- Accueil
- Volume 26 (2023)
- number 3-4
- Past beaked whale diversity in the North Sea: reappraisal through a new Miocene record and biostratigraphic analyses
Visualisation(s): 3064 (32 ULiège)
Téléchargement(s): 1146 (15 ULiège)
Past beaked whale diversity in the North Sea: reappraisal through a new Miocene record and biostratigraphic analyses

Abstract
Extant beaked whales (family Ziphiidae) are deep diving suction feeders and none of them can be considered as a permanent resident of the shallow southern North Sea. The rich fossil record of ziphiids from Neogene deposits of this area is thus surprising. However, chronostratigraphic intervals of most recorded taxa remain poorly constrained, preventing from assessing the evolution of their local diversity. In this work, we describe a new ziphiid cranium from the Neogene of Antwerp (north of Belgium), which is attributed to Caviziphius aff. C. altirostris. Sediment samples were extracted from 15 fossil ziphiid cranial remains from this area (including the one described herein), referred to eight species. The samples were analysed for their palynological content, leading to improved chronostratigraphic ranges for several species. Seven to eight ziphiid species from the southern North Sea, all from the Messapicetus clade, are proposed to originate from a Serravallian to Tortonian (late Middle to early Late Miocene) interval, and three to six more precisely from the mid- to late Tortonian. Added to the fossil record of other regions, these results point to a Late Miocene radiation of members of the Messapicetus clade, possibly related to the synchronous worldwide decline of several hyper-longirostrine dolphin clades.
Table des matières
1. Introduction
1Beaked whales (Ziphiidae) are a successful family of echolocating toothed whales, whose many medium to large-sized extant species are known to feed on deep water oceanic squid and fish that they capture via suction in meso- to benthopelagic regions worldwide (Heyning & Mead, 1996; Tyack et al., 2006; McLeod, 2018). While no extant beaked whale can be considered a permanent resident of the shallow southern North Sea (McLeod et al., 2006), a surprisingly high number of extinct ziphiid species are recorded from Neogene continental shelf deposits of the North Sea, in Belgium (Cuvier, 1823; Melville, 1851; du Bus, 1868; Abel, 1905; Bianucci & Post, 2005; Lambert, 2005; Lambert & Louwye, 2006; Lambert & Louwye, 2016), the Netherlands (Bosselaers, 2014; Post & Reumer, 2016; Bisconti et al., 2019), and Denmark (Ramassamy, 2016; Ramassamy & Lauridsen, 2019). Many of the records from northern Belgium are based on historical, nineteenth-century finds that are generally not associated with precise data on locality and horizon, being often referred to now disused regional stages (du Bus, 1868; Misonne, 1958; Laga et al., 2006). Therefore, the chronostratigraphic range of several species remains poorly constrained (see Lambert, 2005; Bianucci & Post, 2005). A similar shortcoming applies to many fossil ziphiid remains recovered by trawling or long-line fishing from offshore deposits around the world (Bianucci et al., 2007; Bianucci et al., 2013; Gol’din & Vishnyakova, 2011; Lambert et al., 2018), contrasting with inland fossil deposits from only a few regions of the world (e.g., Bianucci et al., 2016a; Buono & Cozzuol, 2013; but see Leidy, 1877, for another inland sample that is similarly poorly chronostratigraphically defined).
2Consequently, despite such an increasingly rich fossil record, it remains difficult to properly assess the evolution of the diversity of this highly successful cetacean family through time, the lack of temporal resolution preventing from adequately testing proposals for radiation events and extinctions. Nevertheless, new developments including the in situ discovery of additional specimens (Bosselaers et al., 2004; Ramassamy, 2016), isotopic analyses of fossil bones and attached minerals (187Os/188Os; Nozaki et al., 2017; 14C; Lambert et al., 2018), and palynological analyses of sediment associated with fossil remains (Lambert & Louwye, 2006; Lambert & Louwye, 2016; Miján et al., 2017) provide directions for future improvements. Focusing on the southern North Sea, recent works suggest that ziphiids occupied this region in a time interval ranging from the Middle Miocene to the Early Pliocene.
3In this study, we describe a ziphiid cranium that was collected in 1974 in Neogene sediments from Deurne (Antwerp eastern suburb, north of Belgium) and was recently donated to the Institut royal des Sciences naturelles de Belgique (IRSNB). In addition, sediment sampled from cavities in 15 fossil ziphiid rostra and partial crania from the Neogene of the Antwerp area (including the specimen described here) and referred to eight species, is analysed for its palynological content. The biostratigraphic interpretations are confronted with updated litho- and chronostratigraphy of the Belgian Neogene (e.g., De Schepper et al., 2009; Goolaerts et al., 2020; Louwye et al., 2020) and past fossil records, allowing for an improvement of our knowledge of the Middle to Late Miocene ziphiid diversity in the southern North Sea.
2. Material and methods
2.1. Institutional abbreviations
4IRSNB, Institut royal des Sciences naturelles de Belgique, Brussels, Belgium; NMB, Natuurhistorisch Museum Boekenberg, Antwerp, Belgium; SGHN, Museo da Natureza da Sociedade Galega de Historia Natural, Ferrol, Spain.
2.2. Anatomical terminology
5Terminology for cranial anatomy is mainly taken from Mead & Fordyce (2009), except for a few features more specific to ziphiids, taken from Lambert (2005) and Bianucci et al. (2013).
2.3. Ziphiid fossil specimens and sediment sampling
6All the ziphiid fossil partial crania (generally including the rostrum and part of the facial region) and isolated rostra from which sediment was sampled for palynological analysis were recovered from the area of Antwerp (north of Belgium). For most of them, the precise geographic locality and horizon were not recorded (for more details on a majority of the specimens, see Lambert, 2005 and below). All the sediment samples were taken with a long needle from deep in the crania, via dorsal infraorbital foramina, major palatine foramina, and/or the tunnel-shaped mesorostral groove. Considering (1) that most of the crania sampled here were collected more than a century ago and underwent several phases of preparation/restoration, and (2) that sediment samples were obtained thanks to the application of some force on the needle, we estimate that the sediment was at least partly indurated/cemented to the bones. It is hypothesized that in most (if not all) cases the sediment recovered from the crania entered there during the initial burial phase; it is indeed difficult to imagine sediment from deep openings being removed and replaced by geologically younger sediment in a second phase of burial (for reworked material). We thus propose that the geological age of the sediment corresponds to the time of initial burial for the ziphiid specimen and makes a reliable source for the age interval of the latter, as proposed earlier for specimens of Beneziphius brevirostris and Mesoplodon posti from the same region (Lambert & Louwye, 2016; Miján et al., 2017). Z1 to Z16 indicate sediment samples analysed for palynology.
7Aporotus recurvirostris: IRSNB 3812-M.1887 (holotype, partial cranium) (Z15); IRSNB 3816-M.1888 (partial rostrum) (Z16)
8Caviziphius aff. Caviziphius altirostris: new specimen IRSNB M.2333 (partial cranium) (Z9)
9Choneziphius planirostris: IRSNB 3774-M.1881 (partial cranium) (Z1); IRSNB 3770 (partial cranium) (Z3); IRSNB 3767 (partial cranium) (Z4); IRSNB ED001 (partial cranium, probably found in Rupelmonde) (Z5); IRSNB 1719c (partial cranium) (Z6)
10Ziphiidae indet. (Choneziphius macrops sensu Lambert, 2005; see also Bianucci et al., 2013): IRSNB 3778-M.1884 (partial cranium) (Z2 + Z10)
11Ziphiidae indet. (holotype of Ziphiopsis servatus sensu du Bus, 1868 and Ziphiidae aff. Eboroziphius sensu Lambert, 2005): IRSNB 3806-M.540 (partial rostrum) (Z14)
12Ziphirostrum marginatum: IRSNB 3846-M.1875 (partial cranium) (Z7); IRSNB 3783-M.1878 (holotype, partial cranium) (Z8)
13Ziphirostrum recurvus: IRSNB 3805-M.544 (holotype, rostrum) (Z13)
14Ziphirostrum turniense: IRSNB 3785-M.539 (lectotype, partial cranium) (Z11); IRSNB 3784-M.1880 (paralectotype, rostrum) (Z12)
2.4. Methodology for palynological analyses
15The samples were prepared following the standard palynological maceration technique described in Louwye et al. (2004). The treatment involves decalcification of the sample with HCl followed by the removal of silicates with HF. The residues were filtered on a 16 μm nylon screen and strew mounted on microscope slides with glycerine jelly. The dinoflagellate cysts were analysed with a ZEISS Axioimager A1 light microscope at 200× and 400× magnifications. The presence and numbers of acritarchs, green algae, pollen, spores, invertebrate remains and other incertae sedis were noted during the systematic count in non-overlapping traverses. The poor preservation of the palynomorphs and the low diversity and richness of the assemblage did not allow to reach the standard count of 200 specimens (cf. infra).
