et al., 2010; Huang et al., 2011; Louis et al., 2012). Existing studies have suggested that induced resistance in plants may be divided into two types: systemic acquired resistance (SAR) and induced systemic resistance (ISR). These types may be differentiated according to the type of bio-aggressor (e.g. pathogens and insect herbivores) and the regulatory pathway used (Vallad et al., 2004; Dicke et al., 2009). The establishment of SAR results in the accumulation of salicylate (SA). In comparison, ISR involves pathways regulated by jasmonate (JA) and ethylene (ET), which lead to the expression of either pathogenesis-related proteins (PR-proteins) or VOCs release in response to various elicitors (Vallad et al., 2004). Both SAR and ISR allow plants to reduce the risk of attack by biotic agents and enhance survival by inducing various signaling pathways (Dicke et al., 2009; Snoeren et al., 2010; De-La-Pena et al., 2012).
2Arabidopsis thaliana has a small genome and is a geographically widespread species; consequently, this plant species has adapted to a wide range of biotic and abiotic environments. It has been shown that the leaves of Arabidopsis contain feeding deterrents, such as glucosinolates (GSs) and proteinase inhibitors, which act against many herbivores (Hirai et al., 2007; Van Poecke, 2007; Hopkins et al., 2009; Louis et al., 2012). Volatile compounds that are emitted following herbivore-feeding activity on Arabidopsis may also attract the natural predators of these herbivores. In addition, recent reports have shown that there is a change in the protein levels within Arabidopsis leaves after being influenced by various elicitors (Huang et al., 2012), and that this altered protein expression results from the resistance response of host plants (Edreva, 2005; De-La-Pena et al., 2012). As both forms of induced resistance (SAR and ISR) exist in Arabidopsis in response to various threats from the outside environment, this plant represents an efficient model to study herbivore-induced resistance responses (Van Poecke, 2007; Snoeren et al., 2010). Depending on the type of insect pest and experimental details, the produced signal molecules allow plants to increase their resistance (Walling, 2000; Mewis et al., 2006; de Vos et al., 2007).
3In this paper, we discuss the results of recent studies on A. thaliana plant-insect interactions. We review:
4– the systemically-induced response of Arabidopsis against herbivorous insects;
5– how herbivores induce volatile emission and PR-protein expression within plants;
6– whether phloem-sap feeders differ from chewers with respect to the elicitation of induced defenses.
2. Herbivore-induced resistance response in Arabidopsis thaliana plants
7Herbivory is one of the most studied biotic factors involved in Arabidopsis-biotic stress interactions. Vallad et al. (2004) showed that, depending on the plant species and type of elicitor, SAR and ISR cause various secondary metabolites to be produced. To date, studies of Arabidopsis responses to insect herbivores have focused on differential VOC emission and gene expression following attack by various pests. Herbivorous pest species are classified into different groups according to the type and degree of (mechanical) wounding, such as tissue-feeding caterpillars, cell-feeding thrips, and phloem-feeding aphids (Mithofer et al., 2005; Gosset et al., 2009). These different forms of attack by insect pests determine the response mechanism (SAR or ISR) implemented by Arabidopsis (Van Poecke, 2007).
8Interactions between many plants and herbivorous insects have been well documented, producing a general picture of plant responsive resistance regulated by a signaling web, in which SA, JA, and ET play key roles. These signaling pathways are activated by specific plant genes that are strongly correlated to the type of pest attack (Walling, 2000). Zheng et al. (2011) recently demonstrated that some silence genes, like AtLOX2 and AtTGG1/TGG2, are important in the response of Arabidopsis to cabbage white butterfly (Pieris rapae) and cabbage moth (Mamestra brassicae). Although it has been shown that SA, JA, and ET are effective at inducing Arabidopsis defense responses, they may have either positive or negative effects on herbivore performance (Van Poecke, 2007; Matthes et al., 2010). For example, it is well-known that glucosinolate production is primarily effective against generalist herbivores, but not specialists (Mewis et al., 2006; Van Poecke, 2007). Moreover, the SA, JA, and ET signaling pathways may interact in Arabidopsis. Indeed, this type of interaction has been reported in response to a pathogen (Pseudomonas syringae) and a caterpillar species (Trichoplusia ni) (Van Poecke, 2007; Koornneef et al., 2008; de Vos et al., 2010). Overall, the induction of SAR and ISR causes an increase in the de novo production of secondary compounds against insect pathogens. Induced resistance may be achieved directly or indirectly by VOCs or PR-proteins (Choudhary et al., 2008). These constituents allow Arabidopsis plants to tolerate, or be protected against, various environmental challenges.
