- Accueil
- Volume 28 (2025)
- number 1-2
- A tiny dolphin (Cetacea, Odontoceti, Kentriodontidae) cranium from the Middle Miocene of the southern North Sea with a rare osteological malformation
Visualisation(s): 806 (9 ULiège)
Téléchargement(s): 381 (2 ULiège)
A tiny dolphin (Cetacea, Odontoceti, Kentriodontidae) cranium from the Middle Miocene of the southern North Sea with a rare osteological malformation

Abstract
Though the Neogene deposits of northern Belgium yielded many marine vertebrate remains, including cetaceans, the fossil record of several clades remains scarce. Among echolocating toothed whales (Odontoceti), cranial fossils of early delphinidans generally referred to the family Kentriodontidae are surprisingly rare in the Miocene units of the Antwerp area. Recently, a large construction pit excavated in the city centre of Antwerp allowed for the lithostratigraphic description of a section in the Kiel and Antwerpen members of the Berchem Formation. In addition to an elasmobranch assemblage of 13 species, a well-preserved, tiny odontocete cranium was discovered in the lower part of the Antwerpen Member (Middle Miocene, lower Langhian). Representing a young individual, this rare fossil displays strong anatomical similarities with the small kentriodontid Kentriodon pernix, originally found in upper Lower to Middle Miocene deposits of the Atlantic Coastal Plain (USA). It is referred here to Kentriodon cf. K. pernix, contributing to the improvement of the scant fossil record of early delphinidans in the North Sea. Associated to a degree of tooth wear that is unexpected in such a young individual, highly unusual anatomical traits, especially in the orbit region, are interpreted as resulting from a malformation that occurred relatively early during cranial development.
Table des matières
1. Introduction
1During the last two centuries, numerous marine vertebrate fossils have been recorded in temporary excavations near the city of Antwerp (northern Belgium, Fig. 1) (e.g. Van Beneden, 1865; Le Hon, 1871; Abel, 1901, 1902, 1905; Leriche, 1926; Misonne, 1958). In this region, the Lower–Middle Miocene Berchem Formation lies at a shallow depth below the surface, making these fossiliferous glauconitic sandy sediments frequently accessible for stratigraphical and palaeontological research (De Meuter et al., 1976; Louwye et al., 2010; Hoedemakers & Dufraing, 2018; Everaert et al., 2020). In particular, the early Langhian to middle Serravallian Antwerpen Member (Berchem Formation) yields many vertebrate remains (Lambert, 2005a; Louwye et al., 2010, 2020; Collareta & Bosselaers, 2022). Most of these are encountered in the lower part of this member, in and around a phosphatic horizon representing a maximum flooding surface (Everaert et al., 2020; Deckers & Everaert, 2022; Deckers et al., 2025). Unfortunately, the stratigraphic origin of most findings of the 19th and early 20th centuries was poorly documented. Hence, new temporary outcrops are still of interest, not only to collect additional fossils but also to better contextualize historical finds.
2In 2023, a large temporary outcrop could be sampled along the Plantin en Moretuslei in the centre of Antwerp. This section not only complements our knowledge of the regional geology, but it also adds one rare vertebrate fossil from the base of the Antwerpen Member. A well-preserved cranium representing a young dolphin from the family Kentriodontidae (Odontoceti) was encountered. Kentriodontidae s.l. were a diversified family of small to large-sized, extinct dolphins with a global distribution, flourishing during the Miocene (e.g. Muizon, 1988b; Dawson, 1996; Bianucci, 2001; Kazár, 2005; Kimura & Hasegawa, 2019; Godfrey & Lambert, 2023). Insights in their phylogeny are rapidly evolving (Lambert et al., 2017; Peredo et al., 2018; Guo & Kohno, 2021, 2023), further taxonomic adjustments are therefore expected. While most kentriodontid findings in Belgium and the Netherlands consist of loose teeth, periotics, and tympanic bullae (Louwye et al., 2010; Everaert et al., 2019; Schouten, 2021), with only a few exceptions (e.g. du Bus, 1872; Abel, 1905), the new cranium allows detailed comparison with amongst others Kentriodon pernix, from the late Early to Middle Miocene of the Atlantic Coastal Plain, USA. In addition, unique anatomical features observed on this specimen are tentatively interpreted as resulting from abnormal cranial development.
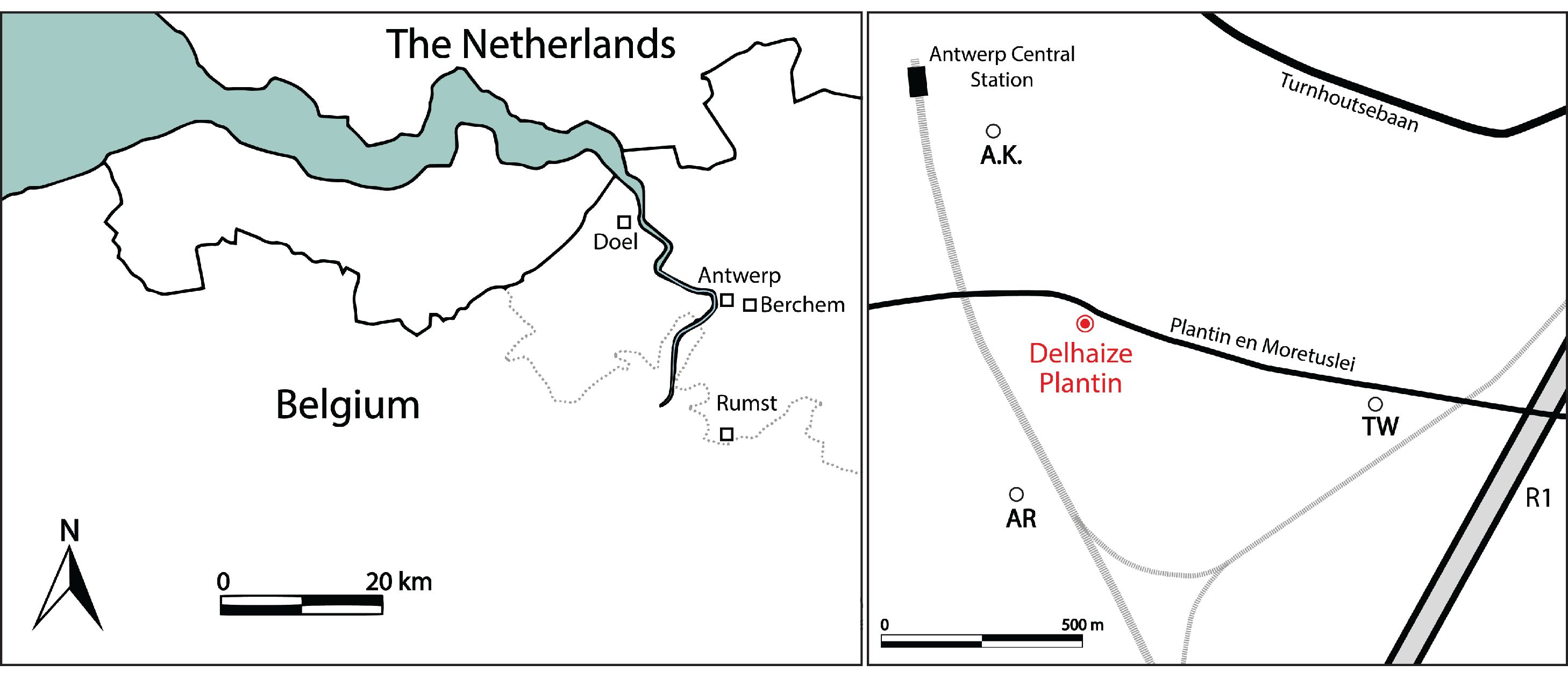
Figure 1. Map of the Antwerp area in northern Belgium (left) and location of the study area in Antwerp (right). Dotted line for southern border of the Lower Miocene after Louwye et al. (2010); black line for country borders. The temporary outcrop Delhaize Plantin, where the kentriodontid specimen RBINS M.2340 was discovered, is indicated in red. Older outcrops are Antwerpen Kievitstraat (A.K.; De Meuter et al., 1976), Argenta and Tweelingenstraat (AR and TW; Everaert et al., 2020).
2. Materials and methods
2.1. Institutional abbreviations
3RBINS, Royal Belgian Institute of Natural Sciences, Brussels, Belgium; USNM, National Museum of Natural History, Smithsonian Institution, Washington, D.C., USA.
2.2. Fossil material
4The studied cetacean fossil was encountered in the base of the Antwerpen Member of the Berchem Formation (Fig. 2) by JVB on May 8th, 2023, in a fragmentary state. All fragments were collected, dried, and impregnated with polyvinyl acetate glue (PVA) diluted in acetone. This is a reversible conservation technique. Subsequently, the treated fragments were reassembled by MB with PVA glue. The specimen has been deposited in the vertebrate palaeontology collection of the Royal Belgian Institute of Natural Sciences with the collection number RBINS M.2340.
5For the description of the cranium RBINS M.2340, the anatomical nomenclature follows Mead & Fordyce (2009). This specimen was compared to other ‘kentriodontids’ s.l. using the available literature, pictures from previous visits to other collections, various casts (including a cast of the cranium of Kentriodon pernix USNM 10670), and the type specimen of Acrodelphis scheynensis IRSNB M.372.
6Additionally, bulk sampling on a 5 mm mesh yielded 13 elasmobranch species. Approximately 1.5 m3 of the base of the Antwerpen Member (Unit 2) was sieved. Only a mesh size of 5 mm was used due to time constraints and the absence of water at the construction site. While this strategy was applied multiple times in the past and allows comparison with older literature, it implies a sampling bias towards taxa characterized by larger fossil remains. The studied elasmobranch remains are housed in the private collection of JVB, as no new species were found compared to published records from other outcrops.