3. Systematic palaeontology
16Order Cetacea Brisson, 1762
17Pelagiceti Uhen, 2008
18Neoceti Fordyce & Muizon, 2001
19Suborder Odontoceti Flower, 1867
20Family Ziphiidae Gray, 1850
21Genus Caviziphius Bianucci & Post, 2005
22Caviziphius aff. Caviziphius altirostris Bianucci & Post, 2005
3.1. Previously referred specimen from the north of Belgium
23NMB 002, partial cranium discovered during the construction of the Antwerp Tower, in 1972, at De Keyserlei 5, Antwerp (Belgium) (Fig. 1). Geographic coordinates: 51°13’05”N - 4°24’59”E. This specimen was briefly described and figured in Lambert (2005) as Ziphiidae aff. Eboroziphius.
3.2. Newly referred specimen
24IRSNB M.2333, partial cranium discovered in April 1974 during the construction works for a school east to Ruggeveldlaan and south to Turnhoutsebaan, in Deurne (eastern suburb of Antwerp, Belgium) (Fig. 1). Geographic coordinates: 51°12’14”N - 4°29’14”E.
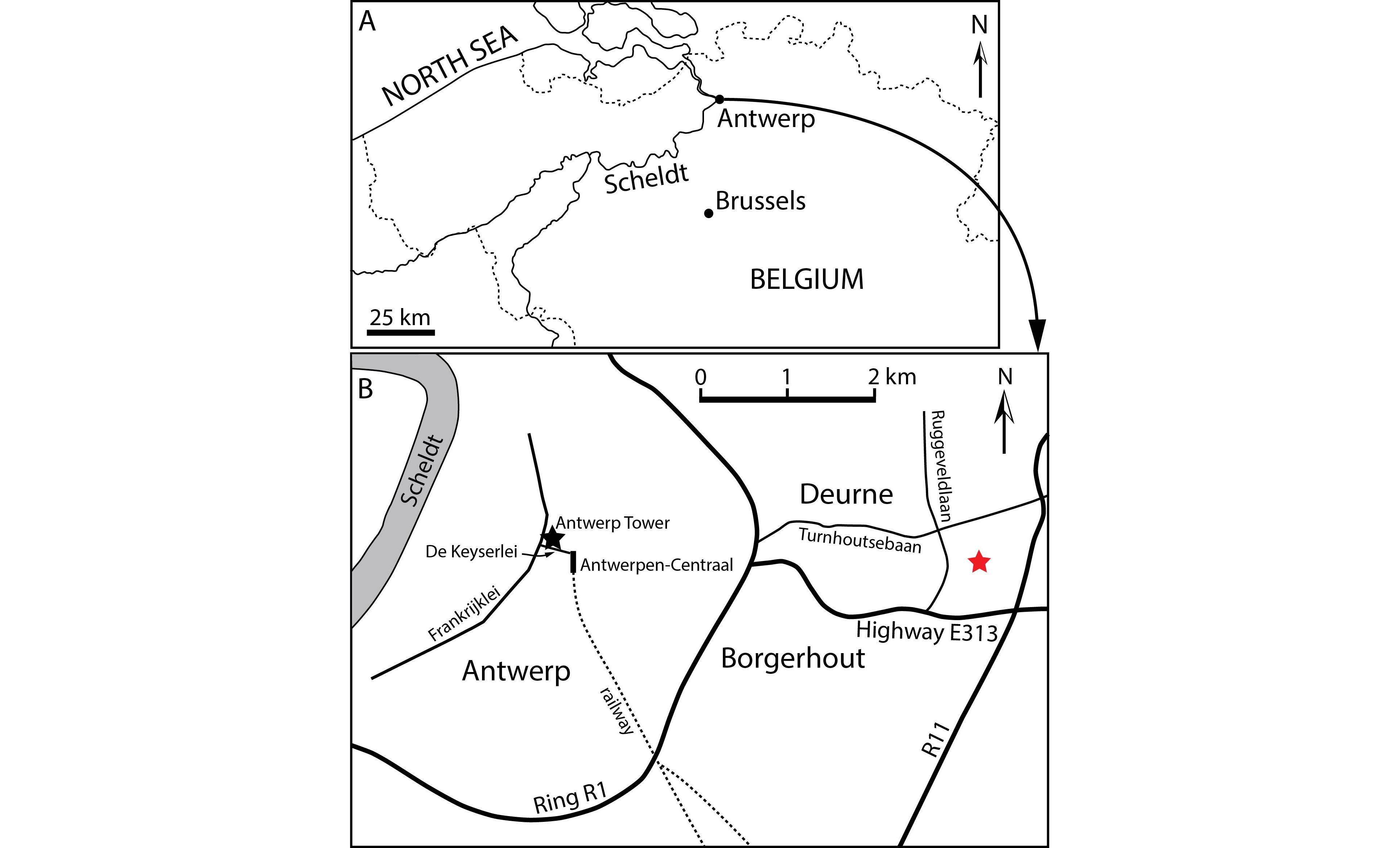
Figure 1. Localities of ziphiid fossil crania discussed in this paper. A. Location of Antwerp in Belgium. B. Detailed map of Antwerp and its eastern suburb showing the localities of Caviziphius aff. Caviziphius altirostris NMB 002, at Antwerp Tower along De Keyserlei in Antwerp (black star), and of Caviziphius aff. Caviziphius altirostris IRSNB M.2333, east to Ruggeveldlaan and south to Turnhoutsebaan in Deurne (red star).
3.3. Horizon and age
25Palynological analysis of a sediment sample (Z9) taken from IRSNB M.2333 yielded a late Langhian to Tortonian age. Based on the presence of Bitectatodinium? arborichiarum, an origin in the Diest Formation (9.54–8.8 Ma, Tortonian) could be tentatively proposed, but we keep a more cautious approach, not excluding a late Langhian to late Serravallian age (see below).
3.4. Brief description and comparison of IRSNB M.2333
26This new specimen (Figs 2, 3), including part of the rostrum (anterior tip and part of the dorsomedial region missing), of the right supraorbital region, and the anterior portion of the premaxillary sac fossae, shares many similarities with NMB 002 (at the level of dimensions, anatomy, and preserved parts), only being slightly more incomplete. Preserved length and maximum width are 608 and 210 mm, respectively, with a rostrum that was originally longer than 390 mm. At mid-preserved length, the robust, cylindrical rostrum is 95.5 mm wide and more than 89 mm high. The lateral margins of the rostrum remain subparallel for most of the posterior half, only diverging abruptly posterolaterally and slightly dorsally as a gradually thinning plate towards the incomplete antorbital region. Minimum width and height at rostrum base are 171 and 119 mm, respectively. In lateral view (Fig. 3), the ventral margin of the rostrum raises markedly anterodorsally, much more than in NMB 002, with the condition in aff. Caviziphius sp. SGHN MA0920 being intermediary.
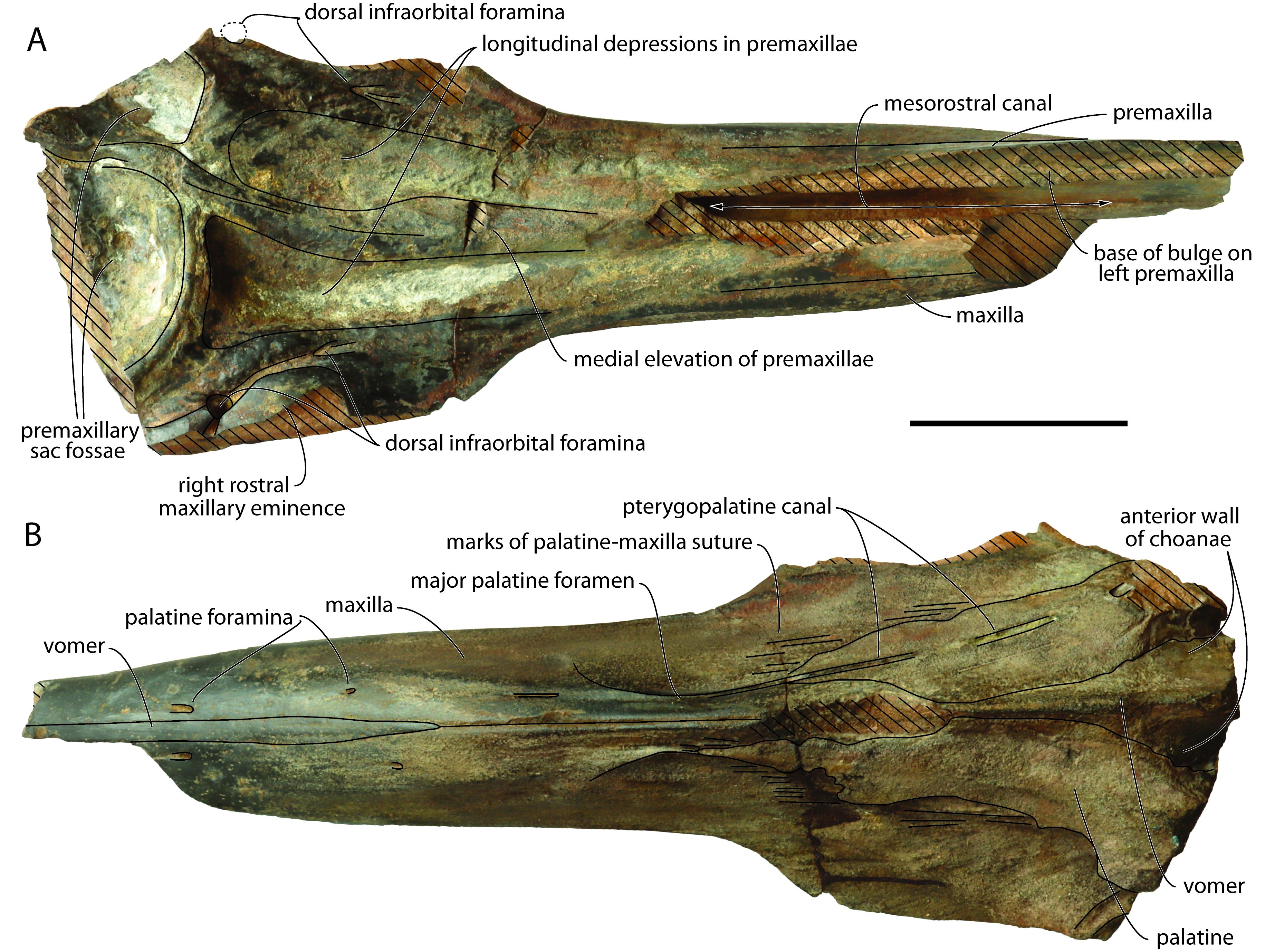
Figure 2. Partial cranium of Caviziphius aff. Caviziphius altirostris IRSNB M.2333, Late Miocene of Deurne (Belgium), in dorsal (A) and ventral (B) views. Oblique hatching indicates major break surfaces. Scale bar = 100 mm.