2.1. Herbivorous insects induce VOCs emission from Arabidopsis thaliana: phloem-sap feeders versus chewers
9Herbivorous insects induce the release of herbivore-induced volatiles (HI-VOC) by Arabidopsis. Similar to other plants in the Brassicaceae family, damaged Arabidopsis plants emit a complex mixture of volatiles into the air from tissue storage sites, often including glucosinolate metabolites, phenolics, and terpenoids (Van Poecke et al., 2001; Aharoni et al., 2003; Chen et al., 2003; Mewis et al., 2005; de Vos et al., 2007; Van Poecke, 2007). These compounds are released as a result of the activity of several biochemical pathways, including the isoprenoid (for terpenes), the shikimic (for phenolics, amino acids), and the lipoxygenase (for green leaf volatiles) pathway, along with the myrosinase-catalyzed degradation of GSs (e.g., Figure 1 illustrates GSs and GS metabolites; Barth et al., 2006; Van Poecke, 2007; Choudhary et al., 2008). The way in which these pathways are activated to synthesize and emit VOCs upon infestation mainly depends on the type of herbivore species and its developmental stage, and may even differ in response to different instars or sexes from the same species (Williams et al., 2005; Sarfraz et al., 2006). For example, Barth et al. (2006) found that the activity of myrosinase enzyme differs in relation to plant organ, the course of leaf development, and the type of insect infestation. These different examples indicate that Arabidopsis–herbivore interactions are complex, and that the interaction of phloem-sap feeders differs to that of chewers in eliciting induced defenses (Bidart-Bouzat et al., 2011; Ali et al., 2012; Louis et al., 2012).
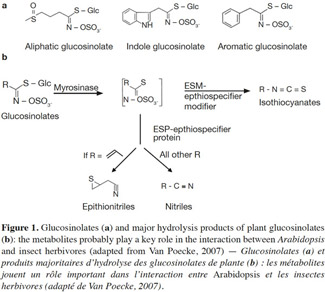
10Phloem-sap feeders. The volatile response of A. thaliana to piercing-sucking insects (phloem-sap feeders) has received much research focus. The induced resistance response relies on different steps. Before inserting the stylet into the phloem and sucking, piercing insects use it to forage around the epidermal and mesophyll cells, causing minor damage to plant foliage. Then, the salivary chemicals and/or proteins of attackers affect plant defenses, and finally lead to the biosynthesis of volatile compounds by the plant (Figure 2a; Walling, 2008; de Vos et al., 2009). Pareja et al. (2012) reported that Myzus persicae causes terpenoids and green leaf volatiles to be emitted by Sinapis alba.
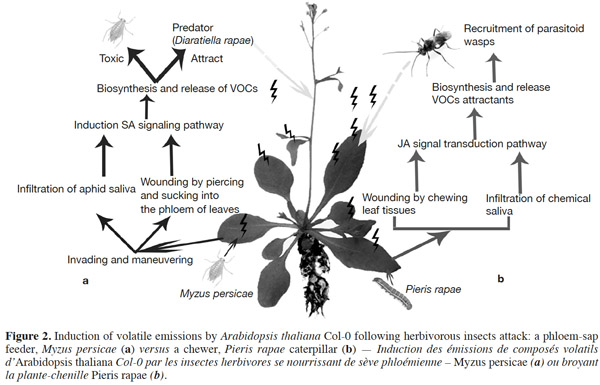
11It has been shown that undamaged Arabidopsis plants do not emit many volatiles; however, infestation by aphids induces the release of terpenoids and GSs metabolites (indole and/or aliphatic). This response is caused by an induced defense response through SA signaling, with SAR induction providing a practical means of counteracting pests (Vallad et al., 2004; Van Poecke, 2007; de Vos et al., 2010; Louis et al., 2012). However, in comparison to other type of herbivores, only a small amount of VOCs are induced. Studies have indicated that green peach aphids feeding on Arabidopsis cause the plant to release phenylpropanoid and isochorismate (Van Poecke, 2007; Louis et al., 2012), which activate the genes involved in SA biosynthesis. For example, the key chemical defenses of Arabidopsis against aphid infestation are GSs and their degradation products. Mewis et al. (2005) demonstrated that volatile derivatives of indolyl-GS from A. thaliana rosettes were induced under aphid infestation. Interestingly, aphid-induced VOCs attract predators, such as Dieretiella rapae (Girling et al., 2008). This phenomenon is probably caused by the occurrence of (E)-β-farnesene (known to act as an aphid alarm pheromone) within the induced blend, which may or may not be combined with other plant volatile compounds, that attracts parasitoids or predators (Francis et al., 2005; de Vos et al., 2010).