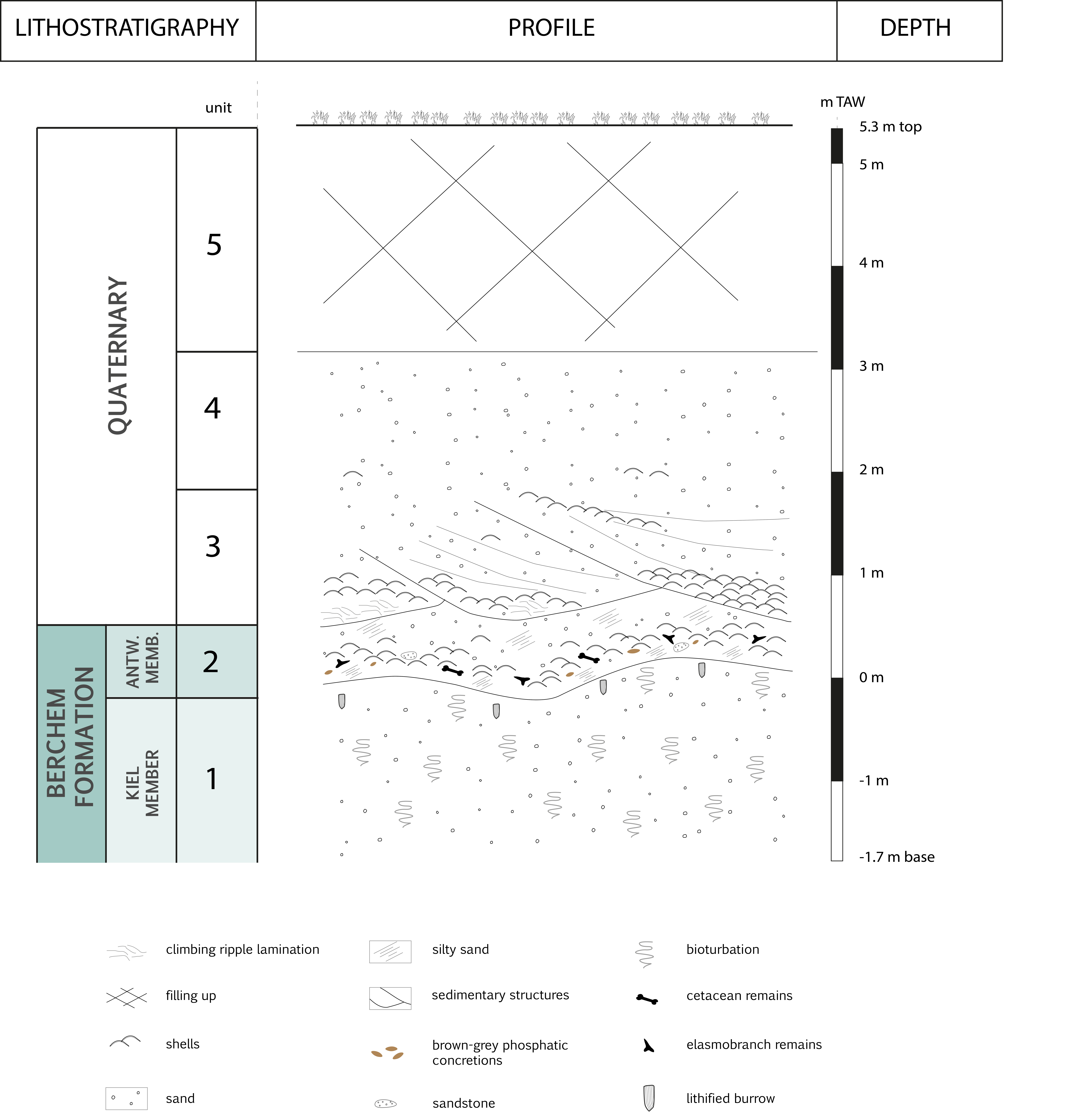
Figure 2. Lithology and lithostratigraphy of the temporary outcrop Delhaize Plantin in Antwerp (northern Belgium). The kentriodontid specimen RBINS M.2340 was recovered from the base of Unit 2 (Antwerpen Member, Berchem Formation). Abbreviations: Antw. Memb. = Antwerpen Member; TAW = Tweede Algemene Waterpassing (Belgian Ordnance Datum).
3. Geological and palaeontological setting
3.1. Locality
7Between April and May 2023, a large construction pit was excavated for a new underground car park in Antwerp (northern Belgium). The temporary outcrop, hereafter referred to as “Delhaize Plantin”, was located between the Plantin en Moretuslei and the Magdalenastraat (WGS84-coordinates 51.2099, 4.4275; Fig. 1). Nine sediment samples are stored at the Belgian Geological Survey (RBINS, BGS 028E0933) and the Geotheek (DOV TO-20230501) of the Flemish Department of Environment.
3.2. Lithological and palaeontological description
8From bottom (-1.7 m) to top (+5.3 m TAW) (TAW = Tweede Algemene Waterpassing (Belgian Ordnance Datum)), the following units are observed (Figs 2–3):
9Unit 1: About 190 cm of fine to medium-fine, relatively well-sorted grey sand with a very high glauconite content. There is nearly no admixture of clay or silt. The sand contains no trace of macrofossils. No sedimentary structures were observed. The sand is bioturbated and contains numerous polychaete traces with cross-sections of only a few millimetres, presumably belonging to Macaronichnus. Occasional crustacean burrows belonging to Ophiomorpha also occur. In the upper 20 cm of this unit, scattered whitish, often circular patches occur with a diameter <3 cm. These are sandstone cross-sections of lithified burrows of O. nodosa. Samples: A (-1.7 m TAW), B (-0.3 m TAW), C (+0.1 m TAW).
10Unit 2: Up to 55 cm thick interval of fine, dark green to blackish sand with a silty admixture and a very high glauconite content. The thickness of this unit is variable due to the erosive contact with the overlying deposits. A clear colour difference can be observed compared to the underlying grey sand. Dispersed shells occur, in addition to a laterally continuous shell bed (dominated by Glycymeris obovata baldii) about 20 cm above the base. Locally, the shell bed converges with the base. Most shells are loose valves with convex-up orientation, although double-valved specimens are also present. Besides molluscs, shark teeth and remains of marine mammals occur scattered along with some rounded brown-grey concretions. Sometimes, the shell bed splits into two thin “sub-layers”. Together with the base of this unit, the shell bed often follows a slightly undulating course (simple load casts). Samples: D (+0.35 m TAW), E (+0.5 m TAW), F (+0.55 m TAW).
11Unit 3: Over 1 metre with grey to light brown/yellowish sand with irregular sorting. The lower part is locally greyish due to the high content of reworked glauconite. The structure of this unit is highly variable and consists of several successive lenses (20–40 cm thick) full of reworked shells and broken shell grit, pebbles, rounded concretions and bone remains (marine Neogene and terrestrial Quaternary). Most of the shells are reworked from Miocene (Glycymeris obovata baldii) and Pliocene deposits (e.g. Angulus benedeni benedeni, Talochlamys harmeri, Ostrea edulis, Digitariopsis obliquata, Astarte incerta). The orientation of the shells is usually very chaotic, both convex-up and convex-down valves occur. The shell lenses are usually located at the base of gullies, with the deepest parts of the gully showing the highest concentration of shell fragments in the thickest part of the layer, thinning towards the edges. The gullies often cut deep into the top of the underlying unit 2, with the latter sometimes being completely removed. Compared to the sand fraction, the volume occupied by shells is very high in the lenses. In between the shell lenses, sand with very fine shell debris deposited in laminations, sometimes visible with subtle cross-bedding, is present. Locally, the base of this unit is not formed by shell gravel, but sometimes by a thin strip of sand with subtle climbing ripple lamination. Samples: K1 (+0.75 m TAW), K2 (+0.9 m TAW), F (+1.1 m TAW).
12Unit 4: About 150 cm, very similar to underlying unit, although lenses with shell remains are scarce and only weakly developed.
13Unit 5: About 200 cm of very heterogenous sediment, oxidized at the base. Towards the top strongly disturbed by anthropogenic activity.
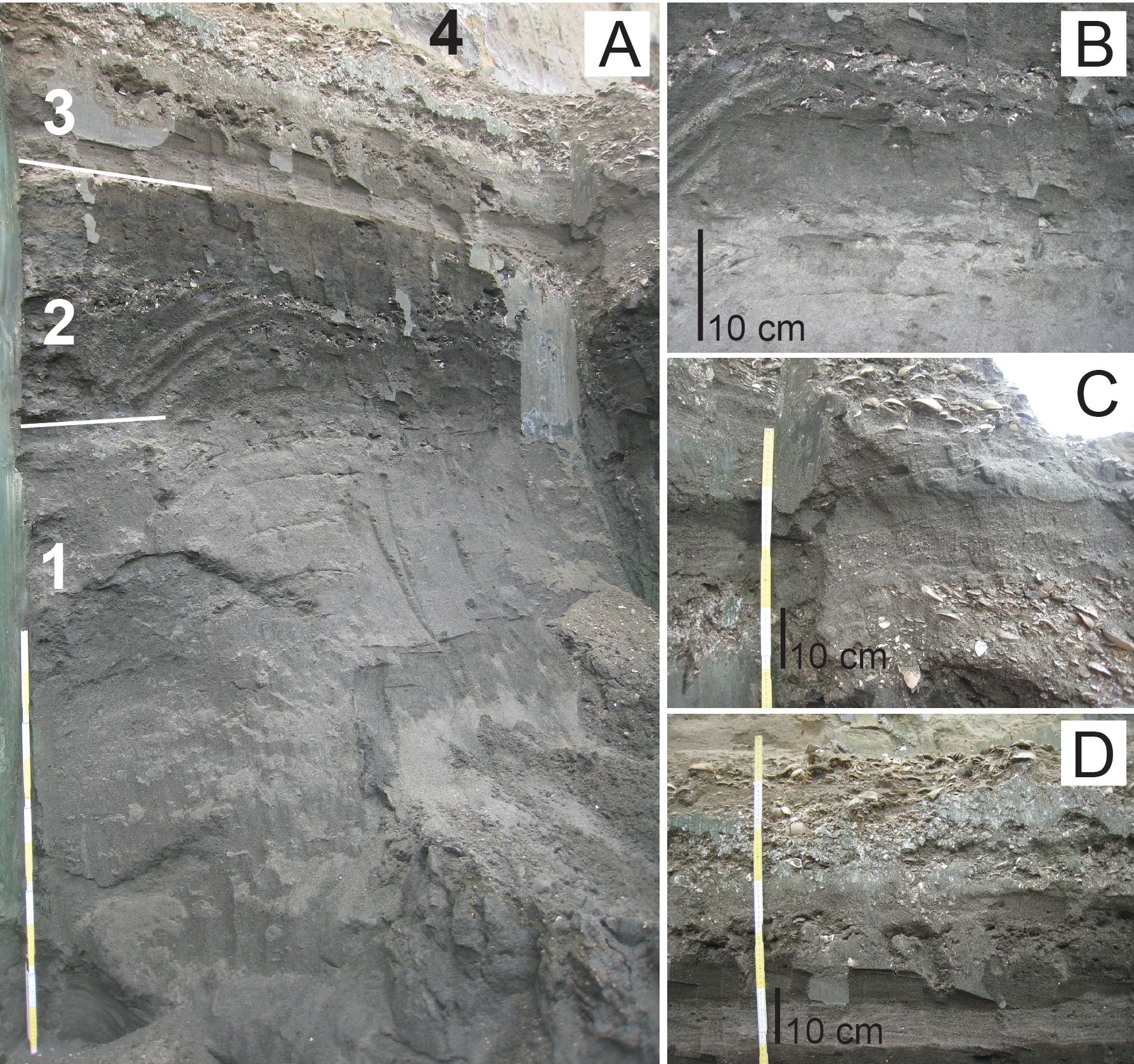
Figure 3. Sampled section of the temporary outcrop Delhaize Plantin, northern wall along the Plantin en Moretuslei, Antwerp (northern Belgium). A. Unit 1 (Kiel Member, Berchem Formation), Unit 2 (Antwerpen Member, Berchem Formation, where the kentriodontid specimen RBINS M.2340 was found), and Units 3–4 (Quaternary deposits). Scale bar = 1 m. B. Detail of the boundary between the middle–late Burdigalian Kiel Member and the lower Langhian Antwerpen Member. Some vague white spots can be observed in the top of the Kiel Member, representing cross-section of lithified Ophiomorpha burrows. C. Detail of Quaternary unit 3. Subtle cross-bedding can be observed between the lenses with reworked Neogene shell debris. D. Other detail of the Quaternary unit 3, with high contents of reworked glauconite and fine climbing ripple lamination in the base. Photographs taken on May 8, 2023.