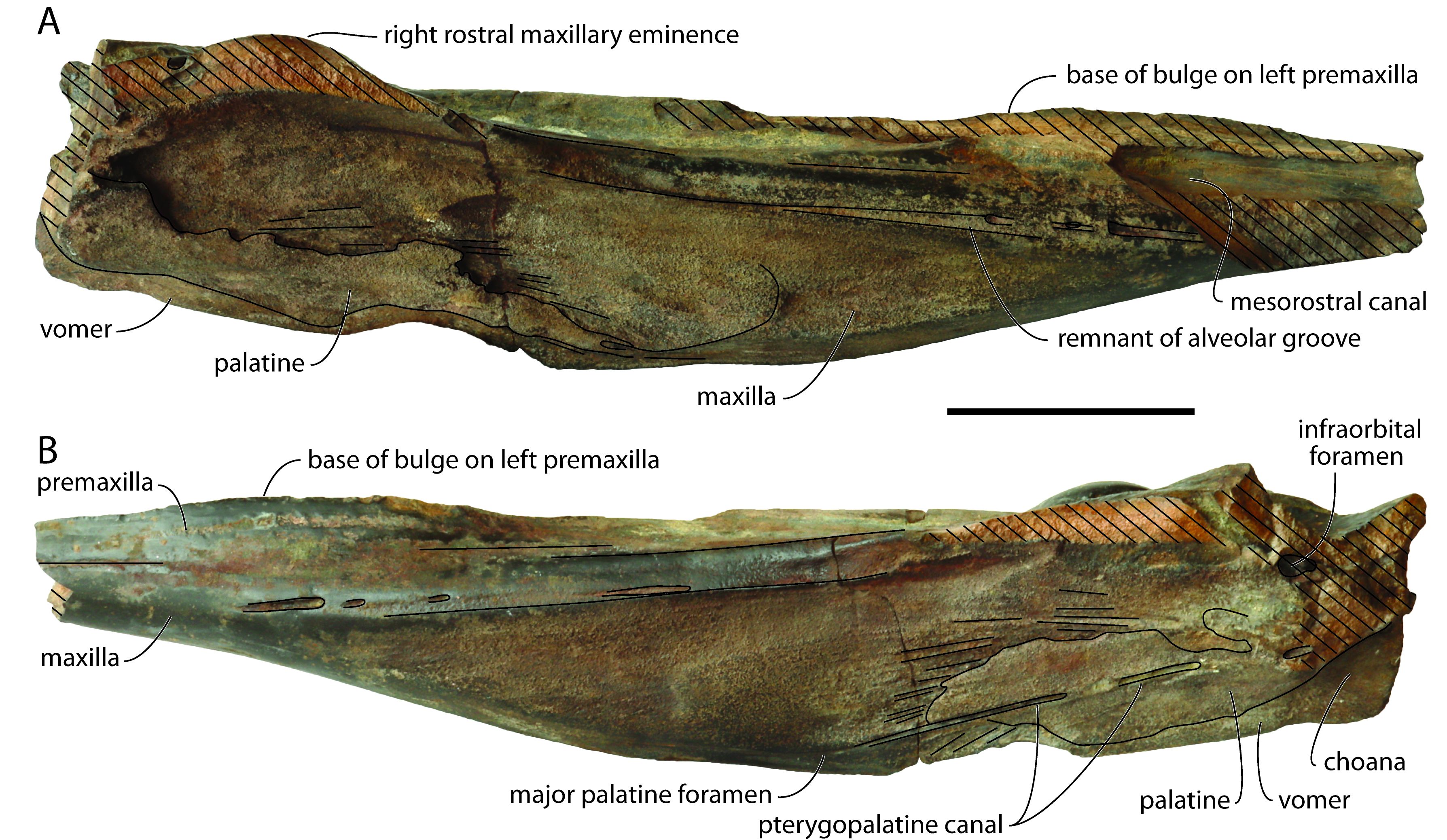
Figure 3. Partial cranium of Caviziphius aff. Caviziphius altirostris IRSNB M.2333, Late Miocene of Deurne (Belgium), in right (A) and left (B) lateral views. Oblique hatching indicates major break surfaces. Scale bar = 100 mm.
27The damage on the dorsomedial portion of each premaxilla on the anterior half of the rostrum reveals the open mesorostral canal, with a transverse diameter of 16 mm at a level 140 mm posterior to the anterior tip of the rostrum (Fig. 2A). The dorsomedial elevation of the incomplete left premaxilla in this region is interpreted as the base of a medial bulge of the premaxillae above the mesorostral canal, as in Tusciziphius atlanticus, aff. Caviziphius sp. SGHN MA0920, and NMB 002 (see Lambert, 2005; Bianucci et al., 2013). Based on the dorsal break surfaces of both premaxillae, the bulge ended posteriorly more than 230 mm anterior to the anterior margin of the right premaxillary sac fossa; this is more anterior than in T. atlanticus (81–128 mm) and, to a lesser extent, SGHN MA0920 (186 mm), closer to the condition in NMB 002 (300 mm). Posterior to the bulge, the medial portion of the premaxillae is dorsally thickened, making a median elevation that broadens posteriorly, up to the approximate level of the antorbital notches, before narrowing and markedly turning towards the left side of the cranium, extending between highly asymmetric premaxillary sac fossae. A similar medial elevation is observed in T. atlanticus, Caviziphius altirostris, Choneziphius leidyi, NMB 002, and SGHN MA0920. Lateral to this elevation, each premaxilla is marked by a longitudinal depression, ending posteriorly just before the corresponding premaxillary sac fossa. The right depression is considerably deeper than the left one and posteriorly defined by a steeper wall; it is also broader and longer than in NMB 002. The maxillary portion of the lateral wall of each depression displays an irregular surface, with pits and humps that are reminiscent of the excrescences observed in this area in Beneziphius spp., Choneziphius spp. and, to a lesser extent T. atlanticus, and interpreted as areas of origin for rostral and facial muscles (Bianucci et al., 2013; Miján et al., 2017). As in many other ziphiids, the right premaxillary sac fossa is much broader than the left. Similar to Caviziphius altirostris, SGHN MA0920, and NMB 002, the anterior margin of each fossa is rounded in dorsal view. Each fossa is similarly transversely and anteroposteriorly deeply concave, with a dorsoventrally thick anterior margin (32 and 29 mm higher than preserved floor of the right and left fossae, respectively). No premaxillary foramen could be detected on the irregular surface of any fossa. Similar to NMB 002 and SGHN MA0920, a rostral maxillary eminence is only preserved on the right side. Either a left eminence was present originally, being lower and more laterally located, as in three specimens of T. atlanticus, or there was no left eminence at all, as in two specimens of the latter species. The right rostral maxillary eminence has a maximum height of 33 mm (taken from the ventral margin of the maxilla), and an anterior slope that is not as steep as in NMB 002. The eminence overhangs a deep and broad sulcus leaving anteriorly from the main dorsal infraorbital foramen, located anterolateral to the premaxillary sac fossa. This sulcus disappears at about the anterior margin of the eminence, whereas a narrower sulcus, though with a similar depth, leaves posterolaterally. A smaller, second dorsal infraorbital foramen is located 40 mm anterior to the large foramen. The right maxilla is similarly pierced by a large foramen anterolateral to the premaxillary sac fossa (partly preserved, anteroposterior diameter estimated at 10 mm) and a second, smaller foramen (transverse diameter smaller than 4 mm) located 57 mm more anteriorly.