12Chewing insects (chewers). In contrast to piercing-sucking insects, chewing insects (chewers) cause extensive damage to plant cells following infestation, with mechanical damage and oral secretions acting as major signals that trigger the release of volatiles from plants (Figure 2b; Snoeren et al., 2010; Ali et al., 2012; Vadassery et al., 2012). The biochemical basis of host plant resistance to chewing insects is divided into two broad categories: those that influence insect behavior or the physiological responses of plants (Ali et al., 2012). It has been suggested that aliphatic GS related metabolites probably play a key role in the interaction between plants and lepidopteran herbivores.
13The cabbage white butterfly (Pieris rapae L.) caterpillar is one chewing insect that naturally frequents Brassicaceae, and causes A. thaliana to emit volatiles. Caterpillars-fed Arabidopsis plants release more volatiles (such as methyl salicylate, terpenoids, green leaf volatiles, sulfides, nitriles, alcohols, and ketones) compared to undamaged plants (Van Poecke et al., 2001; Snoeren et al., 2010; Hirao et al., 2012). Furthermore, volatile emission upon a single infestation event by multiple P. rapae may lead to the recruitment of other insect visitors, such as the parasitoid wasp Cotesia rubecula (Van Poecke et al., 2001; Snoeren et al., 2010), which parasitizes on Pieris caterpillars. Studies on the defense of Arabidopsis against caterpillar feeding have shown that VOC emissions may be induced through the JA signaling pathway by direct or indirect plant defense mechanisms. Direct defense mechanisms involve the production of anti-digestive proteins or toxic secondary metabolites, such as GS by-products that influence the behavior of pests on plants (Van Poecke et al., 2001; Snoeren et al., 2010). For instance, Plutella xylostella (the diamondback moth) caterpillars induce JA-signaling in the defense response of A. thaliana (e.g., Bidart-Bouzat et al., 2011; Savchenko et al., 2013; Zhang et al., 2013). In a previous study, Herde et al. (2008) found that Arabidopsis infested with Plutella xylostella, released methyl salicylate (MeSA), (E,E)-α-farnesene, and the C16-homoterpene TMTT ((E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene). Snoeren et al. (2010) recorded the same emissions, in addition to green-leaf volatiles. Moreover, Savchenko et al. (2013) found that damage caused to P. rapae-infested A. thaliana induced the biosynthesis of green volatiles and JA.
14Overall, studies of the response of Arabidopsis to chewing insects have focused on gene expression, signaling pathways, chemical defense phenotypes, and especially the production of GS volatile metabolites. Moreover, some compounds from volatile blends emitted by plants infested with chewing insects attract natural enemies. For example, P. rapae induces volatile emissions by A. thaliana, which attract the parasitoid Cotesia rubecula (Van Poecke et al., 2001). In another study, Loivamaki et al. (2008) observed the attraction of the parasitic wasp Diadegma semiclausum to the head space of P. rapae-infested Arabidopsis. Barker et al. (2001) reported that P. xylostella accepts A. thaliana as a host plant. However, the change in volatile profiles that are emitted following infestation has received limited study.
15Both P. rapae and P. xylostella are considered to be specialist herbivores of Brassicaceae species. These insects are able to metabolize GSs and their degradation products (Figure 2b). Indeed, when feeding on wild type Col-0 plants, P. rapae converts isothiocynates to nitriles through sulfatase activity. This phenomenon explains why only nitriles, and not isothiocyanates, have been detected in studies to date (Van Poecke et al., 2001; Mewis et al., 2006; Van Poecke, 2007; de Vos et al., 2008).