3.3. Lithostratigraphy, age and palaeoenvironment
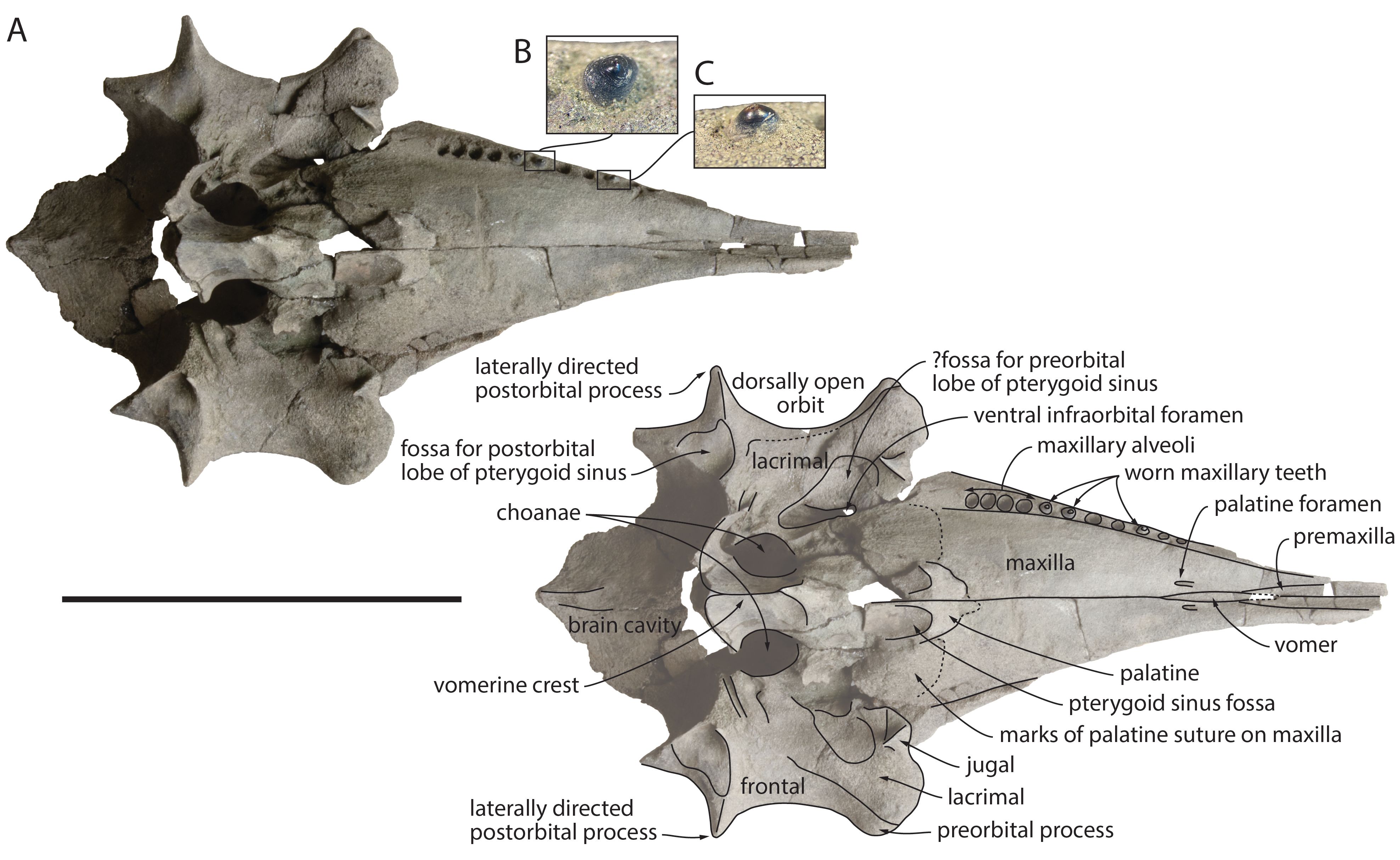
14Given the very high glauconite content (up to 50%) of the fine to medium-fine grained sand of units 1 and 2, these can be attributed to the Berchem Formation (De Meuter & Laga, 1976; Louwye & Deckers, 2023). Due to its grey colour, the near absence of clay and silt, abundant bioturbation and the lack of shells, unit 1 is characteristic of the Kiel Member (De Meuter & Laga, 1976; Everaert et al., 2020; Louwye et al., 2023a). The overlying unit 2 can be assigned to the Antwerpen Member, given its higher silt content and the abundant presence of Glycymeris shells, both dispersed and concentrated in beds (De Meuter et al., 1976; Louwye et al., 2023b). The boundary between both members is similar to nearby sections (Louwye et al., 2010; Hoedemakers & Dufraing, 2018; Everaert et al., 2020, 2024).
15Based on dinoflagellate cyst biostratigraphy, the Kiel Member is of middle to late Burdigalian age (zone M4 of Munsterman & Brinkhuis, 2004 and the E. insigne and C. aubryae zones of Dybkjær & Piasecki, 2010; see Louwye et al., 2020 and Deckers et al., 2025). The overlying lower part of the Antwerpen Member represents an early Langhian age (zone M5 of Munsterman & Brinkhuis, 2004 and the L. truncatum zone of Dybkjær & Piasecki, 2010; see Louwye et al., 2020 and Deckers et al., 2025). Compared to the underlying Kiel Member, the lower Antwerpen Member reflects a calmer, presumably deeper marine environment in which also the silt fraction (16%, Deckers et al., 2023) could settle, below the fair-weather wave base. It was deposited in open neritic conditions during a eustatic sea-level high in the Mid-Miocene Climatic Optimum (Louwye et al., 2010; Deckers et al., 2023).
16Units 3–4 belong to the Quaternary, which can be very variable in this region. The lenses with chaotic shell debris and pebbles are interpreted as channel lag deposits. These are residual concentrations accumulating as discontinuous lenses in deeper parts of channels, present in fluviatile deposits but also in gullies and creeks of intertidal flats (Reineck & Singh, 1975). As the typical clay laminae were not observed, fluvial deposits may be more likely, accumulating large quantities of Neogene shells when the underlying Neogene deposits were eroded. Alternatively, these lenses may represent periglacial deposits, where on the denudation surface, gullies of meltwater locally cut into the substrate and filled it with reworked material of local origin. The outcrops are however not large enough to be conclusive about this (R. Houthuys, pers. comm., 2024). The precise age of these deposits remains to be determined. However, terrestrial and fluviatile molluscs in similar strata near Kruibeke (5 km SW of Antwerp) indicated a Late Pleistocene age (Roosen in Crombé et al., 2022).
3.4. Elasmobranch assemblage
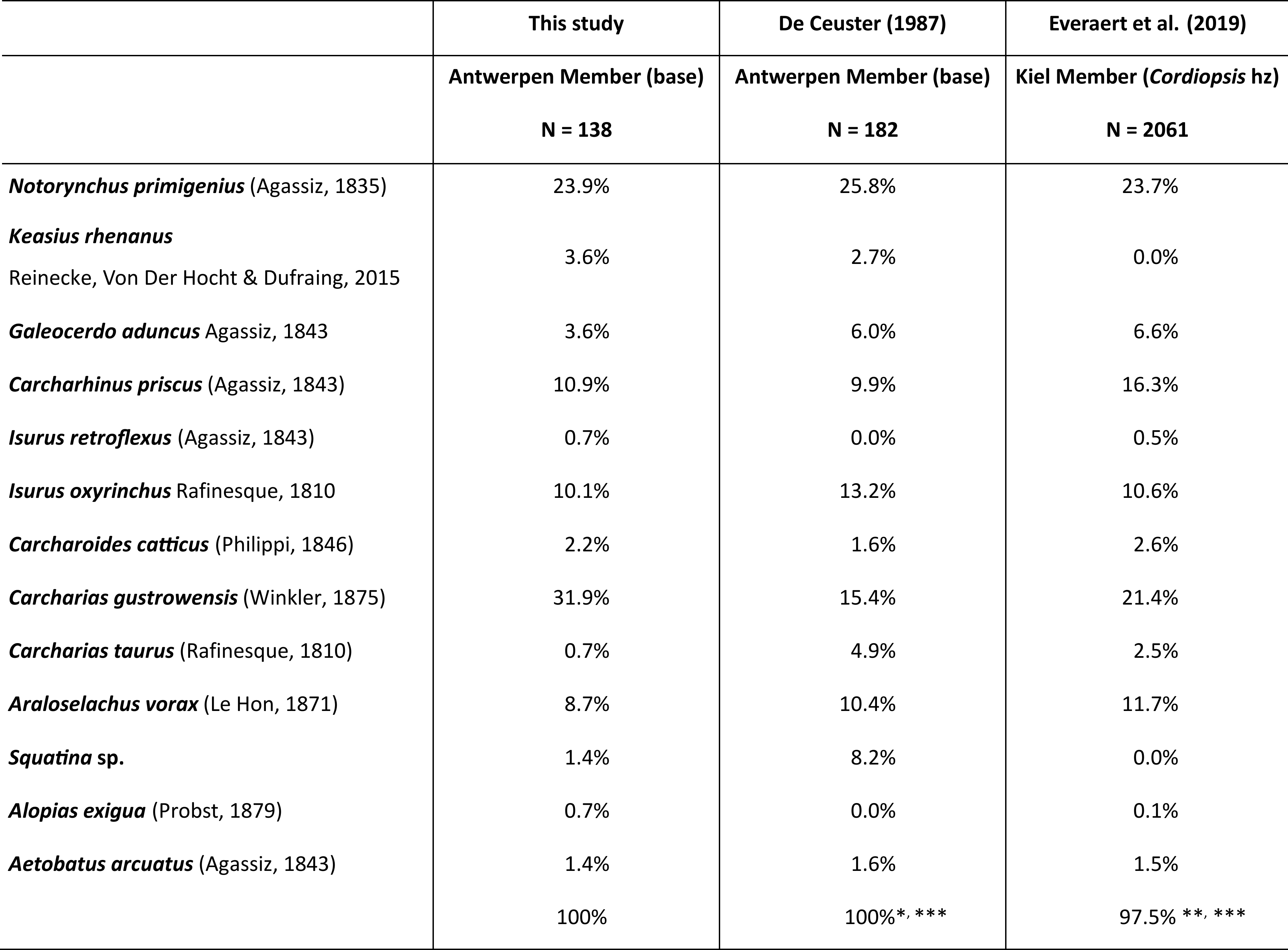
17The base of the Antwerpen Member yielded elasmobranch remains, consistent with observations from other localities such as Posthofbrug (Louwye et al., 2010) and the Antwerpse Hypotheekkas in Berchem (De Ceuster, 1987). At the temporary outcrop Delhaize Plantin, remains of 13 species were collected (12 selachians, 1 batoid) (Table 1). These species are well known from the Berchem Formation (e.g. Leriche, 1926; De Ceuster, 1987; Everaert et al., 2019) and many other Lower–Middle Miocene strata in the southern North Sea Basin (Reinecke et al., 2011; Bor et al., 2012).
18Everaert et al. (2019) discussed the elasmobranchs of the middle Burdigalian ‘Cordiopsis horizon’ in the Kiel Member at the Post X site in Berchem. Hence, two faunal lists are available based on the sampling method, with counts >5 mm and >1 mm, aiding in comparison. Earlier, De Ceuster (1987) provided a faunal overview of several layers of the Berchem Formation at the Antwerpse Hypotheekkas site in Berchem. Similar to the outcrop Delhaize Plantin, his ‘layer 2’ constitutes the base of the Antwerpen Member.