28No individual alveoli could be detected along the highly reduced (though somewhat worn) alveolar groove (Fig. 3). A few foramina, each followed anteriorly by a deep sulcus, are observed just dorsal to this vestigial groove on the anterior half of the rostrum.
29On the palate, ventromedial portions of the two palatines are preserved as a thin plate covering the corresponding maxilla (Figs 2B, 3). Linear marks of the maxilla-palatine suture are observed anteriorly and laterally to these palatine fragments. The pterygopalatine canal is exposed for most of its length on the left side, with its anterior end corresponding to the major palatine foramen. Two pairs of palatine foramina are visible along the ventral exposure of the vomer. The latter has a maximum width of 13 mm and a preserved length of 230 mm. The vomer is also exposed between the palatines at rostrum base; this exposure gradually broadens posterolaterodorsally, along the anteromedial wall of the partly preserved choanae.
3.5. Discussion on systematic affinities
30The many similarities in dimensions and shape of IRSNB M.2333 with NMB 002, also from the area of Antwerp, strongly suggest that these two specimens belong to the same species. Particularly, the extent of the bulge of premaxillae on the rostrum, the presence of a similar elevation and longitudinal depressions anterior to the premaxillary sac fossae, the rounded anterior outline of the highly asymmetric, deeply concave, and thick-margined premaxillary sac fossae, and the morphology of the right rostral maxillary eminence and surrounding features all support a referral to the same taxon. Unfortunately, this new specimen does not allow for a more detailed comparison with the holotype of Caviziphius altirostris, also from the Neogene of the Antwerp area, due to the minimum overlap of the preserved parts. Still, the new specimen has a height of the rostrum at its base that is intermediary between the holotype of C. altirostris and NMB 002, further strengthening the systematic affinities proposed by Bianucci et al. (2013). Pending the discovery of a more complete cranium, including both the rostrum and the vertex, we provisionally refer both NMB 002 and IRSNB M.2333 to Caviziphius aff. C. altirostris and confirm the many similarities with Caviziphius aff. Caviziphius sp. SGHN MA0920 dredged from the seafloor off Galicia (Spain).
4. Biostratigraphy
4.1. Palynological content of sediment samples
31The dinoflagellate cyst diversity and richness are very low to poor in all samples and most of the cysts are furthermore badly preserved. Consequently, only limited number of species could be identified in every sample. Sixteen samples were analysed (Table 1) and three samples (Z13, Z14 and Z16) held no palynomorphs at all. Three other samples (Z1, Z3 and Z6) are considered as almost sterile, holding only a few partly deteriorated, robust specimens of the genera Spiniferites and Achomosphaera. A total of 32 dinoflagellate cyst species, 3 acritarch species, and one green alga species were recorded. The relative dating of the ten samples yielding sufficient marine palynomorphs relies on the presence of seven dinoflagellate cyst index species with a well-known stratigraphic range (Fig. 4). The other dinoflagellate cyst species are long-ranging and have therefore no biostratigraphic significance.
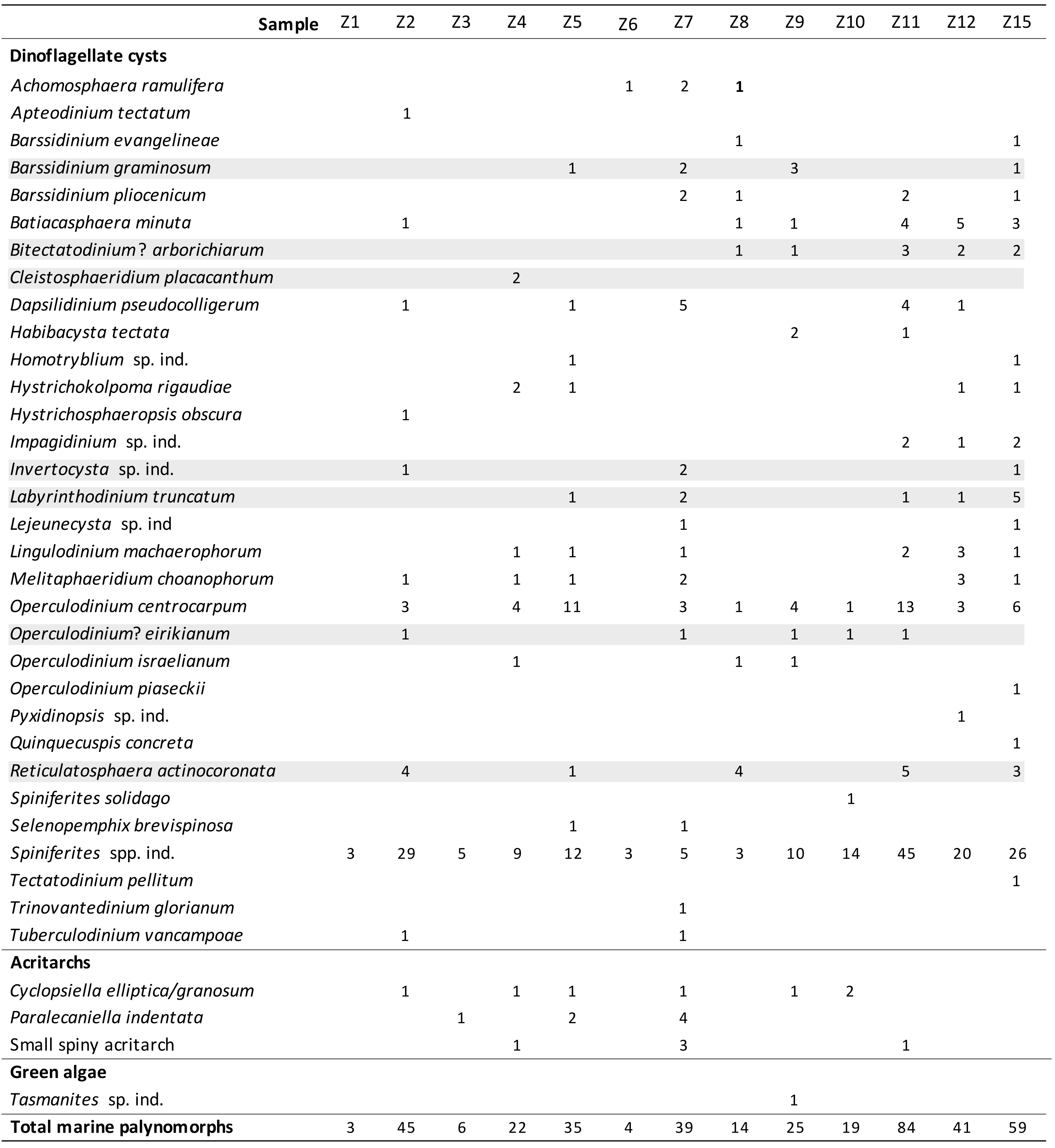
Table 1. Distribution of the marine palynomorphs (raw counts) in samples Z1 to Z15 taken from ziphiid fossil crania and rostra housed in the IRSNB collection.