16Phloem-sap feeders versus chewers. The type of plant response to herbivorous insects is generally determined by insect species and/or its developmental stage. In a recent study, Bidart-Bouzart et al. (2011) noted that chewers cause the greater induction of Arabidopsis plant defense mechanisms compared to phloem-sap feeders in terms of VOCs emission. Hence, the interaction between herbivorous insects and plants is facilitated by signaling pathways and glucosinolate content (Mewis et al., 2005; Mewis et al., 2006; Gols et al., 2009). Indeed, many studies have investigated how the feeding mode influences the induction of various plant defense mechanisms. For example, in the induced resistance response of A. thaliana, chewers (mainly lepidopterans) upregulated different genes in JA-related pathways, leading to an increase in sulfate metabolism and aliphatic metabolites content. In contrast, these genes were all down-regulated by phloem-sap feeders (Bidart-Bouzart et al., 2011). Mewis et al. (2006) showed that phloem-feeding insects (M. persicae) increased the content of aliphatic GSs metabolites in A. thaliana, whereas chewing insects (P. rapae) increased indolyl GS derivative content. Turlings et al. (1998) also found that folivorous caterpillar (Spodoptera littoralis) induced higher levels of VOC emissions compared to the aphid (Rhopalosiphum maidis) on maize.
17Some studies have shown that the emission of plant volatiles is closely associated with herbivore feeding habit. For example, a study using Zea mays found that phloem-sap feeders (corn leaf aphids - Rhopalosiphum maidis) did not increase volatile emissions, whereas chewers (cotton leafworm - Spodoptera littoralis) strongly induced the production of many VOCs (Ali et al., 2012). Similarly, VOCs from cotton plants were induced by chewers, but not by phloem-sap feeders (Ali et al., 2012). A number of studies have compared VOC emissions caused by phloem-feeding insects versus chewing ones (Van Poecke, 2007; de Vos et al., 2008; Herde et al., 2008; Tholl et al., 2011; Ali et al., 2012) (Table 1). In general, phloem-feeding herbivores (like aphids and leafhoppers) cause minor tissue damage, and induce fewer JA-associated genes, whereas chewing insects (such as caterpillars and beetles) induce fewer genes associated with the SA pathway (Ali et al., 2012).
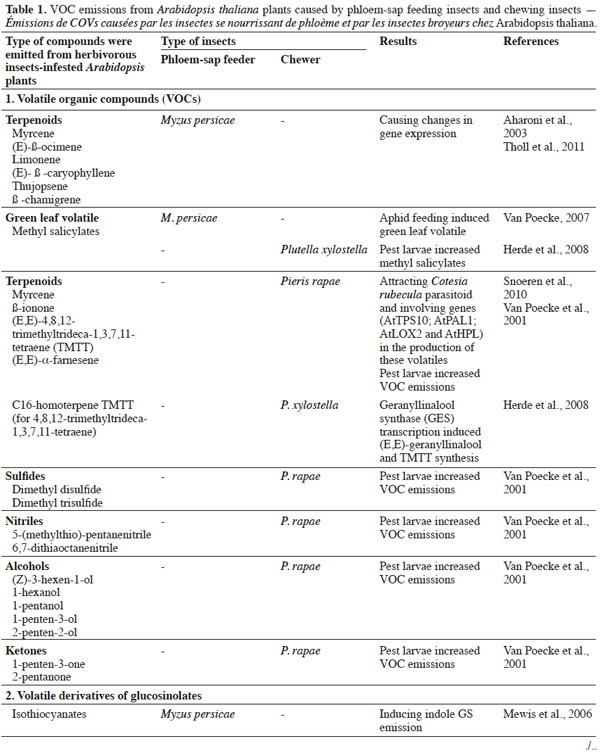
18Although Arabidopsis plant species host many insect types, the comparison of the differences between phloem-sap feeders and chewers, in terms of volatile emission, has received relatively limited research focus. There is much evidence indicating that both phloem-feeding and chewing insects may induce catabolism of GS in Arabidopsis (Mewis et al., 2005; Mewis et al., 2006; Ali et al., 2012); however, further studies are required to additionally verify this observation.