19While small, benthic species (Rajidae, Dasyatidae, Squatina sp.), may represent ca 80% of the fauna (all teeth >1 mm) (Everaert et al., 2019), smaller species are lacking in the Delhaize Plantin section due to sampling bias. In the fraction >5 mm, benthopelagic species dominate. The elasmobranch assemblages at the three locations exhibit similar distributions, with Notorynchus, Carcharias, Isurus, Carcharhinus, and Araloselachus dominating (>70% of teeth >5 mm). Smaller populations of Galeocerdo, Carcharoides, Keasius and Aetobatus are consistently present in the three assemblages. Minor differences between the three faunas occur. Rare taxa in one outcrop are also scarce or even absent in the other outcrops, i.e. Alopias, Otodus, Hemipristis, and Isurus retroflexus.
20Strikingly, the Delhaize Plantin elasmobranch assemblage of the basal Antwerpen Member did not yield any Carcharodon hastalis, while Isurus oxyrinchus dominates. This shift in dominance from I. oxyrinchus to C. hastalis between the Lower to Middle Miocene is well described in previous studies (De Ceuster, 1987; Everaert et al., 2019, 2020). Higher up in the Antwerpen Member, the autochthonous fauna is dominated by C. hastalis. The similarity in faunal composition and the pristine preservation of the elasmobranch teeth suggests a tranquil reworking of fossils from the Kiel Member into the base of the Antwerpen Member, in contrast to other transgressive contacts (e.g. Lutetian Lede and Zanclean Kattendijk formations). This was also observed in the Tweelingenstraat outcrop, approximately 570 m east of the outcrop Delhaize Plantin, where the Antwerpen Member eroded the Cordiopsis horizon of the Kiel Member (Everaert et al., 2020). Also at this locality, the base of the Antwerpen Member yielded several elements reworked from the underlying deposits, including many I. oxyrinchus and brown-grey phosphatic concretions. These observations highlight the variability in palaeontological and lithological content at the contact between both members.
Table 1. Elasmobranch remains from the base of the Antwerpen Member in the temporary outcrop Delhaize Plantin (collection JVB), compared with published records from both the Antwerpen (De Ceuster, 1987) and Kiel members (Everaert et al., 2019). The comparison is made solely based on the species recorded from the temporary outcrop Delhaize Plantin. * Only these species are counted from the data of De Ceuster (1987), as teeth from both small and large fractions (unequal samples) were not separated in this study. ** In contrast, Everaert et al. (2019) provided a fractional representation, not including a granular species count. In the latter study, teeth of Squatina sp. were not included in the fraction >5 mm. *** Some very rare species from both studies (e.g. Hemipristis serra, Otodus megalodon), not recorded at Delhaize Plantin, are not included in this comparison.
4. Systematic palaeontology
21Order Cetacea Brisson, 1762
22Unranked clade Neoceti Fordyce & Muizon, 2001
23Suborder Odontoceti Flower, 1867
24Infraorder Delphinida Muizon, 1984
25Family Kentriodontidae Slijper, 1936 (sensu Godfrey & Lambert, 2023)
26Genus Kentriodon Kellogg, 1927
27Kentriodon cf. Kentriodon pernix Kellog, 1927
28(Figs 4–6)
29Referred specimen. RBINS M.2340, a partial cranium with three maxillary teeth in situ.
30Locality, horizon and age. Temporary outcrop Delhaize Plantin, Antwerp, Belgium (Fig. 1). Base of the Antwerpen Member (Berchem Formation). Middle Miocene, early Langhian. See details in Section 3.
31Description. This well-preserved small cranium (Figs 4–6; Table 2) lacks most of the premaxillae (including the tip of the rostrum), most of the occipital region, and the whole basicranium (including the ear bones). The delicate bones, the pointed rostrum, and the open sutures between maxillae and premaxillae all point to a relatively young individual, probably a juvenile (see Perrin, 1975). However, the strong wear observed in the three preserved maxillary teeth gives a conflicting signal (see below).
32Though most of the premaxillae is lost, the suture marks on the maxillae indicate a laterally convex lateral margin of the premaxilla before the antorbital notch, followed posteriorly by a slight constriction and a slightly convex lateral margin in the facial region (Fig. 4). Based on the preserved fragments and the underlying sulci in the maxilla, the right premaxillary foramen was either at the level of the antorbital notch or slightly posterior. The posterior tip of the ascending process of the left premaxilla displays a broad oblique contact with the corresponding nasal and is distant (14 mm) from the exposure of the frontal on the vertex. In ventral view, the premaxillae are broadly exposed anterior to the vomer in the vomerine trough; they are wider than the maxillae along the preserved anterior section of the rostrum, suggesting that the premaxillae were originally longer than the maxillae (Fig. 6).
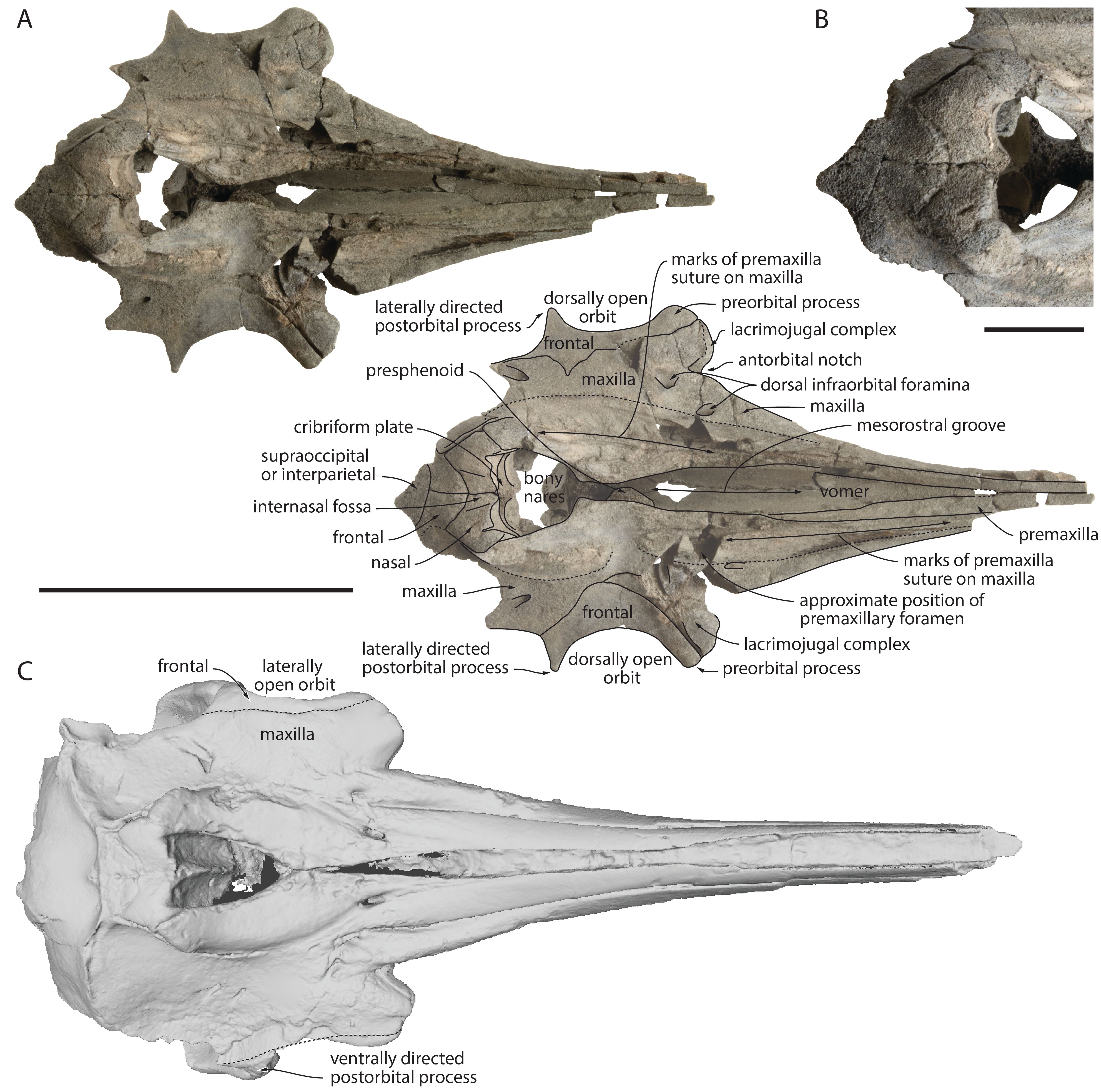
Figure 4. Partial cranium of Kentriodon cf. Kentriodon pernix RBINS M.2340, lower Langhian of Antwerp (Belgium), in dorsal view (A) and detail of the vertex region in dorsal view (B). C. Cranium of Kentriodon pernix USNM 10670, Burdigalian of Maryland (USA) in dorsal view, based on 3D model downloaded from Phenome10k. Stippled lines for suture lines interpreted based on preserved suture marks. Scale bar for A and C = 100 mm, for B = 20 mm.
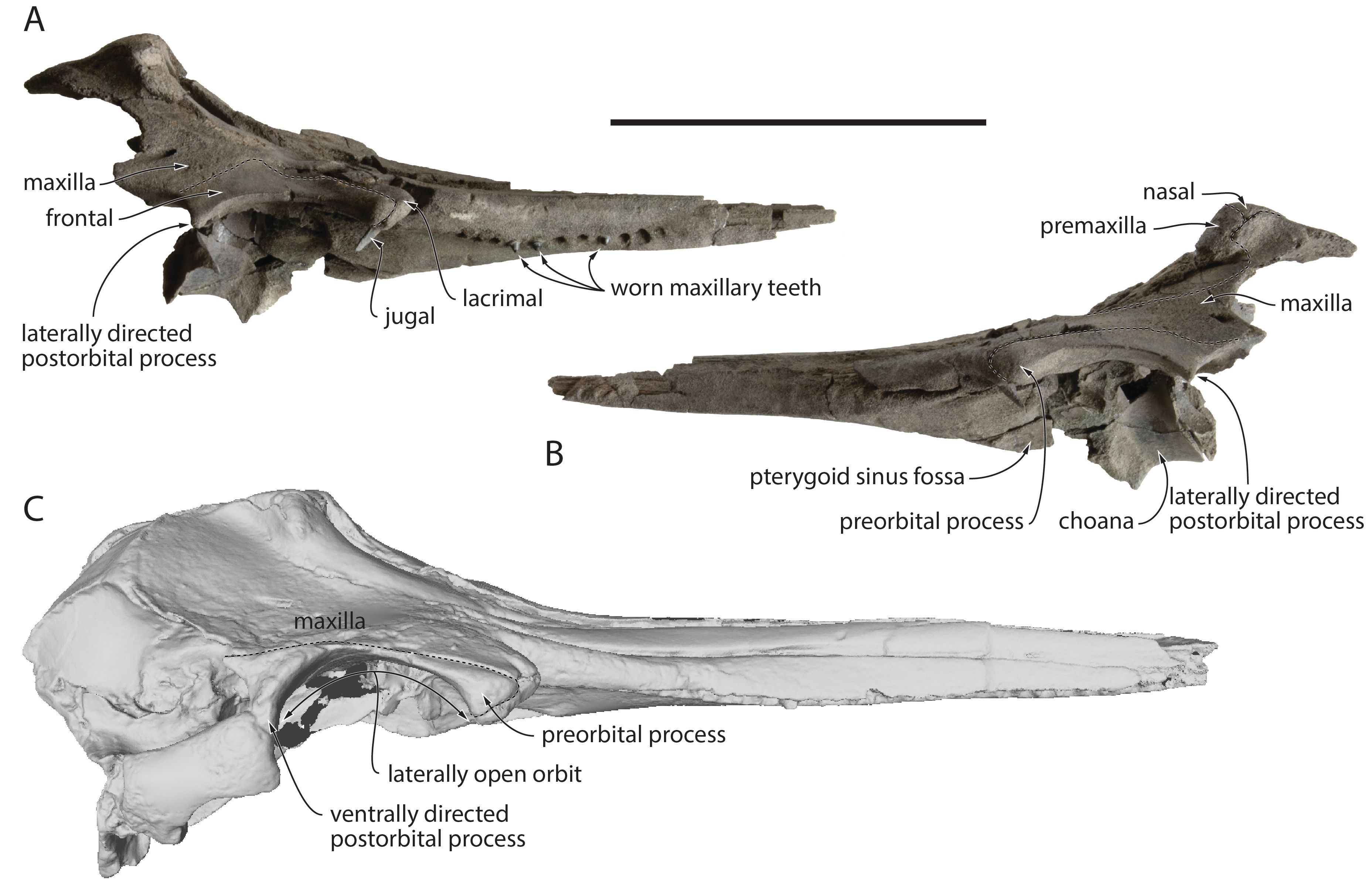
Figure 5. Partial cranium of Kentriodon cf. Kentriodon pernix RBINS M.2340, lower Langhian of Antwerp (Belgium), in right lateral (A) and left lateral (B) view. C. Cranium of Kentriodon pernix USNM 10670, Burdigalian of Maryland (USA), in right lateral view, based on 3D model downloaded from Phenome10k. Stippled lines for suture lines. Scale bar = 100 mm.