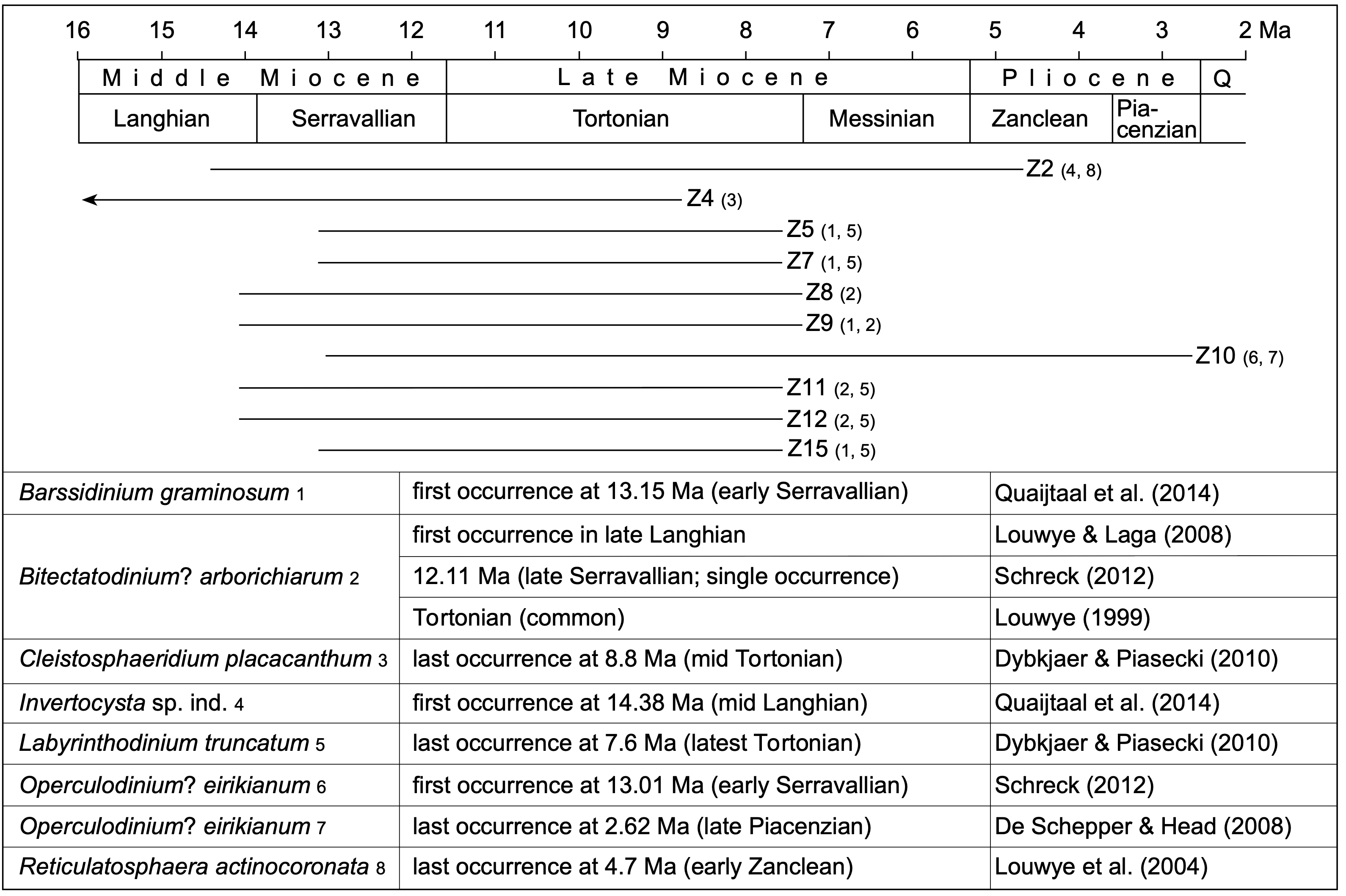
Figure 4. Stratigraphic range of the dinoflagellate cyst index species together with the relative dating of sediment samples taken from ziphiid fossil crania and rostra of the IRSNB collection, based on the range of selected dinoflagellate cyst index species. Z2, Z4, ..., Z15 are sediment samples that yielded sufficient marine palynomorphs for a biostratigraphic assessment. Numbers 1–8 associated to sediment sample numbers Z2, Z4, ..., Z15 correspond to the dinoflagellate cyst index species listed below. Q: Quaternary. Chronostratigraphy is based on the International Chronostratigraphic Chart v2023/04 (International Commission on Stratigraphy).
4.2. Age interval for ziphiid taxa from the Neogene of the North Sea
32As discussed in the material and methods section above, we consider it likely that the geological age of the analysed sediment corresponds to the time of initial burial for the sampled ziphiid specimen. Combining the new ages recovered here (Fig. 4) with data from previous works, the list of temporal ranges for ziphiids of the North Sea Neogene can be updated compared to Bianucci et al. (2016a, table 5) (Fig. 5):
33Aporotus recurvirostris - early Serravallian to Tortonian (13.15–7.6 Ma) - this work (sample Z15). Based on the presence of Bitectatodinium? arborichiarum in the sample, an origin in the Diest Formation (9.54–8.8 Ma, Tortonian) could be tentatively proposed, but we keep a more cautious approach, not excluding an early to late Serravallian age (that would imply a winnowing and reworking phase, unfortunately difficult to test with the data in hand). Fragmentary remains of A. recurvirostris recovered in Kessel (15 km SE from Antwerp) were tentatively referred to the Middle Miocene Antwerpen Sands Member of the Berchem Formation (Lambert, 2005).
34Archaeoziphius microglenoideus - Langhian to early Serravallian (15–13.2 Ma) - Lambert & Louwye (2006). This is the geologically oldest named ziphiid species, a member of the subfamily Berardiinae.
35Beneziphius brevirostris - early to mid-Serravallian (13.2–12.8 Ma) - Miján et al. (2017). This time interval is shorter than the interval for the sister species Beneziphius cetariensis, from the Middle Miocene to Early Pliocene of the seafloor off Galicia (Miján et al., 2017).
36Caviziphius aff. Caviziphius altirostris - late Langhian to Tortonian - this work (sample Z9). Based on the presence of Bitectatodinium? arborichiarum in the sample, an origin in the Diest Formation (9.54–8.8 Ma, Tortonian) could be tentatively proposed, but we keep a more cautious approach, not excluding an early to late Serravallian age. The Belgian specimens share anatomical similarities with aff. Caviziphius sp. SGHN MA0920, recorded by trawling off the Galician coast, but for which no precise age data is available (Bianucci et al., 2013)
37Choneziphius planirostris - early Serravallian to mid-Tortonian (13.15–8.8 Ma) - this work (combining samples Z4 and Z5). This interval overlaps with the report of an in situ find in the Deurne Sands Member (or possibly the recently described Borsbeek Member) of the Diest Formation by an avocational palaeontologist (the late P. Gigase; Lambert, 2005), being older than the proposed interval of the sister species Choneziphius leidyi, from the Atlantic seafloor off Portugal and Spain, tentatively dated from the Messinian to Zanclean (6.1–4.4 Ma) (Bianucci et al., 2013; Antunes et al., 2015).
38Dagonodum mojnum - Tortonian (c. 9.9–7.2 Ma) - Ramassamy (2016). Recovered from the Gram Formation (southern Jutland, Denmark), this longirostrine ziphiid is a member of the Messapicetus clade.
39Mesoplodon posti - Zanclean (4.86–3.9 Ma) - Lambert & Louwye (2016). This species provides an upper calibration point for the divergence date of the species-rich genus Mesoplodon (subfamily Hyperoodontinae), as the only relatively precisely dated extinct species.
40Ziphiidae indet. IRSNB M.3778 - early Serravallian to early Zanclean (13.01–4.7 Ma) - this work (combining samples Z2 and Z10). Previously referred to Choneziphius macrops (Lambert, 2005), this specimen is now considered Ziphiidae indet. due to its fragmentary state (Bianucci et al., 2013). Still, this record indicates the presence of an additional, large species of the Messapicetus clade in the southern North Sea sometime in the late Middle Miocene - earliest Pliocene interval.
41Ziphirostrum marginatum - early Serravallian to latest Tortonian (13.15–7.6 Ma) - this work (combining samples Z7 and Z8). Based on the presence of Bitectatodinium? arborichiarum in sample Z8, an origin in the Diest Formation (9.54–8.8 Ma, Tortonian) could be tentatively proposed, but we keep a more cautious approach, not excluding a Serravallian age. Nevertheless, such an origin in the Diest Formation matches the in situ find of one specimen of Z. marginatum in the Borsbeek Member of the Diest Formation at AZ Monica (previously Middelares Hospital), Deurne (Antwerp suburbs) (Lambert, 2005; Goolaerts et al., 2020).
42Ziphirostrum turniense - late Langhian to latest Tortonian - this work (samples Z11 and Z12). Based on the presence of Bitectatodinium? arborichiarum in the two samples, an origin in the Diest Formation (9.54–8.8 Ma, middle Tortonian) could be tentatively proposed, but we keep a more cautious approach, not excluding a Serravallian age.