2.2. Response to herbivorous insect stress in Arabidopsis: pathogenesis-related protein identification
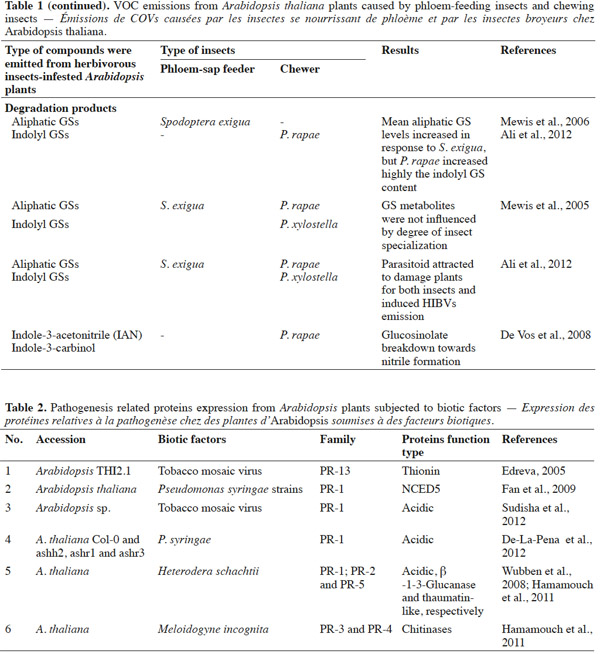
19Several studies have indicated that PR-proteins are host-encoded proteins that accumulate in response to plant pathogens or insects invasion (Edreva, 2005; Liu et al., 2006; De-La-Pena et al., 2012; Sudisha et al., 2012). For instance, acidic PR-1 proteins have been found to accumulate in response to the pathogenic infection of soybean (Phytophthora sojae), cotton (Fusarium oxysporum), alfalfa (Colletotrichum trifolii), and cassava (Xanthomonas axonopodis) (Sudisha et al., 2012). An exhaustive review of the impact of biotic factors on Arabidopsis is presented in table 2; namely whether insect feeding guild (e.g., chewers versus phloem-sap feeders) can generate consistent predictive protein expression. Proteome analyses after different biotic and abiotic stress-responses by Arabidopsis have shown that PR genes are also regulated by stress factors, such as those that mimic biotic, oxidative (Huang et al., 2011), cold (Amme et al., 2006), salt, and sugar (Seo et al., 2008) stresses, thus there is evidence that PR-proteins are expressed by host plants (Thibaud et al., 2004; Amme et al., 2006; Scherer et al. 2006; Seo et al., 2008; Fan et al., 2009). These studies showed that plant defense responses are dependent on changes in protein structure, and that this property is associated with the adaptive evolution of plants.
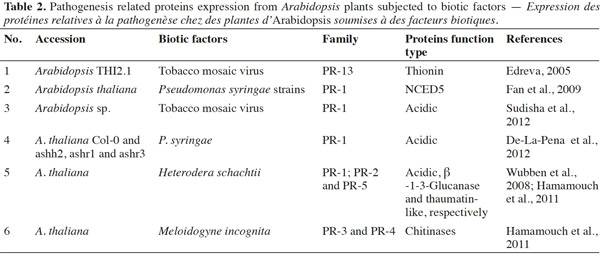
20In conclusion, to date, most knowledge about environmental stress responses of Arabidopsis and PR gene expression derives from studies of temperature, methyl jasmonate, and elicitors (mimickers) (Amme et al., 2006; Huang et al., 2011). However, the identification of PR-proteins upon herbivorous insect feeding has received limited focus. The treatment of excised Arabidopsis leaves with pathogens provided genetic evidence that abscisic acid plays a key role in plant-pathogen interactions, by inducing SAR and systemic pathogenesis-related gene1 in the cds2-1D mutant (Fan et al., 2009) (Figure 2a). Some experiments have shown that PR-proteins may be induced by pathogenic infections, in addition to SA, JA, and ET (Thompson et al., 2006; Sudisha et al., 2012). However, whether particular herbivorous insects are able to induce the synthesis of specific proteins in Arabidopsis, and whether PR-proteins may be expressed in plants infested with insect herbivores, including chewers and phloem-sap feeders, requires investigation in future studies.
3. Conclusions and research perspectives
21Volatile emission and PR-protein expression are fundamental consequences in plant responses to herbivorous insect stresses (Edreva, 2005; Maffei, 2010). In this review, we summarized existing knowledge about the changes in volatile compounds and proteins present in Arabidopsis after challenge by herbivorous attack. Several studies have shown that VOC emissions and PR-protein expression are induced by different stressors (phloem-sap feeders versus chewers), and are regulated by several biosynthetic pathways (the isoprenoid, shikimic and lipoxygenase pathways, along with myrosinase-catalyzed degradation of GSs).