33
Figure 6. Partial cranium of Kentriodon cf. Kentriodon pernix RBINS M.2340, lower Langhian of Antwerp (Belgium), in ventral view (A) with detail of two in situ maxillary teeth in ventromedial view (B, C). Stippled lines for suture lines interpreted based on preserved suture marks. Scale bar for A = 100 mm.
34In the anterior part of the rostrum the maxillae were originally nearly completely hidden by the premaxillae in dorsal view. Their dorsal exposure widens moderately towards the rostral base, where a well-defined maxillary flange has a markedly convex and dorsoventrally thickened lateral margin (Fig. 5). The antorbital notch is V-shaped, drawing an angle of about 60° on the better-preserved left side. On this side, a small (diameter 3 mm) dorsal infraorbital foramen is located along the maxilla-premaxilla suture just anterior to the level of the antorbital notch; a larger (4.5 mm) foramen is located posterolaterally to the first one, at the level of the preorbital process; and a similarly sized (3 mm) third foramen is located posterior to the level of the postorbital process, 8 mm lateral to the maxilla-premaxilla suture (Fig. 4). On the right side, a slightly smaller (2.5 mm) foramen is located anterior to the level of the antorbital notch; at least one, partly preserved larger foramen is located 18 mm posteriorly; and a third posterior foramen (2 mm) is located at the same level as on the left side. The dorsal surface of the left maxilla is slightly thickened in the antorbital region, whereas the maxilla most likely did not cover this region on the right side, leaving part of the lacrimal and a broad part of the preorbital process of the frontal dorsally exposed. On this side, the lateral maxilla-frontal suture nearly reaches medially the level of the maxilla-premaxilla suture, exposing an even broader part of the frontal (16.5 mm), before turning posterolaterally and reaching the lateral margin of the roof of the temporal fossa just behind the postorbital process. The medial projection of the lateral maxilla-frontal suture is not as extensive on the left side. The anterior outline of the bony nares is U-shaped and the slight elevation of the medial edge of each maxilla just anterior to the nares suggests that the maxilla was originally dorsally exposed medial to the premaxilla. Posterior to the nasal, the maxilla projects posteromedially, giving to the posterior part of the vertex a transversely pinched aspect, though with moderately elevated medial margins of the maxillae. In ventral view, a pair of small (diameter 1–1.5 mm) palatal foramina pierces the maxillae just lateral to the ventral exposure of the vomer (Fig. 6). On the better-preserved right side, 11 small alveoli for single-rooted maxillary teeth are counted on a length of 56.5 mm. The anterior alveoli have a transverse diameter of 2.5 mm and interalveolar spaces of 3 mm, whereas the posteriormost alveoli are slightly larger (3–3.5 mm) and more closely spaced (1.2–1.5 mm). The last alveolus is 12 mm anterior to the level of the antorbital notch. The whole palatal region is broad, lacking any well-defined ventromedial keel.
35A thin plate of the vomer floors a large part of the wide and deep mesorostral groove. The cross-section of the latter shifts from V-shaped at rostrum base to U-shaped anteriorly. The dorsal edges of the vomer along the walls of the groove descend in the anterior direction; the anteriormost portion of the vomer makes a narrowing strip between the premaxillae, which originally ended 29.5 mm before the preserved tip of the rostrum. Just anterior to the bony nares, the right flank of the mesorostral groove is covered by a thick plate of the presphenoid. This bone is spongy, and damaged surfaces suggest that it originally extended more dorsally and medially. The narrow, spindle-shaped ventral exposure of the vomer on the rostrum was originally 31 mm-long.
36Anteromedially, the palatines extend on the palate for 15 mm anterior to the level of the antorbital notch. While the anterior tip of each palatine is incomplete, right and left tips were either joined or only separated on a short distance by a narrow ventral exposure of the maxillae. The posterolateral part of both palatines is lost, but suture marks on the maxillae indicate an extension at least to the level of the anterior edge of the ventral infraorbital foramen. Both pterygoids are lost, but the narrow anterior portion of the pterygoid sinus fossa excavates the palatine for 6 mm anterior to the level of the antorbital notch.
37Preserved on both sides, the slender base of the styliform process of the jugal is posteromedial to the antorbital notch (Fig. 6). The lacrimal-jugal suture is visible just posterior to this process, with a mediolateral orientation. Contributing to the lateral edge of the ventral infraorbital foramen, the lacrimal extends posteriorly for at least two thirds of the orbit length (more posteriorly, its sutures with the orbitosphenoid and frontal are difficult to distinguish). The lacrimal ventrally covers a broader portion of the preorbital process of the frontal on the right side, an asymmetric condition that is linked to the narrower right frontal in this region (see below). In lateral view, the lacrimal is exposed for about 9 mm ventral and anteroventral to the preorbital process, with a maximum dorsoventral thickness of 6–8 mm, but without a strong ventral bulge.
38The dorsally swollen anterolateral portion of each nasal constitutes the highest point of the cranium, the two sides being separated by a moderately excavated and broad internasal fossa (Fig. 4). A thin anterolateral spine of the nasal contributes for 4.5–6 mm to the lateral margin of the bony nares; the spine is medially defined by a deep vertical notch. Each nasal sends a posterolateral projection between maxilla and frontal, making a U-shaped suture between nasals and frontals; this projection reaches closer to the nuchal crest on the left side, only separated from the supraoccipital by 7 mm. The cribriform plate is incomplete dorsally but considering the smooth vertical anterior surface of the nasals, it did not extend to the anterodorsal edge of the latter.
39Frontals are much narrower transversely than nasals on the vertex. There, the dorsal surface of the frontals is roughly flat and slopes posteroventrally. As mentioned above, in dorsal view the right frontal is more broadly exposed in the supraorbital region than the left one. Its lateral edge is also much more concave, with a depth of 15 mm vs 9 mm on the right side. Related to this concavity, each postorbital process is a dorsoventrally thin spine that is directed laterally with only a slight ventral deflection. This is a very unusual condition for an odontocete (see discussion below), which corresponds to a relatively flat and dorsolaterally open orbit. In lateral view, the preorbital process is thicker on the left side (10 mm compared to 7 mm on the right side) (Fig. 5). Posterior to the postorbital process, the lateral edge of the frontal is also concave in dorsal view, making a dorsally open anterior part of the roof of the temporal fossa.
40Only a short anteromedial part of the supraoccipital (or interparietal) is preserved posterior to the frontals. Its dorsal surface is continuous with the surface of the frontals, with no nuchal crest developed at this level.
41The three maxillary teeth preserved in situ are heavily worn (Fig. 6B, C). Whereas the apex of the posteriormost tooth displays some degree of postmortem damage, no enamel is seen on the crown for any of these teeth, with a cone shape and several concentric layers indicating that extensive wear removed the enamel and part of the underlying dentine. Diameter at the base of tooth crown and height above the alveolus are respectively 3 and +2.9 mm for the posterior tooth, 3 and 2.6 mm for the intermediate tooth, and 2.5 and 2.3 mm for the anterior tooth, suggesting that wear was more extensive anteriorly.
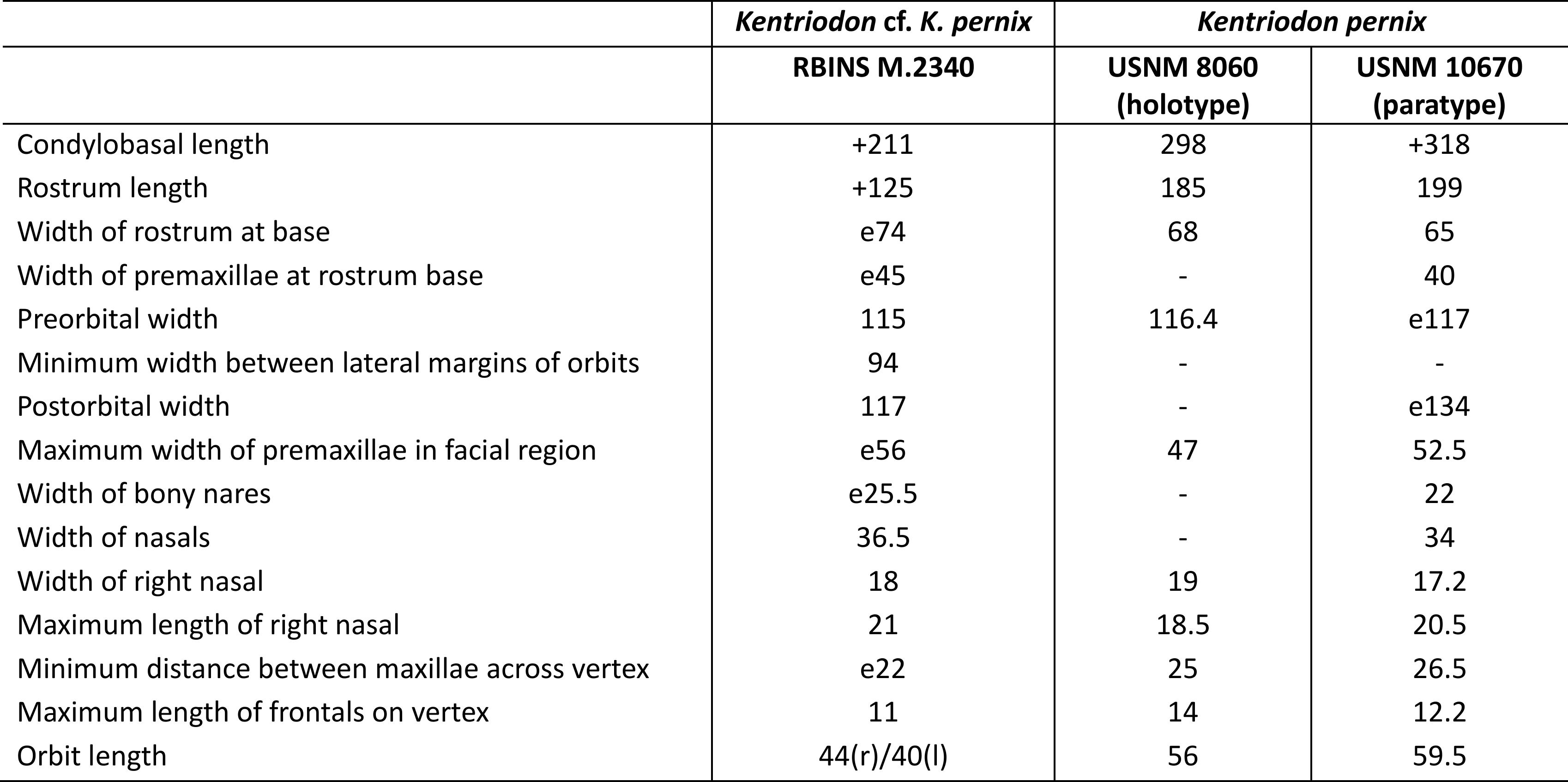
Table 2. Measurements of the partial cranium of Kentriodon cf. Kentriodon pernix RBINS M.2340, Middle Miocene of Antwerp (Belgium), compared with two specimens of Kentriodon pernix, from the Miocene of the Atlantic Coastal Plain (USA). Measurements of the latter taken from Kellogg (1927) and our own measurements. e for estimate, + for incomplete, - for missing data, (l) for left, and (r) for right.