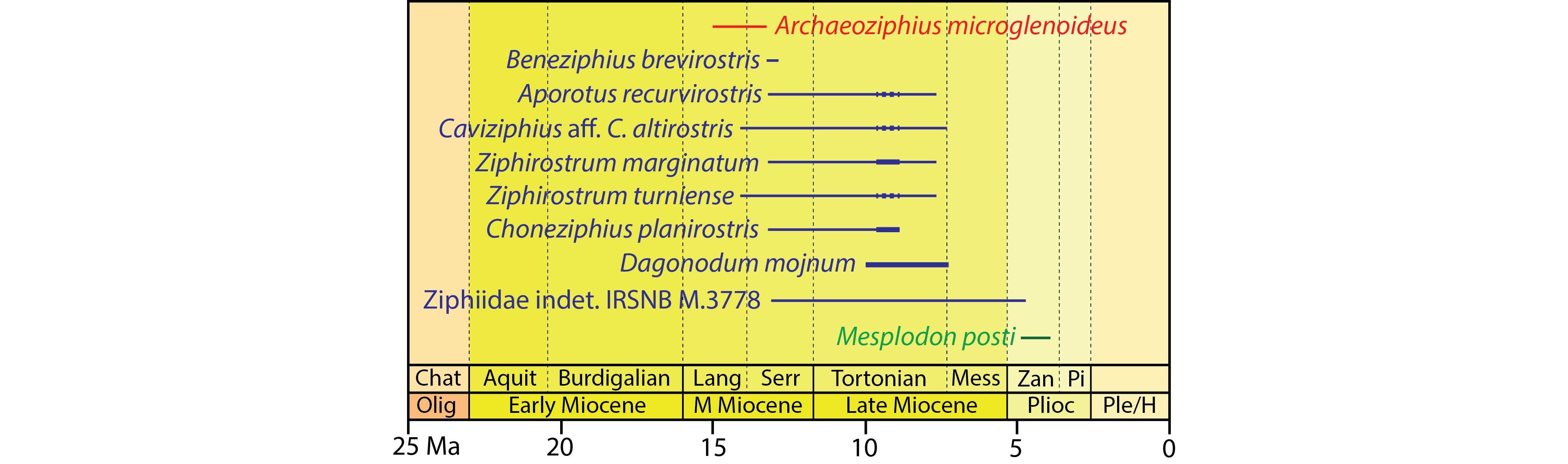
Figure 5. Chronostratigraphic ranges of extinct ziphiid species from the Neogene of the North Sea. Except for Dagonodum mojnum (Jutland, Denmark), all species are based on fossils from the north of Belgium. Ranges are based on past studies (Lambert & Louwye, 2006; Lambert & Louwye, 2016; Ramassamy, 2016; Miján et al., 2017) and new palynological analyses of sediment associated to fossils. Ziphiid species for which no informative age interval is available were omitted. Thin lines correspond to time intervals obtained from index dinoflagellate species (see Fig. 4), thicker dotted lines to more tentative intervals proposed due to the record of Bitectatodinium? arborichiarum, and thicker solid lines to ziphiid specimens found in situ in well-dated lithological units. Red for Berardiinae, blue for members of the Messapicetus clade, green for Hyperoodontinae. Abbreviations: Aquit, Aquitanian; Chat, Chattian; H, Holocene; Lang, Langhian; M, Middle; Mess, Messinian; Olig, Oligocene; Pi, Piacenzian; Ple, Pleistocene; Plioc, Pliocene; Serr, Serravallian; Zan, Zanclean.
5. Discussion
43Most extant ziphiids are deep divers, preying upon meso- to benthopelagic squid and fish (McLeod, 2018). As a consequence, no modern ziphiid species can be considered a permanent resident of the shallow southern North Sea (McLeod et al., 2006), and rare strandings along the Belgian and Dutch coasts generally correspond to two species, Sowerby’s beaked whale Mesoplodon bidens, and, even more rarely, the northern bottlenose whale Hyperoodon ampullatus, whose habitat has to be found farther north in the North Sea or in deep regions of the North Atlantic (De Smet, 1974; Haelters et al., 2018; Keijl et al., 2021 and references therein).
44During the Neogene, the water depth in the southern part of the North Sea changed through time, with among others a marked decrease in the area of deep marine (200–600+ m) and outer shelf deposits, occurring from the Middle Miocene to the Late Miocene and Pliocene, in relation with climate cooling and eustatic sea-level fall following the Mid-Miocene Climatic Optimum (Huuse, 2002; Anell et al., 2012).
45While the oldest ziphiid records from the North Sea originate from Langhian to earliest Serravallian deposits (the small berardiine Archaeoziphius microglenoideus), a higher number of ziphiid species (seven to eight, all of them being members of the Messapicetus clade) is recovered from a late Langhian to Tortonian interval. Based on in situ finds (for Dagonodum mojnum, Choneziphius planirostris, and Ziphirostrum marginatum) and more tentative biostratigraphic intervals (for Aporotus recurvirostris, Caviziphius aff. C. altirostris, and Ziphirostrum turniense), the chronostratigraphic range of three to six of these taxa can be further narrowed to the mid- to late Tortonian. It is suspected that these numbers could be underestimates, as the chronostratigraphic range of several other ziphiid species (Aporotus dicyrtus and at least three additional taxa represented by isolated rostra) from the Belgian Neogene is even more poorly constrained (Lambert, 2005). Noteworthily, several of the Tortonian species (C. planirostris, Z. marginatum, and, more tentatively A. recurvirostris) are known from a relatively high number of specimens in the IRSNB collection (18 for C. planirostris, 17 for Z. marginatum, and 10 for A. recurvirostris), all originating from the north of Belgium (Lambert, 2005). Though the preservation of so many specimens is at least partly due to the high compactness and thickness (= pachyosteosclerosis) of rostral and facial bones in these species (Lambert et al., 2011), recovering a higher ziphiid diversity in the North Sea at times of local shallowing may sound counterintuitive considering the deep, pelagic habitat of most extant beaked whales. It is therefore likely that at least part of the Neogene ziphiids, especially in the Messapicetus clade, were not deep divers, a hypothesis that has been previously supported by sedimentological and taphonomic data from ziphiid-rich localities of the Late Miocene of Peru and Italy (Bianucci et al., 2010; Lambert et al., 2015; Bianucci et al., 2016b). Interestingly, with the exception of the longirostrine Dagonodum mojnum, all the ziphiids from the Late Miocene of the North Sea share anatomical features (vestigial upper alveolar groove, located relatively high along the lateral surface of the rostrum, and generally lacking any deep marks for individual alveoli) that suggest a strong reduction of the dentition and point to a suction feeding strategy (Lambert, 2005; Johnston & Berta, 2011; Ramassamy, 2016). Among extant odontocetes, such a feeding strategy is often associated with deep diving, not only in ziphiids, but also in physeteroids and some large delphinids (Werth, 2006; Watwood et al., 2006; Soto et al., 2008). Nevertheless, suction feeding is also recorded in odontocetes that are known to feed at various depths, including shallower waters (e.g., the delphinid Grampus griseus and the monodontids Delphinapterus leucas and Monodon monoceros; Johnston & Berta, 2011), and specialization towards suction feeding should not be considered too strictly as a proxy for deep diving in toothed whales. Still, it is worth noting that, as mentioned above, most of these North Sea specimens display various degrees of pachyosteosclerosis in cranial bones, a character that has been proposed to have evolved convergently in at least two ziphiid lineages, possibly in relation with the acquisition of deep diving abilities (Gol’din, 2014; Bianucci et al., 2016a).
46One may ask which physical and/or biological parameters drove this local increase in ziphiid diversity. On a broader geographic scale, taxonomic similarities were noted between Miocene ziphiid faunas from (1) the North Sea and the northeastern Atlantic off the Iberian Peninsula, with several genera from the Messapicetus clade recovered in both areas (Beneziphius, Choneziphius, and more tentatively Caviziphius and Ziphirostrum), (2) the Mediterranean, the northeastern Atlantic off the Iberian Peninsula, and the east coast of USA (Tusciziphius), and (3) the Mediterranean and the southeastern Pacific (Messapicetus) (Bianucci et al., 2013; Bianucci et al., 2016b; Miján et al., 2017; Bianucci et al., 2019). Furthermore, when available, chronostratigraphic data point to a Tortonian to Messinian age for a majority of the fossil records of the Messapicetus clade (Bianucci, 1997; Bianucci et al., 2016a; Bianucci et al., 2016b; Ramassamy, 2016; this work). A more global Late Miocene radiation and long-distance dispersion of this clade may thus be proposed, pending further improvement of the chronostratigraphic context for fossils recovered from the seafloor off the Iberian Peninsula and the east coast USA. If confirmed, such a radiation should be confronted to Middle to Late Miocene global climatic and oceanographic changes, including temperature and sea level drops and the establishment of the Northern Hemisphere ice sheets (Zachos et al., 2001). In this context, it is worth noting the synchronous decline of several clades of hyper-longirostrine dolphins (Marx et al., 2016; Lambert & Goolaerts, 2022), which may have freed ecological niches for beaked whales of the Messapicetus clade in coastal environments, as represented by the East Pisco basin in Peru, the southern North Sea basin, and part of the Mediterranean. More palaeontological, palaeoecological, and biostratigraphic data will be needed to investigate on the one side the extinction of the Messapicetus clade during the Plio-Pleistocene and, on the other side, the mode and tempo of the shift of crown beaked whales to deeper, meso- to benthopelagic feeding grounds, focusing for example on the rich ziphiid assemblages from deep sea deposits of the Southern Ocean.
6. Acknowledgements
47For having generously donated the specimen of Caviziphius aff. Caviziphius altirostris IRSNB M.2333 to the IRSNB, we would like to warmly thank the following persons from the Natuurhistorisch Museum Boekenberg (Antwerp): Koen Maes (president of the board), Bert Spilthoorn (member of the board), and Jan Peeters. We extend our thanks to the late Herman Peeters, Myrjam Buelens, and Kris Van Oosterwijck for providing valuable information about the localities, the geological context, and the dates of discovery of IRSNB M.2333 and NMB 002, to Sabine Van Cauwenberghe for the palynological maceration of the samples, and to the two reviewers, Dirk Munsterman and Giovanni Bianucci, for their constructive comments.
Author contribution
48O.L. and S.L. conceived and designed the project. O.L. performed the description and comparison of the fossil ziphiid material. S.L. analysed the palynological content of the sediment samples and made the biostratigraphic interpretations. M.B. collected data on the geographic origin of several fossil ziphiid specimens and the context of their discovery. O.L. and S.L. wrote the first draft of the manuscript. The three authors discussed the results and reviewed the manuscript.