22The functional role of volatile blends produced by plants in response to ecological factors has been considered in recent years by different approaches such as plant-plant interactions, plant-insect interactions, and plant-abiotic factors interactions (Maffei, 2010). These approaches focused on the impact of single abiotic and biotic stresses, independent of each other. However, studies that combine both abiotic and biotic stresses are required, to assess how these factors interact. Moreover, plants that grow in continental environments that contain a high diversity of organisms might be subject to the simultaneous attack of various invaders, especially herbivores. Such multiple stresses are probably natural, and may influence plant photosynthesis and different defense responses, producing a variety of volatile profiles (Holopainen et al., 2010). Therefore, it is important to quantify these phenomena to increase existing knowledge about the resistance responses of plants under natural environmental conditions.
23The relationship between Arabidopsis and its elicitors has also been studied in terms of PR-protein expression; however, only a limited number of stresses have been considered (e.g., cold, oxidative, and mimicked biotic stress). The interaction of herbivorous insects, or a combination of pests and abiotic factors, may potentially alter protein biosynthesis. However, experimental evidence remains limited to reach any wide-ranging conclusions on this topic, which requires further analysis.
24Overall, knowledge about the effects of biotic and/or abiotic factors on volatile emission and PR-proteins expression from A. thaliana plants could help us to determine the natural occurrence of such reaction products (VOCs and PR-proteins) and their importance in plant defense strategies. This review present the different ways of defense mechanisms by Arabidopsis against attackers; therefore, studies investigating the relationship of different plant-herbivorous insect interactions and the production of various components and proteins are of interest for comparison purposes.
25Abbreviations
26ET: ethylene
27GSs: glucosinolates
28ISR: induced systemic resistance
29JA: jasmonate
30NCED5: nine-cis-epoxycarotenoid dioxygenase5
31PR-protein: pathogenesis-related proteins
32SA: salicylate
33SAR: systemic acquired resistance
34VOCs: volatile organic compounds
35Acknowledgements
36Dieu-Hien Truong is recipient of a PhD scholarship from Ministry of Education and Training Vietnam. We specially thank to Dr. Van Poecke who authorizes the replication of Figure2.
Bibliographie
Aharoni A. et al., 2003. Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell, 15, 2866-2884.
Ahuja I., Rohloff J. & Bones A.M., 2010. Defence mechanisms of Brassicaceae: implications for plant-insect interactions and potential for integrated pest management. Agron. Sustainable Dev., 2, 623-670.
Ali G.J. & Agrawal A.A., 2012. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci., 17(5), 293-302.
Amme S., Matros A., Schlesier B. & Mock P.-H., 2006. Proteome analysis of cold stress response in Arabidopsis thaliana using DIGE-technology. J. Exp. Bot., 57(7), 1537-1546.
Barker J., Poppy G. & Payen C., 2001. Arabidopsis thaliana as a model host plant for Plutella xylostella. In: Endersby N.M. & Ridland P.M., eds. Proceedings of the 4th International workshop, The management of diamondback moth and other crucifer pests, Nov. 2001, Melbourne, Australia, 147-151. Gosford, UK: The Regional Institute Ltd.
Barth C. & Jander G., 2006. Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. Plant J., 46, 549-562.
Bidart-Bouzat G.M. & Kliebenstein D., 2011. An ecological genomic approach challenging the paradigm of differential plant responses to specialist versus generalist insect herbivores. Oecologia, 167, 677-689.
Chen F. et al., 2003. Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell, 15, 481-494.
Choudhary K.D., Johri N.B. & Prakash A., 2008. Volatiles as priming agents that initiate plant growth and defence responses. Curr. Sci., 94(5), 598-604.
de Vos M., Jae H.K. & Jander G., 2007. Biochemistry and molecular biology of Arabidopsis–aphid interactions. BioEssays, 29, 871-883.
de Vos M., Kriksunov L.K. & Jander G., 2008. Indole-3-acetonitrile prodution from indole glucosinolates deter oviposition by Pieris rapae. Plant Physiol., 146, 916-926.
de Vos M. & Jander G., 2009. Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Environ., 32, 1548-1560.
de Vos M. & Jander G., 2010. Volatile communication in plant–aphid interactions. Biotic interactions. Plant Biol., 13, 366-371.