5. Comparison of the cranium RBINS M.2340 and systematic affinities
42The combination of several morphological features of the vertex observed in RBINS M.2340, including the absence of a contact between premaxilla and frontal (also present in many other delphinidans), dorsally inflated nasals, an internasal fossa, a deep vertical notch on the anterior wall of each nasal, and a U-shaped outline of the sutures between nasals and frontals, identifies this specimen as a member of the family Kentriodontidae sensu Godfrey & Lambert (2023) (see Barnes, 1978; Muizon, 1988a; Ichishima et al., 1995), though the content and possible non-monophyly of this group remain debated (Lambert et al., 2017; Peredo et al., 2018; Guo & Kohno, 2021, 2023). Among kentriodontids, strong similarities with Kentriodon pernix, from the Lower to Middle Miocene of the Calvert Formation (Maryland and Virginia, USA; Kellogg, 1927; Godfrey & Lambert, 2023), are noted at the level of the vertex, rostrum base, and maxillary alveoli. The number, position, and size of the dorsal infraorbital foramina, as well as the shape of the nasals and frontals, and the number and spacing of alveoli are for example strikingly similar to the paratype of K. pernix, USNM 10670 (Figs 4–7). In addition, except for dimensions related to the orbit (see below), most cranial measurements are close to this latter specimen and the holotype of K. pernix USNM 8060 (Table 2). The rostrum is more pointed and was most likely originally shorter in RBINS M.2340, but such differences can be easily explained by ontogenetic changes, with younger individuals being characterized by a shorter and more pointed rostrum in many extant odontocete species (e.g. Perrin, 1975; Galatius et al., 2011).
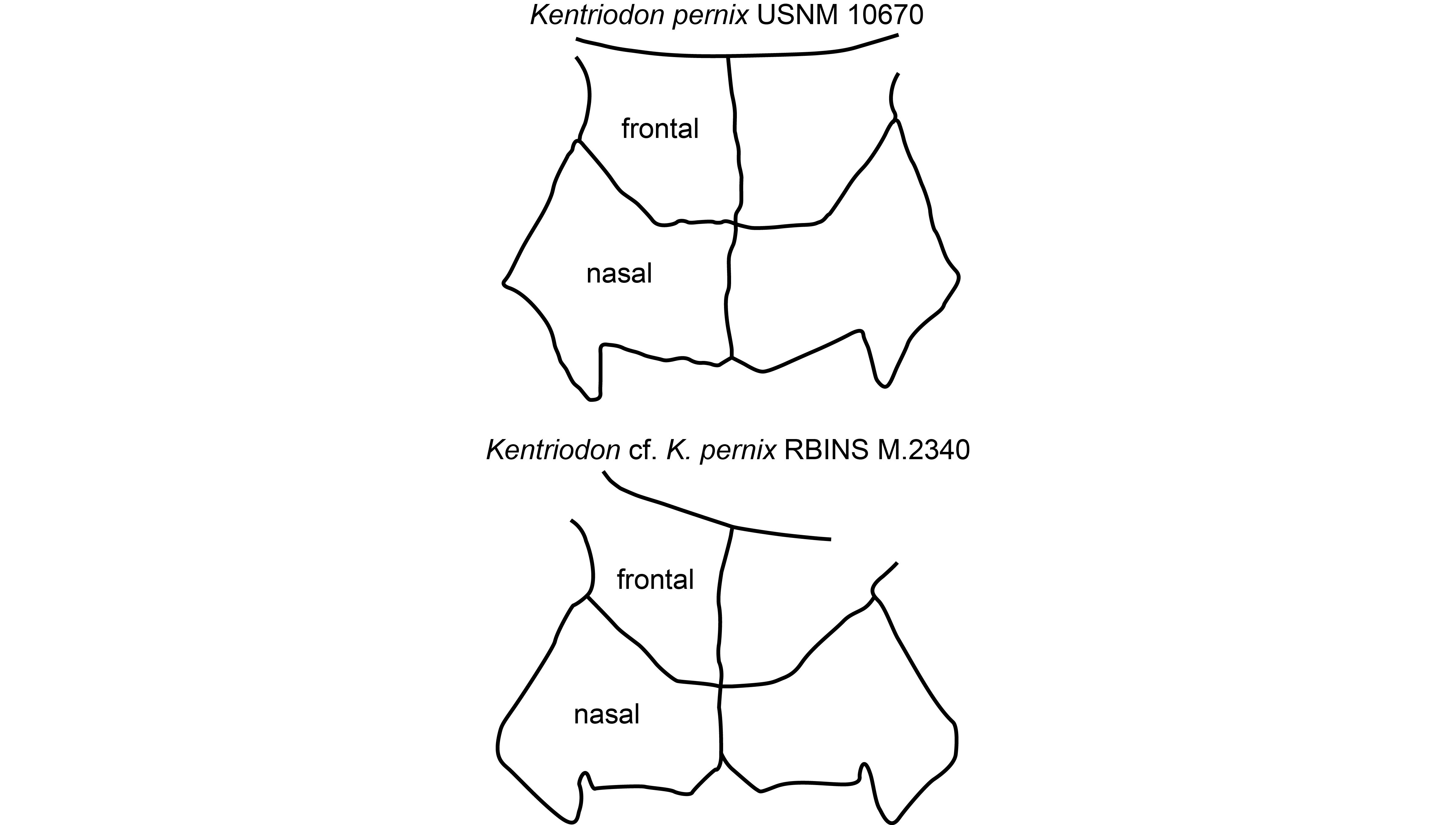
Figure 7. Comparison of the outline of frontals and nasals on the vertex in Kentriodon pernix USNM 10670 (Burdigalian of Maryland, USA), based on Godfrey & Lambert (2023, fig. 2.33b), and Kentriodon cf. Kentriodon pernix RBINS M.2340 (lower Langhian of Antwerp, Belgium), in dorsal view. Schematic drawings were scaled at the same width of the nasals.
43RBINS M.2340 also shares some similarities with specimens from the Miocene of Europe that have been attributed (sometimes tentatively) to the genus Kentriodon. The partial cranium RBINS M.372, from an unknown locality and horizon of the area of Antwerp, was originally placed in the species Phocaenopsis scheynenis (du Bus, 1872) and later recombined as Acrodelphis sheynensis (Abel, 1905). Subsequently, Kellogg (1927) noted similarities for this specimen with K. pernix, and Fordyce & Muizon (2001) proposed that it is close to or within the genus Kentriodon. The systematic status of this specimen is currently being reassessed in a separate work. Cranial dimensions are generally close to RBINS M.2340, but the preserved left nasal is proportionally narrower than in the latter, and the left frontal sends a longer, more pointed anteromedial projection.
44The outline of the nasals of RBINS M.2340 is reminiscent of Kentriodon hoepfneri, from the Middle to Late Miocene of North Germany (Kazár & Hampe, 2014), but the latter displays frontal-nasal sutures with an outline that is less convex anteriorly, and its nasals are distinctly wider. Unfortunately, the state of preservation of the holotype of K. hoepfneri does not allow for further comparison with other cranial regions (see also comments on the systematic attribution of this specimen in Nobile et al., 2024).
45A partial cranium from the Early Miocene of northeastern Italy, recently attributed to Kentriodon sp. (Nobile et al., 2024), differs in the broad dorsal exposure of the maxillae along the bony nares (proposed to be absent in RBINS M.2340 based on suture marks) and the larger cranial dimensions.
6. Discussion on unusual anatomical features of the cranium RBINS M.2340
46As mentioned in the description, the unusual shape of the two orbit regions in RBINS M.2340 departs markedly from other delphinidans, including Kentriodon pernix and the other specimens compared just above. Indeed, in these delphinidans the supraorbital process of the frontal is nearly completely covered by the maxilla, the postorbital process is directed ventrolaterally, and the lateral margin of the orbit roof is thus only moderately concave in dorsal view (e.g. Abel, 1905; Kellogg, 1927; Nobile et al., 2024; Figs 4C, 5C), much less than in RBINS M.2340. Among odontocetes, a reduced cover of the frontal by the maxilla is mostly observed in stem odontocete taxa, for example the Oligocene simocetids and xenorophids (Fordyce, 2002; Boessenecker & Geisler, 2023); in the holotype of Simocetus rayi, the dorsal exposure of the frontal is even more similar to RBINS M.2340, with a laterally concave lateral margin of the maxilla and a long posterior extent of this bone. However, in both simocetids and xenorophids, this morphology has been interpreted as representing a plesiomorphic condition, whereas more crownward odontocetes (including delphinidans) are all characterized by a nearly complete cover of the frontal by the maxilla. Furthermore, in these early odontocetes the more limited cover is not associated with a laterally directed postorbital process, the latter feature being, to our knowledge, not found in any other odontocete, even in juvenile individuals.
47The asymmetric development of these features in RBINS M.2340 (both the dorsal exposure of the frontal and the lateral concavity are greater on the right side) and the highly unusual resulting morphology (leading for example to a bizarre dorsolateral opening of the orbits) point to an abnormal condition. Its development on both orbits and the lack of evidence for a healed fracture, abscess, or any other local lesion indicate that it did not result from a local trauma or infection. Infestation by Crassicauda nematodes, often occurring in the orbit region of several extant odontocete species, can also be excluded considering the very different aspect of the bone, much more irregular, resulting from the related trabecular osteolytic lesions (e.g. Van Bressem et al., 2020).