Data availability
49All fossil ziphiid specimens studied in this work are curated in official, public collections at the IRSNB, NMB, and SGHN, guaranteeing their long-term safekeeping and availability to other researchers for future studies.
References
50Abel, O., 1905. Les Odontocètes du Boldérien (Miocène supérieur) d’Anvers. Mémoires du Musée royal d’Histoire naturelle de Belgique, 3, 1–155.
51Anell, I., Thybo, H. & Rasmussen, E., 2012. A synthesis of Cenozoic sedimentation in the North Sea. Basin Research, 24, 154–179. https://doi.org/10.1111/j.1365-2117.2011.00517.x
52Antunes, M.T., Legoinha, P. & Balbino, A., 2015. Megalodon, mako shark and planktonic foraminifera from the continental shelf off Portugal and their age. Geologica Acta, 13, 181–190. https://doi.org/10.1344/GeologicaActa2015.13.3.1
53Bianucci, G., 1997. The Odontoceti (Mammalia Cetacea) from Italian Pliocene. The Ziphiidae. Palaeontographia italica, 84, 163–192.
54Bianucci, G. & Post, K., 2005. Caviziphius altirostris, a new beaked whale from the Miocene southern North Sea basin. Deinsea, 11, 1–6.
55Bianucci, G., Lambert, O. & Post, K., 2007. A high diversity in fossil beaked whales (Odontoceti, Ziphiidae) recovered by trawling from the sea floor off South Africa. Geodiversitas, 29, 5–62.
56Bianucci, G., Lambert, O. & Post, K., 2010. High concentration of long-snouted beaked whales (genus Messapicetus) from the Miocene of Peru. Palaeontology, 53, 1077–1098. https://doi.org/10.1111/j.1475-4983.2010.00995.x
57Bianucci, G., Miján, I., Lambert, O., Post, K. & Mateus, O., 2013. Bizarre fossil beaked whales (Odontoceti, Ziphiidae) fished from the Atlantic Ocean floor off the Iberian Peninsula. Geodiversitas, 35, 105–153. https://doi.org/10.5252/g2013n1a6
58Bianucci, G., Di Celma, C., Urbina, M. & Lambert, O., 2016a. New beaked whales from the late Miocene of Peru and evidence for convergent evolution in stem and crown Ziphiidae (Cetacea, Odontoceti). PeerJ, 4, e2479. https://doi.org/10.7717/peerj.2479
59Bianucci, G., Collareta, A., Post, K., Varola, A. & Lambert, O., 2016b. A new record of Messapicetus from the Pietra leccese (Late Miocene, Southern Italy): antitropical distribution in a fossil beaked whale (Cetacea, Ziphiidae). Rivista Italiana di Paleontologia e Stratigrafia, 122, 63–74.
60Bianucci, G., Llàcer, S., Cardona, J.Q., Collareta, A. & Florit, A. R., 2019. A new beaked whale record from the upper Miocene of Menorca, Balearic Islands, based on CT-scan analysis of limestone slabs. Acta Palaeontologica Polonica, 64, 291–302. https://doi.org/10.4202/app.00593.2019
61Bisconti, M., Munsterman, D.K. & Post, K., 2019. A new balaenopterid whale from the late Miocene of the Southern North Sea Basin and the evolution of balaenopterid diversity (Cetacea, Mysticeti). PeerJ, 7, e6915. https://doi.org/10.7717/peerj.6915
62Bosselaers, M., 2014. Westerschelde geeft zeer zeldzame fossiele dolfijn prijs. Zeeland, 23, 101–102.
63Bosselaers, M., Herman, J., Hoedemakers, K., Lambert, O., Marquet, R. & Wouters K., 2004. Geology and palaeontology of a temporary exposure of the Late Miocene Deurne Sand Member in Antwerpen (N. Belgium). Geologica Belgica, 7, 27–39.
64Brisson, M.-J., 1762. Regnum Animale in classes IX. Distributum, sive synopsis methodica. Apud Theodorum Haak, Lugduni Batavorum, 296 p.
65Buono, M.R. & Cozzuol, M.A., 2013. A new beaked whale (Cetacea, Odontoceti) from the Late Miocene of Patagonia, Argentina. Journal of Vertebrate Paleontology, 33, 986–997. https://doi.org/10.1080/02724634.2013.752377
66Cuvier, G., 1823. Recherches sur les ossements fossiles, tome 5 (1ère partie). G. Dufour et E. D’Ocagne, Paris, 405 p.
67De Schepper, S. & Head, M.J., 2008. Age calibration of dinoflagellate cyst and acritarch events in the Pliocene–Pleistocene of the eastern North Atlantic (DSDP Hole 610A). Stratigraphy, 5/2, 137–161.
68De Schepper, S., Head, M.J. & Louwye, S., 2009. Pliocene dinoflagellate cyst stratigraphy, palaeoecology and sequence stratigraphy of the Tunnel-Canal Dock, Belgium. Geological Magazine, 146, 92–112. https://doi.org/10.1017/S0016756808005438
69De Smet, W.M.A., 1974. Inventaris of de walvisachtigen (Cetacea) van de Vlaamse kust en de Schelde. Bulletin de l’Institut royal des Sciences naturelles de Belgique, 50, 1–156.
70du Bus, B.A.L., 1868. Sur différents Ziphiides nouveaux du Crag d’Anvers. Bulletin de l’Académie royale des Sciences, des Lettres et des Beaux-Arts de Belgique, 25, 621–630.
71Dybkjær, K. & Piasecki, S., 2010. Neogene dinocyst zonation for the eastern North Sea Basin, Denmark. Review of Palaeobotany and Palynology, 161, 1–29. https://doi.org/10.1016/j.revpalbo.2010.02.005
72Flower, W.H., 1867. Description of the skeleton of Inia geoffrensis and the skull of Pontoporia blainvillii, with remarks on the systematic position of these animals in the Order Cetacea. Transactions of the Zoological Society of London, 6, 87–116. https://doi.org/10.1111/j.1096-3642.1867.tb00572.x
73Fordyce, R.E. & Muizon, C. de., 2001. Evolutionary history of cetaceans: a review. In Mazin, J.-M. & Buffrénil, V. de (eds), Secondary Adaptation of Tetrapods to Life in Water. Verlag Dr. Friedrich Pfeil, München, 169–233.
74Gol’din, P., 2014. ‘Antlers inside’: are the skull structures of beaked whales (Cetacea: Ziphiidae) used for echoic imaging and visual display? Biological Journal of the Linnean Society, 113, 510–515. https://doi.org/10.1111/bij.12337
75Gol’din, P.E. & Vishnyakova K.A., 2011. Africanacetus from the sub-Antarctic region: the southernmost record of fossil beaked whales. Acta Palaeontologica Polonica, 58, 445–452. https://doi.org/10.4202/app.2011.0097
76Goolaerts, S., De Ceuster, J., Mollen, F.H., Gijsen, B., Bosselaers, M., Lambert, O., Uchman, A., Van Herck, M., Adriaens, R., Houthuys, R., Louwye, S., Bruneel, Y., Elsen, J. & Hoedemakers, K., 2020. The upper Miocene Deurne Member of the Diest Formation revisited: unexpected results from the study of a large temporary outcrop near Antwerp International Airport, Belgium. Geologica Belgica, 23, 219–252. https://doi.org/10.20341/gb.2020.011
77Gray, J.E., 1850. Catalogue of the specimens of Mammalia in the collections of the British Museum. Part I – Cetacea. London, 153 p.
78Haelters, J., Kerckhof, F. & Jauniaux, T., 2018. Strandings of cetaceans in Belgium from 1995 to 2017. Lutra, 61/1, 107–126.
79Heyning, J.E. & Mead, J.G., 1996. Suction feeding in beaked whales: morphological and observational evidence. Contributions in Science, Natural History Museum of Los Angeles County, 464, 1–12. https://doi.org/10.5962/p.226802
80Huuse, M., 2002. Late Cenozoic palaeogeography of the eastern North Sea Basin: climatic vs tectonic forcing of basin margin uplift and deltaic progradation. Bulletin of the Geological Society of Denmark, 49, 145–170.
81Johnston, C. & Berta, A., 2011. Comparative anatomy and evolutionary history of suction feeding in cetaceans. Marine Mammal Science, 27, 493–513. https://doi.org/10.1111/j.1748-7692.2010.00420.x
82Keijl, G.O., Bakker Paiva, M.F., IJsseldijk, L.L. & Kamminga, P., 2021. Cetaceans stranded in the Netherlands in 2015-2019. Lutra, 64/1, 19–44.
83Laga, P., Louwye, S. & Mostaert, F., 2006. Disused Neogene and Quaternary regional stages from Belgium: Bolderian, Houthalenian, Antwerpian, Diestian, Deurnian, Kasterlian, Kattendijkian, Scaldisian, Poederlian, Merksemian and Flandrian. Geologica Belgica, 9, 215–224.