De-La-Pena C., Rangel-Cano A. & Alvarez-Venegas R., 2012. Regulation of disease-responsive genes mediated by epigenetic factors: interaction of Arabidopsis–Pseudomonas. Mol. Plant Pathol., 13(4), 388-398.
Dicke M., van Loon J.A.J. & Soler R., 2009. Chemical complexity of volatiles from plants induced by multiple attacks. Nat. Chem. Biol., 5, 317-324.
Edreva A., 2005. Pathogenesis-related proteins: research progress in the last 15 years. Gen. Appl. Plant Physiol., 31(1-2), 105-124.
Fan J. et al., 2009. Abscisic acid has a key role in modulating diverse plant pathogen interactions. Plant Physiol., 150, 1750-1761.
Francis F., Martin T., Lognay G. & Haubruge É., 2005. Role (E)-ß-farnesene in systematic aphid prey location by Episyrphus balteatus larvae (Diptera: Syrphidae). Eur. J. Entomol., 102, 431-436.
Girling R.D., Hassall M., Turner J.G. & Poppy G.M., 2008. Behavioural response of the aphid parasitoid Dieretiella rapae to volatiles from Arabidopsis thaliana induced by Myzus persicae. Entomol. Exp. Appl., 120, 1-9.
Gols R. & Harvey J.A., 2009. Plant-mediated effects in the Brassicaceae on the performance and behaviour of parasitoids. Phytochemistry, 8(1), 187-206.
Gosset V. et al., 2009. Attacks by a piercing-sucking insect (Myzus persicae Sultzer) or a chewing insect (Leptinotarsa decemlineata Say) on potato plants (Solanum tuberosum L.) induce differential changes in volatile compound release and oxylipin synthesis. J. Exp. Bot., 60(4), 1231-1240.
Hamamouch N. et al., 2011. Expression of Arabidopsis pathogenesis-related genes during nematode infection. Mol. Plant Pathol., 12(4), 355-364.
Herde M. et al., 2008. Identification and regulation of TPS04/GES, an Arabidopsis geranyllinalool synthase catalyzing the first step in the formation of the insect-induced volatile C16-homoterpene TMTT. Plant Cell, 20(4), 1152-1168.
Hirai M.Y. et al., 2007. Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc. Natl. Acad. Sci., 104(15), 6478-6483.
Hirao T. et al., 2012. Green leaf volatiles enhance methyl jasmonate response in Arabidopsis. J. Biosci. Bioeng., 114(5), 540-545.
Holopainen K.J. & Gershenzon J., 2010. Multiple stress factors and the emission of plant VOCs. Special issue: induced biogenic volatile organic compounds from plants. Trends Plant Sci., 15(3), 176-184.
Hopkins R.J., van Dam N.M. & van Loon J.J., 2009. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol., 54, 57-83.
Huang C. et al., 2011. Response to biotic and oxidative stress in Arabidopsis thaliana: analysis of variably phosphorylated proteins. J. Protemics, 74, 1934-1949.
Huang M. et al., 2012. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-ß-caryophyllene, is a defense against a bacterial pathogen. New Phytol., 193, 997-1008.
Koornneef A. & Pieterse C.M., 2008. Cross talk in defense signaling. Plant Physiol., 146(3), 839-844.
Liu J.-J. & Ekramoddoullah K.M.A., 2006. The family 10 of plant pathogenesis-related proteins: their structure, regulation, and function in response to biotic and abiotic stresses. Physiol. Mol. Plant Pathol., 68, 3-13.
Loivamaki M., Mumm R., Dicke M. & Schnitzler J.P., 2008. Isoprene interferes with the attraction of bodyguards by herbaceous plants. Proc. Natl. Acad. Sci. USA, 105(45), 17430-17435.
Loreto F. & Schnitzler J.-P., 2010. Special issue: induced biogenic volatile organic compounds from plants: abiotic stresses and induced BVOCs. Trends Plant Sci., 15(3), 154-166.
Louis J., Singh V. & Shah J., 2012. Arabidopsis thaliana-aphid interaction. Am. Soc. Plant Biologists, 10, 1-19.
Maffei M.E., 2010. Sites of synthesis, biochemistry and functional role of plant volatiles. S. Afr. J. Bot., 76, 612-631.
Matthes M.C. et al., 2010. The transcriptome of cis-jasmone-induced resistance in Arabidopsis thaliana and its role in indirect defence. Planta, 232, 1163-1180.
Mewis I. et al., 2005. Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol., 138, 1149-1162.