48A similar reduction of the cover of the supraorbital process of the frontal by the maxilla was observed in one cranium of the Miocene ziphiid Ziphirostrum marginatum (Lambert, 2005b, fig. 7; RBINS M.542), but the less complete state of preservation of this specimen prevents us from describing the outline of the underlying frontal and the degree of asymmetry of this condition. This cranial morphology is preliminarily interpreted here as a developmental/congenital malformation (e.g. Dabin et al., 2004; Van Bressem et al. 2007; and references therein). Comparing the condition of RBINS M.2340 to foetuses of the extant dolphin Stenella attenuata (Moran et al., 2011), similarities with individuals at Carnegie stages 20–22 are noted at the level of the outline of the postorbital process (slender and pointed) and the limited cover of the frontal by the maxilla. These similarities may suggest that the malformation occurred relatively early during embryogenesis. An in-depth literature and collection search for records of similar malformations in extant odontocetes and other mammals, and an investigation of the inner bone structure in the modified regions of RBINS M.2340 (via microCT-scanning) would allow for testing this hypothesis and may provide clues for potential causes and the developmental mechanisms involved (e.g. Kyomen et al., 2023). Interestingly, the observed cranial morphology is associated with an unusual degree of tooth wear, which should probably be taken into account to discuss the aetiology of this condition. One may, for example, hypothesize that the high apical wear observed in this young individual could be related to the absence of enamel cover on the crown (see Randall et al., 2024 for comments on the relaxed selection of genes critical to enamel development in odontocetes, in relation to a reduced use of teeth for prey capture).
7. Conclusions
49Pending a better understanding of the developmental context, and though most anatomical differences with adult individuals of the kentriodontid Kentriodon pernix could be interpreted either as resulting from the earlier ontogenetic stage represented by RBINS M.2340 or as a developmental malformation, we prefer a cautious systematic attribution of this specimen to Kentriodon cf. K. pernix. This new southern North Sea record further strengthens the odontocete faunal similarities noted between the Berchem Formation and the Calvert Formation (Lower to Middle Miocene, Atlantic Coastal Plain, USA), where K. pernix was originally described (see Godfrey & Lambert, 2023 and references therein). Despite more than 150 years of palaeontological research in the Antwerp area, temporary outcrops keep yielding new, scientifically informative cetacean fossils. The description in this work of a rare kentriodontid cranium found in situ in the lower part of the Antwerpen Member of the Berchem Formation improves our knowledge of the diversity and palaeogeographic distribution of early delphinidans in the North Atlantic realm. Furthermore, the new fossil described here not only informs us on the content of the local Langhian assemblage, but also on unusual anatomical features that are interpreted as cranial malformations related to abnormal individual development. Detailed monitoring of future construction works in the Antwerp region, in close collaboration with citizen scientists, will undoubtedly lead to new, scientifically relevant palaeontological discoveries.
Acknowledgements
50We would like to thank the following people for their cooperation in this study: Maarten D’haeyer (Willemen Construct), Raf De Proost (Aertssen Infra), Joppe Geentjens (Aertssen Infra), Ward Segers (Aertssen Infra), and Rik Bergen (Smet Group) for providing access to the temporary outcrop and for their help during fieldwork, Gunther Cleemput (Gooik) for the graphical assistance in the making of the map and the drawing of the lithological section, Rik Houthuys (Geoconsultant, Halle) and Jef Deckers (VITO) for critical remarks on the stratigraphical part, and Katrien de Nil (Geotheek, VPO) and Marleen De Ceukelaire (RBINS, Belgian Geological Survey) for preserving the sediment samples. We also wish to thank the two reviewers, Stephen J. Godfrey and Toshiyuki Kimura, as well as the editor Annick Anceau, for their constructive comments on an earlier version of this work.
Author contribution
51Stijn Everaert, Mark Bosselaers, and Olivier Lambert conceptualized this research project. Jeroen Van Boeckel, Mark Bosselaers, Bert Gijsen, and Stijn Everaert participated to fieldwork at the temporary outcrop Delhaize Plantin. Jeroen Van Boeckel discovered the studied dolphin cranium, which was prepared by him and Mark Bosselaers. Stijn Everaert described the local lithological section. Jeroen Van Boeckel, Bert Gijsen, and Stijn Everaert took part to the identification of the elasmobranch remains. Olivier Lambert described the dolphin cranium, which was compared and identified by him and Pieter Van Rompaey. Olivier Lambert, Jeroen Van Boeckel, Mark Bosselaers, and Stijn Everaert wrote the initial draft of the manuscript, which was revised by all authors.
Data availability
52The studied dolphin cranium is curated in the Palaeontological collections of the Royal Belgian Institute of Natural Sciences (RBINS) in Brussels (Belgium), guaranteeing its long-term safekeeping and availability to other researchers for future studies. Sediment samples from the described lithological section are permanently stored at the Belgian Geological Survey (RBINS) and the Geotheek of the Flemish Department of Environment.
References
53Abel, O., 1901. Les dauphins longirostres du Boldérien (Miocène supérieur) des environs d’Anvers. I. Mémoires du Musée royal d’Histoire naturelle de Belgique, 1, 1–95.
54Abel, O., 1902. Les dauphins longirostres du Boldérien (Miocène supérieur) des environs d’Anvers. II. Mémoires du Musée royal d’Histoire naturelle de Belgique, 2, 99–190.
55Abel, O., 1905. Les Odontocètes du Boldérien (Miocène supérieur) des environs d’Anvers. Mémoires du Musée royal d’Histoire naturelle de Belgique, 3, 1–155.
56Barnes, L.G., 1978. A review of Lophocetus and Liolithax and their relationships to the delphinoid family Kentriodontidae (Cetacea: Odontoceti). Contributions in Science, Natural History Museum of Los Angeles County, 28, 1–35.
57Bianucci, G., 2001. A new genus of kentriodontid (Cetacea: Odontoceti) from the Miocene of south Italy. Journal of Vertebrate Paleontology, 21/3, 573–577. https://doi.org/10.1671/0272-4634(2001)021[0573:ANGOKC]2.0.CO;2
58Boessenecker, R.W. & Geisler, J.H., 2023. New skeletons of the ancient dolphin Xenorophus sloanii and Xenorophus simplicidens sp. nov. (Mammalia, Cetacea) from the Oligocene of South Carolina and the ontogeny, functional anatomy, asymmetry, pathology, and evolution of the earliest Odontoceti. Diversity, 15/11, 1154. https://doi.org/10.3390/d15111154
59Bor, T., Reinecke, T. & Verschueren, S., 2012. Miocene Chondrichthyes from Winterswijk - Miste, the Netherlands. Palaeontos, 21, 136 p.
60Brisson, M.-J., 1762. Regnum Animale in classes IX distributum, sine synopsis methodica. Theodorum Haak, Paris, 296 p.
61Collareta, A. & Bosselaers, M., 2022. A new record of Gunnellichnus moghraensis from the Middle Miocene of Belgium, with some remarks on the origin of this seemingly uncommon ichnospecies. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 305/3, 237–243. https://dx.doi.org/10.1127/njgpa/2022/1088
62Crombé, P., Halbrucker, E, Vandendriessche, H., Mol, D., Roosen, M.T. & Bulteel, T., 2022. (Middle) Palaeolithic finds from the Argex quarry at Kruibeke (East Flanders, BE). Notae Phaehistoricae, 42, 103–110.
63Dabin, W., Cesarini, C., Clemenceau, I., Dhermain, F., Jauniaux, T., Van Canneyt, O. & Ridoux, V., 2004. Double-faced monster in the bottlenosed dolphin (Tursiops truncatus) found in the Mediterranean sea. Veterinary Record, 154/10, 306–308. https://doi.org/10.1136/vr.154.10.306
64Dawson, S.D., 1996. A new kentriodontid dolphin (Cetacea; Delphinoidea) from the middle Miocene Choptank Formation, Maryland. Journal of Vertebrate Paleontology, 16/1, 135–140. https://doi.org/10.1080/02724634.1996.10011291
65Deckers, J. & Everaert, S., 2022. Distinguishing the Miocene Kiel and Antwerpen Members (Berchem Formation) and their characteristic horizons using cone penetration tests in Antwerp (northern Belgium). Geological Journal, 57, 2129–2143.
66Deckers, J., De Koninck, R., Everaert, S., Adriaens, R. & Verhaegen, J., 2023. Granulometry, carbonate and glauconite content as stratigraphic tools to distinguish the Kiel Member and lower Antwerpen Member (Berchem Formation) in the City of Antwerp area (Belgium). Geologica Belgica, 26, 127–141. https://doi.org/10.20341/gb.2023.008
67Deckers, J., Louwye, S., Munsterman, D., De Koninck, R., De Nil, K., Verhaegen, J., Algoe, C., Vergauwen, I., Adriaens, R., Everaert, S., 2025. Characterization of the Miocene successions in the Schoten borehole (southern North Sea Basin, northern Belgium) and regional correlation with the Netherlands. Geological Magazine, 162, e9. https://doi.org/10.1017/S0016756825000020
68De Ceuster, J., 1987. A little known odontaspid shark from the Antwerp Sand Member (Miocene, Hemmoorian) and some stratigraphical remarks on the shark-teeth of the Berchem Formation (Miocene, Hemmoorian) at Antwerp (Belgium). Mededelingen Werkgroep voor Tertiaire en Kwartaire Geologie, 24, 231–246.
69De Meuter, F.J. & Laga, P.G., 1976. Lithostratigraphy and biostratigraphy based on benthonic foraminifera of the Neogene deposits of Northern Belgium. Bulletin de la Société belge de Géologie, 85/4, 133–152.
70De Meuter, F., Wouters, K. & Ringelé, D., 1976. Lithostratigraphy of Miocene sediments from temporary outcrops in the Antwerp city area: Pl. Antwerpen 28 W, Pl. Borgerhout 28 E. Service géologique de Belgique, Professional Papers, 1976/3, 1–19.
71du Bus, B.A.L., 1872. Mammifères nouveaux du Crag d’Anvers. Bulletin de l’Académie royale des Sciences, des Lettres et des Beaux-Arts de Belgique, 34, 491–509.
72Dybkjær, K. & Piasecki, S., 2010. Neogene dinocyst zonation for the eastern North Sea Basin, Denmark. Review of Palaeobotany and Palynology, 161/1-2, 1–29. https://doi.org/10.1016/j.revpalbo.2010.02.005
73Everaert, S., De Schutter, P., Mariën, G., Cleemput, G., Van Boeckel, J., Rondelez, D. & Bor, T., 2019. Een vroeg-miocene fauna uit het Zand van Kiel (Formatie van Berchem) bij Post X in Berchem (Antwerpen). Afzettingen van de Werkgroep voor Tertiaire en Kwartaire Geologie, 40, 83–100.
74Everaert, S., Munsterman, D., De Schutter, P., Bor, T., Bosselaers, M., Van Boeckel, J., Cleemput, G. & Bor, T.J. 2020. Stratigraphy and palaeontology of the lower Miocene Kiel Sand Member (Berchem Formation) in temporary exposures in Antwerp (northern Belgium). Geologica Belgica, 23/3-4, 167–198. https://doi.org/10.20341/gb.2020.025
75Everaert, S., Deckers, J., Bosselaers, M., Schiltz, M. & Louwye, S., 2024. A Neogene succession in the city of Antwerp (Belgium): stratigraphy, palaeontology and geotechnics of the Rubenshuis temporary outcrop. Geologica Belgica, 27/1-2, 47–70. https://doi.org/10.20341/gb.2024.005
76Flower, W.H., 1867. Description of the skeleton of Inia geoffrensis and the skull of Pontoporia blainvillii, with remarks on the systematic position of these animals in the Order Cetacea. Transactions of the Zoological Society of London, 6, 87–116. https://doi.org/10.1111/j.1096-3642.1867.tb00572.x
77Fordyce, R.E., 2002. Simocetus rayi (Odontoceti: Simocetidae, new family): a bizarre new archaic Oligocene dolphin from the eastern North Pacific. Smithsonian Contributions to Paleobiology, 93, 185–222. https://doi.org/10.5479/si.00810266.93.185
78Fordyce, R.E. & Muizon, C. de, 2001. Evolutionary history of cetaceans: a review. In Mazin, J.-M. & Buffrénil, V. de (eds), Secondary Adaptation of Tetrapods to Life in Water. Verlag Dr. Friedrich Pfeil, München, 169–233.