84Lambert, O., 2005. Systematics and phylogeny of the fossil beaked whales Ziphirostrum du Bus, 1868 and Choneziphius Duvernoy, 1851 (Cetacea, Odontoceti), from the Neogene of Antwerp (North of Belgium). Geodiversitas, 27, 443–497.
85Lambert, O. & Goolaerts, S., 2022. Late Miocene survival of a hyper-longirostrine dolphin and the Neogene to Recent evolution of rostrum proportions among odontocetes. Journal of Mammalian Evolution, 29, 99–111. https://doi.org/10.1007/s10914-021-09573-6
86Lambert, O. & Louwye, S., 2006. Archaeoziphius microglenoideus, a new primitive beaked whale (Mammalia, Cetacea, Odontoceti) from the Middle Miocene of Belgium. Journal of Vertebrate Paleontology, 26, 182–191. https://doi.org/10.1671/0272-4634(2006)26[182:AMANPB]2.0.CO;2
87Lambert, O. & Louwye S., 2016. A new early Pliocene species of Mesoplodon: a calibration mark for the radiation of this species-rich beaked whale genus. Journal of Vertebrate Paleontology, 36, e1055754. https://doi.org/10.1080/02724634.2015.1055754
88Lambert, O., Buffrénil, V. de & Muizon, C. de, 2011. Rostral densification in beaked whales: diverse processes for a similar pattern. Comptes Rendus Palevol, 10, 453–468. https://doi.org/10.1016/j.crpv.2011.03.012
89Lambert, O., Collareta, A., Landini, W., Post, K., Ramassamy, B., Di Celma, C., Urbina, M. & Bianucci, G., 2015. No deep diving: evidence of predation on epipelagic fish for a stem beaked whale from the Late Miocene of Peru. Proceedings of the Royal Society B, 282, 20151530. https://doi.org/10.1098/rspb.2015.1530
90Lambert, O., Muizon, C. de, Duhamel, G. & van der Plicht J., 2018. Neogene and Quaternary fossil remains of beaked whales (Cetacea, Odontoceti, Ziphiidae) from deep-sea deposits off Crozet and Kerguelen islands, Southern Ocean. Geodiversitas, 40, 135–160. https://doi.org/10.5252/geodiversitas2018v40a6
91Leidy, J., 1877. Description of vertebrate remains, chiefly from the Phosphate Beds of South Carolina. Journal of the Academy of Natural Sciences of Philadelphia, 8, 209–261.
92Louwye, S., 1999. New species of organic-walled dinoflagellates and acritarchs from the Upper Miocene Diest Formation, northern Belgium (southern North Sea Basin). Review of Palaeobotany and Palynology, 107/1-2, 109–123. https://doi.org/10.1016/S0034-6667(99)00012-3
93Louwye, S. & Laga, P., 2008. Dinoflagellate cyst stratigraphy and palaeoenvironment of the marginal marine Middle and Upper Miocene of the eastern Campine area, northern Belgium (southern North Sea Basin). Geological Journal, 43/1, 75–94. https://doi.org/10.1002/gj.1103
94Louwye, S., Head, M.J. & De Schepper, S., 2004. Dinoflagellate cyst stratigraphy and palaeoecology of the Pliocene in northern Belgium, southern North Sea Basin. Geological Magazine, 141/3, 353–378. https://doi.org/10.1017/S0016756804009136
95Louwye, S., Deckers, J., Verhaegen, J., Adriaens, R. & Vandenberghe, N., 2020. A review of the lower and middle Miocene of northern Belgium. Geologica Belgica, 23, 137–156. https://doi.org/10.20341/gb.2020.010
96Marx, F.G., Lambert O. & Uhen, M.D., 2016. Cetacean Paleobiology. John Wiley & Sons, Chichester, 319 p. https://doi.org/10.1002/9781118561546
97McLeod, C.D., 2018. Beaked whales, overview. In Würsig, B., Thewissen, J.G.M. & Kovacs, K.M. (eds), Encyclopedia of Marine Mammals. 3rd ed. Academic Press, London, 80–83. https://doi.org/10.1016/B978-0-12-804327-1.00062-5
98McLeod, C.D., Perrin, W.F., Pitman, R., Barlow, J., Ballance, L., D’Amico, A., Gerrodette, T., Joyce, G., Mullin, K.D., Palka, D.L. & Waring, G.T., 2006. Known and inferred distributions of beaked whale species (Cetacea: Ziphiidae). Journal of Cetacean Research and Management, 7, 271–286. https://doi.org/10.47536/jcrm.v7i3.737
99Mead, J.G. & Fordyce, R.E., 2009. The therian skull: a lexicon with emphasis on the odontocetes. Smithsonian Contributions to Zoology, 627, 1–248. https://doi.org/10.5479/si.00810282.627
100Melville, H., 1851. Moby-Dick or, The Whale. Harper & Brothers Publishers, New York, 635 p.
101Miján, I., Louwye, S. & Lambert, O., 2017. A new Beneziphius beaked whale from the ocean floor off Galicia, Spain and biostratigraphic reassessment of the type species. Acta Palaeontologica Polonica, 62, 211–220. https://doi.org/10.4202/app.00309.2016
102Misonne, X., 1958. Faune du Tertiaire et du Pléistocène inférieur de Belgique (Oiseaux et Mammifères). Bulletin de l’Institut royal des Sciences naturelles de Belgique, 34/5, 1–36.
103Nozaki, T., Takaya, Y., Toyofuku, T., Tokumaru, A., Goto, K.T., Chang, Q., Kimura, J.I., Kato, Y., Suzuki, K., Augustin, A.H. & Kitazato, H., 2017. Depositional age of a fossil whale bone from São Paulo Ridge, South Atlantic Ocean, based on Os isotope stratigraphy of a ferromanganese crust. Resource Geology, 67, 442–450. https://doi.org/10.1111/rge.12138
104Post, K. & Reumer, J.W., 2016. History and future of paleontological surveys in the Westerschelde Estuary (Province of Zeeland, the Netherlands). Deinsea, 16, 1–9.
105Quaijtaal, W., Donders, T., Persico, D. & Louwye, S., 2014. Characterizing the middle Miocene Mi-events in the Eastern North Atlantic realm: A first high-resolution marine palynological record from the Porcupine Basin. Palaeogeography, Palaeoclimatology, Palaeoecology, 399, 140–159. https://doi.org/10.1016/j.palaeo.2014.02.017
106Ramassamy, B., 2016. Description of a new long‐snouted beaked whale from the Late Miocene of Denmark: evolution of suction feeding and sexual dimorphism in the Ziphiidae (Cetacea: Odontoceti). Zoological Journal of the Linnean Society, 178, 381–409. https://doi.org/10.1111/zoj.12418
107Ramassamy, B. & Lauridsen, H., 2019. A new specimen of Ziphiidae (Cetacea, Odontoceti) from the late Miocene of Denmark with morphological evidence for suction feeding behaviour. Royal Society Open Science, 6, 191347. https://doi.org/10.1098/rsos.191347
108Schreck, M., 2012. Biostratigraphy and Paleoenvironment in the Neogene of the High Northern Latitudes: Insights from the palynomorph record of ODP Hole 907A in the Iceland Sea. Unpublished Ph.D. Thesis, University of Bremen, Bremerhaven, 220 p.
109Soto, N.A., Johnson, M.P., Madsen, P.T., Díaz, F., Domínguez, I., Brito, A. & Tyack, P., 2008. Cheetahs of the deep sea: deep foraging sprints in short-finned pilot whales off Tenerife (Canary Islands). Journal of Animal Ecology, 77, 936–947. https://doi.org/10.1111/j.1365-2656.2008.01393.x
110Tyack, P.L., Johnson, M., Soto, N.A., Sturlese, A. & Madsen, P.T., 2006. Extreme diving of beaked whales. Journal of Experimental Biology, 209, 4238–4253. https://doi.org/10.1242/jeb.02505
111Uhen, M.D., 2008. New protocetid whales from Alabama and Mississippi, and a new cetacean clade, Pelagiceti. Journal of Vertebrate Paleontology, 28, 589–593. https://doi.org/10.1671/0272-4634(2008)28[589:NPWFAA]2.0.CO;2
112Watwood, S.L., Miller, P.J.O., Johnson, M., Madsen, P.T. & Tyack, P.L., 2006. Deep‐diving foraging behaviour of sperm whales (Physeter macrocephalus). Journal of Animal Ecology, 75, 814–825. https://doi.org/10.1111/j.1365-2656.2006.01101.x
113Werth, A.J., 2006. Mandibular and dental variation and the evolution of suction feeding in Odontoceti. Journal of Mammalogy, 87, 579–588. https://doi.org/10.1644/05-MAMM-A-279R1.1
114Zachos, J., Pagani, M., Sloan, L., Thomas, E. & Billups, K., 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292, 686–693. https://doi.org/10.1126/science.1059412
115Manuscript received 22.06.2023, accepted in revised form 30.08.2023, available online 12.10.2023.