Mewis I. et al., 2006. Gene expression and glucosinolate accumulation in Arabidopsis thaliana in response to generalist and specialist herbivores of different feeding guilds and the role of defense signaling pathways. Phytochemistry, 67, 2450-2462.
Mithofer A., Wanner G. & Boland W., 2005. Effects of feeding Spodoptera littoralis on lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiol., 137, 1160-1168.
Pareja M. et al., 2012. Herbivory by a phloem-feeding insect inhibits floral volatile production. PlosOne, 7(2), e31971, 1-11.
Sarfraz M., Dosdall M.L. & Keddie A.B., 2006. Diamondback moth–host plant interactions: implications for pest management. Crop Prot., 25, 625-639.
Savchenko T. et al., 2013. Insect herbivores selectively suppress the HPL branch of the oxylipin pathway in host plants. Plant J., 73(4), 653-662.
Scherer M.E. et al., 2006. Evolutionary analysis in pathogenesis-related proteins. Comput. Biophys. Syst. Biol., 34, 193-196.
Seo J.P., Lee A.-K., Xiang F. & Park C.-M., 2008. Molecular and functional profiling of Arabidopsis pathogenesis-related genes: insights into their roles in salt response of seed germination. Plant Cell Physiol., 49(3), 334-344.
Snoeren A.L.T. et al., 2010. Natural variation in herbivore-induced volatiles in Arabidopsis thaliana. J. Exp. Bot., 61(11), 3041-3056.
Sudisha J. et al., 2012. Pathogenesis related proteins in plant defense response. Prog. Biol. Control, 12, 379-403.
Thibaud C.-M., Gineste S., Nussaume L. & Robadlia C., 2004. Sucrose increases pathogenesis-related PR-2 gene expression in Arabidopsis thaliana through an SA-dependent but NPR1-independent signaling pathway. Plant Physiol. Biochem., 42, 81-88.
Tholl D. & Lee S., 2011. Terpene specialized metabolism in Arabidopsis thaliana. Arabidopsis Book, 9, e0143.
Thompson E.C. et al., 2006. Molecular modeling of pathogenesis-related proteins of family 5. Cell Biochem. Biophys., 44, 385-395.
Turlings T.C.J. et al., 1998. The induction of volatile emissions in maize by three herbivore species with different feeding habits: possible consequences for their natural enemies. Biol. Control, 11, 122-129.
Vadassery J., Reichelt M. & Mithofer A., 2012. Direct proof of ingested food regurgitation by Spodoptera littoralis caterpillars during feeding on Arabidopsis. J. Chem. Ecol., 38(7), 865-872.
Vallad E.G. & Goodman M.R., 2004. Review and interaction: systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci., 44, 1920-1934.
Van Poecke R.M.P., 2007. Arabidopsis-insect interactions. Arabidopsis Book, 5, e0107.
Van Poecke R.M.P., Posthumus A.M. & Dicke M., 2001. Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioral, and gene-expression analysis. J. Chem. Ecol., 27(10), 1911-1928.
Walling L.L., 2000. The myriad plant response to herbivore. J. Plant Growth Regul., 19, 195-216.
Walling L.L., 2008. Avoiding effective defences: strategies employed by phloem-feeding insects. Plant Physiol., 146, 859-866.
Williams III L., Rodriguez-Saona C., Pare W.P. & Cradts-Brandner J.S., 2005. The piercing-sucking herbivores Lygus hesperus and Nezara viridula induce volatile emissions in plants. Arch. Insect Biochem. Physiol., 58, 84-96.
Wubben M.J.E., Jin J. & Baum T.J., 2008. Cyst nematode parasitism of Arabidopsis thaliana is inhibited by salicylic acid (SA) and elicits uncoupled SA-independent pathogenesis-related gene expression in roots. Mol. Plant-Microbe Interact., 21, 424-432.
Zhang P.J. et al., 2013. Jasmonate and ethylene signaling mediate whitefly-induced interference with indirect plant defense in Arabidopsis thaliana. New Phytol., 197(4), 1291-1299.
Zheng S.J., Zhang P.J., van Loon J.J. & Dicke M., 2011. Silencing defense pathways in Arabidopsis by heterologous gene sequences from Brassica oleracea enhances the performance of a specialist and a generalist herbivorous insect. J. Chem. Ecol., 37(8), 818-829.