79Galatius, A., Berta, A., Frandsen, M.S. & Goodall, R.N.P., 2011. Interspecific variation of ontogeny and skull shape among porpoises (Phocoenidae). Journal of Morphology, 272/2, 136–148. https://doi.org/10.1002/jmor.10900
80Godfrey, S.J. & Lambert, O., 2023. Miocene toothed whales (Odontoceti) from Calvert Cliffs, Atlantic Coastal Plain, USA. In Godfrey, S.J. (ed.), The Geology and Vertebrate Paleontology of Calvert Cliffs, Maryland, USA – Volume 2: Turtles and Toothed Whales. Smithsonian Contributions to Paleobiology, 107, 49–186. https://doi.org/10.5479/si.23847438
81Guo, Z. & Kohno, N., 2021. A new kentriodontid (Cetacea: Odontoceti) from the early to middle Miocene of the western North Pacific and a revision of kentriodontid phylogeny. PeerJ, 9, e10945. https://doi.org/10.7717/peerj.10945
82Guo, Z. & Kohno, N., 2023. An Early Miocene kentriodontoid (Cetacea: Odontoceti) from the western North Pacific, and its implications for their phylogeny and paleobiogeography. PLoS ONE, 18/2, e0280218. https://doi.org/10.1371/journal.pone.0280218
83Hoedemakers, K. & Dufraing, L., 2018. Een profiel bij Posthofbrug (Antwerpen). Afzettingen Werkgroep voor Tertiaire en Kwartaire Geologie, 39/2, 65–80.
84Ichishima, H., Barnes, L.G., Fordyce, R.E., Kimura, M. & Bohaska, D.J., 1995. A review of kentriodontine dolphins (Cetacea; Delphinoidea; Kentriodontidae): systematics and biogeography. The Island Arc, 3, 486–492. https://doi.org/10.1111/j.1440-1738.1994.tb00127.x
85Kazár, E., 2005. A new kentriodontid (Cetacea: Delphinoidea) from the Middle Miocene of Hungary. Mitteilungen aus dem Museum für Naturkunde Berlin, Geowissenschaftliche Reihe, 8, 53–73. https://doi.org/10.1002/mmng.200410004
86Kazár, E. & Hampe, O., 2014. A new species of Kentriodon (Mammalia, Odontoceti, Delphinoidea) from the middle/late Miocene of Groß Pampau (Schleswig-Holstein, North Germany). Journal of Vertebrate Paleontology, 34/5, 1216–1230. https://doi.org/10.1080/02724634.2014.857347
87Kellogg, R., 1927. Kentriodon pernix, a Miocene porpoise from Maryland. Proceedings of the United States National Museum, 69/10, 1–55. https://doi.org/10.5479/si.00963801.69-2645.1
88Kimura, T. & Hasegawa, Y., 2019. A new species of Kentriodon (Cetacea, Odontoceti, Kentriodontidae) from the Miocene of Japan. Journal of Vertebrate Paleontology, e1566739. https://doi.org/10.1080/02724634.2019.1566739
89Kyomen, S., Murillo-Rincón, A.P. & Kaucká, M., 2023. Evolutionary mechanisms modulating the mammalian skull development. Philosophical Transactions of the Royal Society B, 378/1880, 20220080. https://doi.org/10.1098/rstb.2022.0080
90Lambert, O., 2005a. Phylogenetic affinities of the long-snouted dolphin Eurhinodelphis (Cetacea, Odontoceti) from the Miocene of Antwerp, Belgium. Palaeontology, 48/3, 653–679. http://doi.org/10.1111/j.1475-4983.2005.00472.x
91Lambert, O., 2005b. Systematics and phylogeny of the fossil beaked whales Ziphirostrum du Bus, 1868 and Choneziphius Duvernoy, 1851 (Cetacea, Odontoceti), from the Neogene of Antwerp (North of Belgium). Geodiversitas, 27, 443–497.
92Lambert, O., Bianucci, G., Urbina, M. & Geisler, J.H., 2017. A new inioid (Cetacea, Odontoceti, Delphinida) from the Miocene of Peru and the origin of modern dolphin and porpoise families. Zoological Journal of the Linnean Society, 179/4, 919–946.
93Le Hon, H., 1871. Préliminaires d’un mémoire sur les poissons tertiaires de Belgique. H. Merzbach, Bruxelles, 15 p.
94Leriche, M., 1926. Les poissons néogènes de la Belgique. Mémoires du Musée royal d’Histoire naturelle de Belgique, 32, 367–472.
95Louwye, S. & Deckers, J., 2023. The Berchem Formation, 01/09/2023. National Commission for Stratigraphy Belgium. http://ncs.naturalsciences.be/lithostratigraphy/Berchem-Formation, accessed 15/03/2024.
96Louwye, S., Marquet, R., Bosselaers, M. & Lambert, O. 2010. Stratigraphy of an Early–Middle Miocene Sequence near Antwerp in Northern Belgium (Southern North Sea Basin). Geologica Belgica, 13/3, 269–284.
97Louwye, S., Deckers, J., Verhaegen, J., Adriaens, J. & Vandenberghe, N., 2020. A review of the lower and middle Miocene of northern Belgium. Geologica Belgica, 23/3-4, 137–156. https://doi.org/10.20341/gb.2020.010
98Louwye, S., Adriaens, R., Deckers, J., Everaert, S., Vandenberghe, N. & Verhaegen, J., 2023a. The Kiel Member, 01/09/2023. National Commission for Stratigraphy Belgium. http://ncs.naturalsciences.be/lithostratigraphy/Kiel-Member, accessed 15/03/2024.
99Louwye, S., Adriaens, R., Deckers, J., Everaert, S., Vandenberghe, N. & Verhaegen, J., 2023b. The Antwerpen Member, 01/09/2023. National Commission for Stratigraphy Belgium. http://ncs.naturalsciences.be/lithostratigraphy/Antwerpen-Member, accessed 15/03/2024.
100Mead, J.G. & Fordyce, R.E., 2009. The therian skull: a lexicon with emphasis on the odontocetes. Smithsonian Contributions to Zoology, 627, 1–248. https://doi.org/10.5479/si.00810282.627
101Misonne, X., 1958. Faune du Tertiaire et du Pléistocène inférieur de Belgique (Oiseaux et Mammifères). Bulletin de l’Institut royal des Sciences naturelles de Belgique, 34/5, 1–36.
102Moran, M.M., Nummela, S. & Thewissen, J.G.M., 2011. Development of the skull of the pantropical spotted dolphin (Stenella attenuata). The Anatomical Record, 294/10, 1743–1756. https://doi.org/10.1002/ar.21388
103Muizon, C., de., 1984. Les vertébrés de la Formation Pisco (Pérou). Deuxième partie : Les Odontocètes (Cetacea, Mammalia) du Pliocène inférieur de Sud-Sacaco. Travaux de l’Institut français d’Etudes andines, 27, 1–188.
104Muizon, C., de., 1988a. Les relations phylogénétiques des Delphinida. Annales de Paléontologie, 74, 159–227.
105Muizon, C., de., 1988b. Les vertébrés fossiles de la Formation Pisco (Pérou). Troisième partie : Les Odontocètes (Cetacea, Mammalia) du Miocène. Travaux de l’Institut français d’Etudes andines, 42, 1–244.
106Munsterman, D.K. & Brinkhuis, H., 2004. A southern North Sea Miocene dinoflagellate cyst zonation. Netherlands Journal of Geosciences/Geologie en Mijnbouw, 83, 267–285. https://doi.org/10.1017/S0016774600020369
107Nobile, F., Collareta, A., Perenzin, V., Fornaciari, E., Giusberti, L. & Bianucci, G., 2024. Dawn of the Delphinidans: New remains of Kentriodon from the Lower Miocene of Italy shed light on the early radiation of the most diverse extant cetacean clade. Biology, 13/2, 114. https://doi.org/10.3390/biology13020114
108Peredo, C.M., Uhen, M.D. & Nelson, M.D., 2018. A new kentriodontid (Cetacea: Odontoceti) from the early Miocene Astoria Formation and a revision of the stem delphinidan family Kentriodontidae. Journal of Vertebrate Paleontology, 38/2, e1411357. https://doi.org/10.1080/02724634.2017.1411357
109Perrin, W.F., 1975. Variation of spotted and spinner porpoise (genus Stenella) in the eastern Pacific and Hawaii. Bulletin of the Scripps Institution of Oceanography University of California, San Diego La Jolla, California, 21, 1–206.
110Randall, J.G., Gatesy, J., McGowen, M.R. & Springer, M.S., 2024. Molecular evidence for relaxed selection on the enamel genes of toothed whales (Odontoceti) with degenerative enamel phenotypes. Genes, 15/2, 228. https://doi.org/10.3390/genes15020228
111Reineck, H.E. & Singh, I.B., 1975. Depositional Sedimentary Environments: with Reference to Terrigenous Clastics. Springer-Verlag Berlin, Heidelberg, 439 p.
112Reinecke, T., Louwye, S., Havekost, U. & Moths, H., 2011. The elasmobranch fauna of the late Burdigalian, Miocene, at Werder-Uesen, Lower Saxony, Germany, and its relationship with early Miocene faunas on the North Atlantic, Central Paratethys and Mediterranean. Palaeontos, 20, 1–170.
113Schouten, S., 2021. Determinatiegids voor het determineren van periotica van tandwalvissen (Odonticeti) uit Nederland. Cranium, 38/2, 43–55.
114Slijper, E.J., 1936. Die Cetaceen Vergleichend-Anatomisch und Systematisch. Capita Zoologica, 7, 1–590.
115Van Beneden, P.J., 1865. Recherches sur les ossements provenant du Crag d’Anvers : les Squalodons. Mémoires de l’Académie royale des Sciences, des Lettres et des Beaux-Arts de Belgique, 35, 3–85. https://doi.org/10.3406/marb.1865.3567
116Van Bressem, M.-F., Reyes, J., Félix, F., Echegaray, M., Siciliano, S., Di Beneditto, A., Flach, L., Viddi, F., Avila, I. & Herrera, J., 2007. A preliminary overview of skin and skeletal diseases and traumata in small cetaceans from South American waters. Latin American Journal of Aquatic Mammals, 6, 7–42. https://doi.org/10.5597/lajam00108
117Van Bressem, M.-F., Duignan, P., Raga, J. A., Van Waerebeek, K., Fraija-Fernández, N. & Plön, S., 2020. Cranial crassicaudiasis in two coastal dolphin species from South Africa is predominantly a disease of immature individuals. Diseases of Aquatic Organisms, 139, 93–102. https://doi.org/10.3354/dao03468
118Manuscript received 11.04.2025, accepted in revised form 18.08.2025, available online 29.10.2025.






